Abstract
The prevalence of autism spectrum disorders (ASDs) differs substantially between males and females, suggesting that sex-related neurodevelopmental factors are central to ASD pathogenesis. Numerous studies have suggested that abnormal brain specialization patterns and poor regional cooperation contribute to ASD pathogenesis, but relatively little is known about the related sex differences. Therefore, this study examined sex differences in brain functional specialization and cooperation among children with ASD. The autonomy index (AI) and connectivity between functionally homotopic voxels (CFH) derived from resting-state functional magnetic resonance imaging (rs-fMRI) were compared between 58 male and 13 female children with ASD. In addition, correlations were examined between regional CFH values showing significant sex differences and symptom scores on the autism behavior checklist (ABC) and childhood autism rating scale (CARS). Male children with ASD demonstrated significantly greater CFH in the left fusiform gyrus (FG) and right opercular part of the inferior frontal gyrus (IFGoperc) than female children with ASD. In addition, the CFH value of the left FG in male children with ASD was negatively correlated with total ABC score and subscale scores for sensory and social abilities. In contrast, no sex differences were detected in brain specialization. These regional abnormalities in interhemispheric cooperation among male children with ASD may provide clues to the neural mechanisms underlying sex differences in ASD symptomatology and prevalence. Autism spectrum disorders, sex, resting-state functional magnetic resonance imaging, cerebral specialization, interhemispheric cooperation.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental condition characterized by impaired communication and social interaction skills, stereotyped repetitive behaviors, and restricted interests1. While numerous ASD therapies have been developed targeting language, socialization, behavioral control, and motor skills, these autistic traits and behaviors frequently endure into adulthood, limiting functional independence and occupational success. The overall prevalence of ASD is also increasing. According to a 2021 report from the Centers for Disease Control and Prevention, 1 in 44 children will be diagnosed with ASD by age 82. Notably, ASD is at least four times more common in males than females. Despite the identification of numerous risk genes and neurological biomarkers unique to ASD, there is still no comprehensive model of disease pathogenesis or the biological basis for male predominance3,4. Previous magnetic resonance imaging (MRI) studies have revealed abnormalities in brain structure and function among children with ASD, as well as differences in neural activity patterns between males and females with ASD5,6. These findings suggest that investigating sex differences in brain function will help reveal the pathogenic mechanisms of ASD.
Most neuroimaging studies on sex differences among children with ASD have focused on regional brain structure6,7,8. However, complex brain functions such as language and social behavior are subserved by activity within hierarchical neural networks spanning cortical and subcortical regions. Thus, ASD may arise from defects in network connectivity within and between hemispheres. Cerebral specialization and inter-hemispheric cooperation are two valuable parameters for monitoring and evaluating functional connections between brain hemispheres. Brain lateralization refers to the structural and functional asymmetry between the two cerebral hemispheres9. This asymmetry is often disrupted in neuropsychiatric disorders. For example, some studies found significant alterations in lateralization patterns in patients with Parkinson’s disease and obsessive–compulsive disorder, whereas these changes in lateralization patterns are correlated with their pathological symptoms10,11. In turn, the capacity to integrate information from bilateral hemispheres is termed interhemispheric cooperation. Executive functions, such as sensory processing and motor control, depend on integration of information by homotopic regions12, while abnormalities in executive function are clinical markers for ASD13.
Previous studies have reported abnormalities in both brain lateralization and interhemispheric cooperation among individuals with ASD14,15,16. For instance, a structural MRI study revealed abnormal asymmetry in regions related to language and social/face processing among individuals with ASD, and these structural abnormalities may be linked to the cardinal symptoms of autism17. In addition, a functional MRI (fMRI) study reported altered temporal dynamics of intra- and interhemispheric functional connectivity (FC) among brain regions within the social network of ASD patients18.
