Abstract
Lidamycin (LDM) has been confirmed to have a strong anti-pancreatic cancer effect and can affect the mitochondrial function of pancreatic cancer cells. Mitofusin-2 (Mfn2) is located in the outer membrane of mitochondria, and Mfn2 is currently believed to play a role in cancer inhibition in pancreatic cancer. In order to explore whether the anti-pancreatic cancer effect of LDM is related to Mfn2-mediated mitophagy, Bioinformatics and in vitro cell experiments are used for experimental research. The experimental results demonstrated that Mfn2 