However, there is only limited evidence supporting the possibility of sex differences in interhemispheric functional connectivity associated with ASD. One study utilizing voxel-mirrored homotopic connectivity (VMHC), a measure reflecting interhemispheric homotopic relations, found weaker connectivity in the dorsolateral occipital cortex of females than males with autism, while neurotypical females had higher VMHC than neurotypical males19. A sex difference was also found in corpus callosum volume of ASD patients, which is closely related to interhemispheric FC20. In summary, there is a lack of studies employing direct objective indicators to assess sex differences in hemispheric function among individuals with ASD.
In the current study, we aimed to investigate sex differences in brain functional specialization and interhemispheric cooperation among preschool-aged children with ASD. We employed the autonomy index (AI) to assess cerebral specialization and connectivity between functionally homotopic voxels (CFH) to evaluate interhemispheric cooperation. The AI is an innovative measure of functional specialization that can be applied at the voxel level, with higher regional AI values indicating greater brain specialization in the ipsilateral hemisphere. The AI has been proposed as a reliable metric for characterizing functional specialization in both healthy and patient cohorts21,22. The CFH is unbiased by hemispheric asymmetry, as the functional homotopic region of a given voxel is defined as the point with the strongest FC value in the contralateral hemisphere regardless of anatomic location. Again, higher CFH values indicate greater communication between regions across hemispheres11,23. Based on previous studies on abnormal interhemispheric functional connectivity in ASD, we hypothesized that there are significant sex differences in the functional specialization index (i.e., AI) and the interhemispheric cooperation index (i.e., CFH). In addition, distinct clinical symptoms among children with ASD may be linked to structural or functional abnormalities in specific regions24,25, so we also examined whether regional CFH values were correlated with scores on the autism behavior checklist (ABC) and childhood autism rating scale (CARS).
Methods
Participants
Seventy-one children diagnosed with ASD (58 males and 13 females, 23 to 87 months of age, mean ± standard deviation 47.42 ± 6.48 months) were recruited from the Anhui Hospital Affiliated to the Pediatric Hospital of Fudan University between June 2021 and November 2023. Each child received an independent diagnosis by two pediatric psychiatrists based on DSM-5 criteria. Exclusion criteria were (1) neurological syndromes or focal neurological signs, (2) birth asphyxia, (3) significant sensory impairment (e.g., blindness, deafness), (4) known monogenic syndromes associated with ASD (e.g., fragile X syndrome, Rett syndrome, and tuberous sclerosis complex), and (5) other mental disorders such as schizophrenia and affective disorders. All children with ASD filled out questionnaires on their clinical characteristics, including the CARS26, the ABC27, the Griffiths development scales-Chinese (GDS-C)28,29, and the Infants-junior middle school students’ social-life abilities scale (S-M scale)30. Informed written consent was obtained from the parents of all participants. A statement to confirm that all methods were carried out in accordance with relevant guidelines and regulations of the Ethics Committee of Anhui Hospital Affiliated to the Pediatric Hospital of Fudan University (approval number: EYLL-2023-026).
MRI data acquisition
All functional and structural MRI datasets were collected at the Anhui Hospital Affiliated to the Pediatric Hospital of Fudan University using a 3.0-T scanner (Philips Ingenia CX). During resting-state fMRI scanning, participants were administered a 10% chloral hydrate enema at a dosage of 0.5 mL/kg (maximum dose ≤ 10 mL) due to the limited cooperation of children with ASD. Functional images (240 volumes) were obtained using an echo-planar imaging sequence (repetition/echo time: 2000/30 ms; flip angle: 90°). Images of 46 transverse slices (field of view: 192 × 192 mm; matrix: 64 × 64; slice thickness: 3 mm with no inter-slice gap; voxel size: 3 × 3 × 3 mm) were acquired parallel to the anteroposterior commissure line. Subsequently, high spatial resolution T1-weighted anatomic images were acquired with the following parameters: repetition/echo time: 6.77/3.07 ms; flip angle: 8°; field of view: 256 × 256 mm2; matrix: 256 × 256; slice thickness: 1 mm with no inter-slice gap; voxel size: 1 × 1 × 1 mm3; 176 slices.
RS-fMRI data preprocessing
Images were processed and analyzed using the WhiteMatter toolkit (https://github.com/jigongjun/Neuroimaging-and-Neuromodulation), which incorporates functions from AFNI31, SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12), and FSL (http://fsl.fmrib.ox.ac.uk/fsl). Preprocessing steps included (1) deletion of the first five volumes, (2) slice timing and realignment, (3) co-registering individual functional images to structural images and segmenting structural images into gray matter, white matter, and cerebrospinal fluid, (4) regression of interfering signals including 24 head motion parameters as well as mean signals in the whole brain, white matter, and cerebrospinal fluid, (5) smoothing of functional images with a 4 mm isotropic Gaussian kernel, and (6) temporal band-pass filtering (0.01–0.1 Hz). Image series with evidence of head movements exceeding 3 mm of translation or 3° of rotation were discarded.
AI calculation
The autonomy index (AI) was computed for the entire brain using the equation AI = (Ni∕Hi)−(Nc∕Hc), where Ni and Nc are the numbers of voxels significantly correlated with a given voxel (r > 0.25, p < 0.001) in the ipsilateral (i) and contralateral (c) hemispheres, respectively, and Hi and Hc denote the total number of voxels in the ipsilateral and contralateral hemisphere24. Subsequently, an AI map was generated for each participant.
CFH computation
To address the limitations of conventional inter-hemispheric connectivity analysis based on structural symmetry, we calculated the connectivity between functionally homotopic regions by (1) defining homotopic regions, and (2) computing homotopic connectivity maps. For a given voxel, we performed seed-to-whole brain FC analysis and averaged the resulting maps across all participants. The voxel with the maximal FC value in the contralateral hemisphere was defined as the homotopic voxel of the seed voxel. The CFH value of each voxel was then defined as Pearson’s correlation coefficient with the homotopic voxel in the contralateral hemisphere. Subsequently, the CFH map was normalized by dividing all values by the mean value for the whole brain, thereby enhancing comparability between groups.
Statistical analysis
Demographic and clinical characteristics were compared between male and female ASD groups using the Mann–Whitney U–test. To identify sex differences in cerebral specialization and interhemispheric cooperation, we compared AI and CFH maps between male and female ASD groups within a gray matter mask (probability threshold > 0.2, from SPM12) using the SPM12 permutation test function statistical non-parametric mapping (SnPM13)32 with age and CARS score as covariates. To control the family-wise error in multiple comparisons, we initially set a voxel-level threshold of P < 0.001. Then, only clusters larger than 29 voxels (for AI analysis) and 17 voxels (for CFH analysis) were considered to have survived the cluster-level correction at Pcorr < 0.05. Subsequently, CFH values of the clusters displaying significant differences between male and female ASD groups were extracted, and Pearson’s or Spearman’s correlation analysis was employed to test the relationships with clinical symptoms as measured by the CARS score, ABC total score, and the five ABC subscale scores. A P-value of < 0.05 was considered statistically significant. Additional supplementary analyses are available in the supplementary materials.
Results
Demographic and clinical features of male and female children with ASD
The demographic and clinical characteristics of male and female ASD groups are summarized in Table 1. No significant differences were found in age, CARS score, GDS-C score, ABC total score, ABC subscores, and S-M scale score.
Differences in regional AI between male and female ASD groups
Functional specialization maps exhibited similar patterns in male and female ASD groups (Fig. 1A, B), and no significant differences were found in AI.
Differences in regional CFH between male and female ASD groups
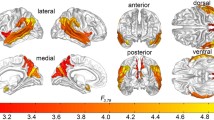
The interhemispheric cooperation maps of males and females with ASD also revealed similar regional CFH patterns (Fig. 2A, B), but the male ASD group exhibited significantly greater CFH by the left fusiform gyrus (FG) (Fig. 2C, peak t-value = 5.43, Montreal Neurological Institute [MNI] coordinates = [− 24, − 57, − 15], cluster size = 26 voxels) and right opercular part of inferior frontal gyrus (IFGoperc) (Fig. 2C, peak t-value = 5.06, MNI coordinates=[9, 36, 39], cluster size = 37 voxels).
Correlations of CFH values with clinical variables
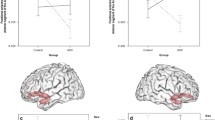
The CFH value of the left FG was not significantly correlated with CARS score in either the male or female ASD group (Fig. 3A , r= − 0.186, p = 0.161; r = − 0.358, p = 0.209). However, the CFH value of the left FG was significantly and negatively correlated with total ABC score in the male ASD group (Fig. 3B , r = − 0.430, p = 0.001), although not in the female ASD group (Fig. 3B , r = − 0.165, p = 0.573). Moreover, among the five ABC subscales, CFH value at the left FG of the male ASD group was significantly and negatively correlated with the sensory features subscale (Fig. 3C , r = − 0.460, p = 0.001) and the relating subscale (Fig. 3D , r = − 0.429, p = 0.002). The CFH value at the right IFGoperc was not significantly correlated with CARS score in either the male or female ASD group (Fig. 4A , r = − 0.095, p = 0.480; r = − 0.077, p = 0.793). The CFH value at the right IFGoperc was marginally associated with total ABC score in the female ASD group (Fig. 4B, r= 0.471, p = 0.089), but not in the male ASD group (Fig. 4B, r = 0.066, p = 0.629). These imaging-related analyses are detailed in Table 2 and the supplementary materials (Fig. S3).
Correlations between sex-specific CFH values at the left FG and clinical characteristics of ASD. (A) Correlations between CARS scores and CFH values. (B) Correlations between ABC total scores and CFH values. (C) Correlations between sensory subscale scores and CFH values. (D) Correlations between relating subscale scores and CFH values. M male ASD, F female ASD.
Discussion
Male children with ASD exhibited stronger interhemispheric cooperation of the left FG and right IFGoperc than female children with ASD as measured by the connectivity between functionally homotopic voxels (CFH). Moreover, greater CFH values in the left FG of 58 male children were associated with less severe autistic symptoms as measured by the ABC total score, sensory subscore, and relating subscore. Alternatively, no sex differences were observed in hemispheric lateralization. These findings suggest that interhemispheric cooperation of the left FG may be associated with ASD prevalence and symptom severity in males.
Hemispheric specialization is a fundamental organizational principle of the human brain. It emerges during childhood33, and dynamic modulation is still observed in some brain networks during adulthood, underscoring the importance of hemispheric specialization for supporting higher cognitive functions. Both extreme rightward and leftward deviations in hemispheric specialization, particularly in language, motor, and visuospatial regions, have been observed in individuals with autism, and these deviations were associated with symptom severity17,34, strongly suggesting contributions to pathogenesis35,36. However, we found similar patterns of ASD lateralization in male and female children, and no significant sex-related regional differences according to AI calculations. Thus, the contribution of altered hemispheric specialization to ASD appears sex-independent, at least in the current preschool sample. In contrast, sex differences in hemispheric specialization are substantial among neurotypical individuals37,38. For instance, neurotypical males typically demonstrate stronger lateralization, while neurotypical females tend to display a more symmetric distribution39. That is, the current results do not overlap with known patterns of sex differences, but this atypical lateralization may reflect neurodevelopmental abnormalities associated with the emergence of autism. Indeed, abnormalities in structural and functional lateralization can be detected in infants at high risk for autism as early as one year of age40,41. Our results suggest that the sex differences in lateralization among individuals with ASD may not be fully developed in early childhood.
Higher-level brain functions such as complex emotional processing and cognition require the sharing of information between hemispheres12,42, and deficits in interhemispheric coordination have been detected in ASD43,44. Consistent with previous observations, we found stronger interhemispheric cooperation by the left FG and right IFGoperc of male children with ASD compared to female children with ASD. Previous studies have also found sex differences in the structure and function of brain regions involved in social information processing among individuals with ASD45,46, such as abnormal frontal connectivity47. The current findings further suggest that the interhemispheric cooperation of the social brain network also differs between male and female children with ASD, and that the degree of interhemispheric cooperation by the FG in male children is associated with reduced clinical symptom severity. This finding is in accord with previous studies reporting abnormalities in FG gray matter volume and function associated with ASD symptom severity48,49. The FG is involved in facial perception, and extensive evidence suggests a correlation between fusiform gyrus dysfunction and social impairment in ASD50,51. Additionally, the strength of fusiform gyrus–amygdala connectivity is also closely related to social function52,53. Differences in interhemispheric cooperation by the FG between males and females with ASD may also influence social brain network development, leading to sex differences in ASD symptoms54. The degree of hemispheric cooperation by the FG was associated with the social aspects of symptoms a sufficient sample size of male ASD children. This suggests that atypical interhemispheric cooperation may impact clinical symptoms in males, thereby supporting, to some extent, the view that abnormalities in social brain function contribute to the greater vulnerability of males to ASD55. In addition, the results of the correlation analysis for the female group may be limited due to the small sample size, making it difficult to draw definitive conclusions about the relationship between the hemispheric function and clinical symptoms in female ASD children.
The inferior frontal gyrus (IFG) is a component of the semantic network involved in the integration of semantic content with emotional signals for communication56. Previous studies have found structure and functional abnormalities in the IFGoper of individuals with ASD compared to normally developing individuals57,58, with significant correlations between IFGoper gray matter volume and communication skill scores59. Moreover, one study supposed that gender differences in the language abilities of preschoolers with ASD may be linked to structural imbalances in the development of Broca’s area and Wernicke’s area, particularly Broca’s area60. Our research further confirmed the gender differences in interhemispheric cooperation in the IFGoper located in Broca's area in ASD, mainly found that the level of interhemispheric cooperation in the right IFGoper is higher in males than in females. This finding may help to better explain the gender differences in speech skill performance in individuals with ASD from a brain function perspective61.
Limitations
This study has several limitations, including the relatively small sample of female children with ASD, which limited statistical power for detecting relevant correlations with clinical symptoms. To address this, we conducted supplementary analyses to determine whether the mismatch in sample sizes affected the results of the inter-group comparisons, ensuring the stability of our findings. However, it is imperative to conduct follow-up studies with group matching, possibly by recruiting children from multiple centers. In addition, there was no control group of typically developing children for distinguishing inherent sex differences from sex differences specific to ASD. Additionally, hemispheric lateralization and cooperativity were evaluated using single indicators. Future research will assess multiple indicators to more comprehensively characterize differences in interhemispheric organization and communication among children with ASD.
Conclusion
This study compared hemispheric lateralization and interhemispheric cooperativity between male and female children with ASD to reveal potential shared and sex-specific pathomechanisms. Our findings revealed stronger interhemispheric cooperation by the left fusiform gyrus (FG) and right inferior frontal gyrus (IFGoperc) among male children with ASD, and further than the strength of hemispheric cooperation by the left fusiform gyrus in male children was associated with sensory and social symptoms. These results not only highlight sex differences in social brain organization and function among children with ASD, but also suggest that maldevelopment and dysfunction of interhemispheric pathways within the social brain network contribute to ASD onset and symptom severity.
Data availability
Due to reasons of data protection, the preprocessed data is available only upon reasonable request to the corresponding author.
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fifth edition (DSM-5). (The American Psychiatric Association, Arlington, 2013).
Maenner, M. J. et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR Surveill. Summ. 70(11), 1 (2021).
Zhang, Y. et al. Biological implications of genetic variations in autism spectrum disorders from genomics studies. Biosci. Rep. 41(7), BSR20210593 (2021).
Manoli, D. S. & State, M. W. Autism spectrum disorder genetics and the search for pathological mechanisms. Am. J. Psychiatry 178(1), 30–38 (2021).
Yue, X. et al. Regional dynamic neuroimaging changes of adults with autism spectrum disorder. Neuroscience 523, 132–139 (2023).
Deng, Z. & Wang, S. Sex differentiation of brain structures in autism: Findings from a gray matter asymmetry study. Autism Res. 14(6), 1115–1126 (2021).
Walsh, M. J. M. et al. Sex-related brain connectivity correlates of compensation in adults with autism: Insights into female protection. Cereb. Cortex 33(2), 316–329 (2023).
Retico, A. et al. The effect of gender on the neuroanatomy of children with autism spectrum disorders: A support vector machine case-control study. Mol. Autism 7, 5 (2016).
Toga, A. W. & Thompson, P. M. Mapping brain asymmetry. Nat. Rev. Neurosci. 4(1), 37–48 (2003).
Liu, Y. et al. Brain functional specialization in obsessive-compulsive disorder associated with neurotransmitter profiles. J. Affect. Disord. 329, 477–482 (2023).
Sun, J. et al. Brain functional specialization and cooperation in Parkinson’s disease. Brain Imaging Behav. 16(2), 565–573 (2022).
Davis, S. W. & Cabeza, R. Cross-hemispheric collaboration and segregation associated with task difficulty as revealed by structural and functional connectivity. J. Neurosci. Off. J. Soc. Neurosci. 35(21), 8191–8200 (2015).
Rodgaard, E. M., Jensen, K., Vergnes, J. N., Soulieres, I. & Mottron, L. Temporal changes in effect sizes of studies comparing individuals with and without autism: A meta-analysis. JAMA Psychiatry 76(11), 1124–1132 (2019).
Lee, J. M., Kyeong, S., Kim, E. & Cheon, K. A. Abnormalities of inter- and intra-hemispheric functional connectivity in autism spectrum disorders: A study using the autism brain imaging data exchange database. Front. Neurosci. 10, 191 (2016).
Hahamy, A., Behrmann, M. & Malach, R. The idiosyncratic brain: Distortion of spontaneous connectivity patterns in autism spectrum disorder. Nat. Neurosci. 18(2), 302–309 (2015).
Anderson, J. S. et al. Decreased interhemispheric functional connectivity in autism. Cereb. Cortex 21(5), 1134–1146 (2011).
Herbert, M. R. et al. Abnormal asymmetry in language association cortex in autism. Ann. Neurol. 52(5), 588–596 (2002).
Guo, X. et al. Altered inter- and intrahemispheric functional connectivity dynamics in autistic children. Hum. Brain Mapp. 41(2), 419–428 (2020).
Floris, D. L. et al. Towards robust and replicable sex differences in the intrinsic brain function of autism. Mol. Autism 12(1), 19 (2021).
Nordahl, C. W. et al. Sex differences in the corpus callosum in preschool-aged children with autism spectrum disorder. Mol. Autism 6, 26 (2015).
Mueller, S., Wang, D., Pan, R., Holt, D. J. & Liu, H. Abnormalities in hemispheric specialization of caudate nucleus connectivity in schizophrenia. JAMA Psychiatry 72(6), 552–560 (2015).
Wang, D., Buckner, R. L. & Liu, H. Functional specialization in the human brain estimated by intrinsic hemispheric interaction. J. Neurosci. Off. J. Soc. Neurosci. 34(37), 12341–12352 (2014).
He, K. et al. Abnormal interhemispheric functional cooperation in schizophrenia follows the neurotransmitter profiles. J. Psychiatry Neurosci. 48(6), E452–E460 (2023).
Xu, M. X. & Ju, X. D. Abnormal brain structure is associated with social and communication deficits in children with autism spectrum disorder: A voxel-based morphometry analysis. Brain Sci. 13(5), 779 (2023).
Li, G. et al. Reduction in grey matter volume and its correlation with clinical symptoms in Chinese boys with low functioning autism spectrum disorder. J. Intellect. Disabil. Res. JIDR 63(2), 113–123 (2019).
Schopler, E., Reichler, R. J., DeVellis, R. F. & Daly, K. Toward objective classification of childhood autism: Childhood autism rating scale (CARS). J. Autism Dev. Disord. 10(1), 91–103 (1980).
Krug, D. A., Arick, J. & Almond, P. Behavior checklist for identifying severely handicapped individuals with high levels of autistic behavior. J. Child Psychol. Psychiatry Allied discip. 21(3), 221–229 (1980).
Tso, W. W. Y. et al. The Griffiths development scales-Chinese (GDS-C): A cross-cultural comparison of developmental trajectories between Chinese and British children. Child Care Health Dev. 44(3), 378–383 (2018).
Li, P.-Y. et al. The Griffiths development scales-Chinese (GDS-C): A reliable and valid neurodevelopmental assessment tool in children with ASD aged 3–8 years old in Tianjin, China. Asian J. Psychiatry 52, 102144 (2020).
Mao, S., Shen, J., Xu, F. & Zou, C. Quality of life in caregivers of young children with Prader-Willi syndrome. World J. Pediatr. 15(5), 506–510 (2019).
Cox, R. W. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173 (1996).
Nichols, T. E. & Holmes, A. P. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain Mapp. 15, 1–25 (2002).
Olulade, O. A. et al. The neural basis of language development: Changes in lateralization over age. Proc. Natl. Acad. Sci. U. S. A. 117(38), 23477–23483 (2020).
Floris, D. L. et al. Atypical brain asymmetry in autism-a candidate for clinically meaningful stratification. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 6(8), 802–812 (2021).
Floris, D. L. & Howells, H. Atypical structural and functional motor networks in autism. Prog. Brain Res. 238, 207–248 (2018).
Carper, R. A., Treiber, J. M., DeJesus, S. Y. & Muller, R. A. Reduced hemispheric asymmetry of white matter microstructure in autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry 55(12), 1073–1080 (2016).
Dumitru, M. L. Brain asymmetry is globally different in males and females: Exploring cortical volume, area, thickness, and mean curvature. Cereb. Cortex 33(24), 11623–11633 (2023).
Tian, L., Wang, J., Yan, C. & He, Y. Hemisphere- and gender-related differences in small-world brain networks: A resting-state functional MRI study. Neuroimage 54(1), 191–202 (2011).
Hirnstein, M., Hugdahl, K. & Hausmann, M. Cognitive sex differences and hemispheric asymmetry: A critical review of 40 years of research. Laterality 24(2), 204–252 (2019).
Keehn, B., Vogel-Farley, V., Tager-Flusberg, H. & Nelson, C. A. Atypical hemispheric specialization for faces in infants at risk for autism spectrum disorder. Autism Res. 8(2), 187–198 (2015).
Rolison, M., Lacadie, C., Chawarska, K., Spann, M. & Scheinost, D. Atypical intrinsic hemispheric interaction associated with autism spectrum disorder is present within the first year of life. Cereb. Cortex 32(6), 1212–1222 (2022).
Weissman, D. H. & Banich, M. T. The cerebral hemispheres cooperate to perform complex but not simple tasks. Neuropsychology 14(1), 41–59 (2000).
Li, Q. et al. Decreased interhemispheric functional connectivity rather than corpus callosum volume as a potential biomarker for autism spectrum disorder. Cortex J. Devoted Stud. Nerv. Syst. Behav. 119, 258–266 (2019).
Berkins, S. et al. Morphometric analysis of corpus callosum in autistic and typically developing Indian children. Psychiatry Res. Neuroimaging 328, 111580 (2023).
Hall, J. et al. Social cognition, the male brain and the autism spectrum. PLoS One 7(12), e49033 (2012).
Lee, J. K. et al. Altered development of amygdala-connected brain regions in males and females with Autism. J. Neurosci. 42(31), 6145–6155 (2022).
Zeestraten, E. A. et al. Sex differences in frontal lobe connectivity in adults with autism spectrum conditions. Transl. Psychiatry 7, e1090 (2017).
Zhao, X. et al. Abnormalities of gray matter volume and its correlation with clinical symptoms in adolescents with high-functioning autism spectrum disorder. Neuropsychiatr. Dis. Treat. 18, 717–730 (2022).
Bai, C. et al. Abnormal gray matter volume and functional connectivity patterns in social cognition-related brain regions of young children with autism spectrum disorder. Autism Res. 16(6), 1124–1137 (2023).
Suzuki, K. et al. Reduced acetylcholinesterase activity in the fusiform gyrus in adults with autism spectrum disorders. Arch. Gen. Psychiatry 68(3), 306–313 (2011).
Sato, W., Toichi, M., Uono, S. & Kochiyama, T. Impaired social brain network for processing dynamic facial expressions in autism spectrum disorders. BMC Neurosci. 13(1), 99 (2012).
Kleinhans, N. M. et al. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain 131(4), 1000–1012 (2008).
Dziobek, I., Bahnemann, M., Convit, A. & Heekeren, H. R. The role of the fusiform-amygdala system in the pathophysiology of autism. Arch. Gen. Psychiatry 67(4), 397–405 (2010).
Guo, X. et al. Mapping progressive gray matter alterations in early childhood autistic brain. Cereb. Cortex 31(3), 1500–1510 (2021).
Hall, J. et al. Social cognition, the male brain and the autism spectrum. PLoS One 7(12), e49033 (2012).
Briggs, R. G. et al. Anatomy and white matter connections of the inferior frontal gyrus. Clin. Anat. 32(4), 546–556 (2019).
Yang, Q. et al. Mapping alterations of gray matter volume and white matter integrity in children with autism spectrum disorder: Evidence from fMRI findings. Neuroreport 29(14), 1188–1192 (2018).
Lu, H. et al. Decreased functional concordance in male children with autism spectrum disorder. Autism Res. 16, 2263–2274 (2023).
Duan, Y. et al. Identifying and predicting autism spectrum disorder based on multi-site structural MRI With machine learning. Front. Hum. Neurosci. 15, 765517 (2021).
Zhang, Y. et al. Sex differences of language abilities of preschool children with autism spectrum disorder and their anatomical correlation with Broca and Wernicke areas. Front. Pediatr. 10, 762621 (2022).
Neef, N. E., Angstadt, M., Koenraads, S. P. C. & Chang, S. E. Dissecting structural connectivity of the left and right inferior frontal cortex in children who stutter. Cereb. Cortex 33(7), 4085–4100 (2023).
Acknowledgements
We would like to thank all participants.
Funding
This research was funded by the National Natural Science Foundation of China (grant number 32271134, 82090034, 91432301), the National Key Research and Development Program (program number 2021YFC3300500), the University Synergy Innovation Program of Anhui Province (program number GXXT-2021-003), and the Health Research Project of Anhui Province (program number AHWJ2022b061), the Anhui Medical University Research Fund Project (program number 2021xkj230).
Author information
Authors and Affiliations
Contributions
H.L. and Q.Z. wrote the main manuscript text and preformed the administrative tasks for this study. Q.Z. and Q.H. analyzed the data. G.J., K.W. and C.Z. conceptualized the study. H.L., D.L., K.W. and C.Z. acquired the funding. T.D. and J.L. planned the MRI measurements and monitored the collection of the questionnaire data. L.S. conducted the MRI measurements. All authors critically reviewed and revised the manuscript. All authors contributed to and have approved the final manuscript. All authors agreed the possible publication of this article on Scientific Reports. The participant has consented to the submission of the article to the journal.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was conducted under the approval of the Ethics Committee of Anhui Hospital Affiliated to the Pediatric Hospital of Fudan University (approval number: EYLL-2023-026). This article does not contain any studies with animals performed by any of the authors. The participants and their parents were asked to sign an informed consent prior to their participation in the study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, H., Zhang, Q., Duan, T. et al. Sex differences in brain functional specialization and interhemispheric cooperation among children with autism spectrum disorders. Sci Rep 14, 22096 (2024). https://doi.org/10.1038/s41598-024-72339-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72339-6
- Springer Nature Limited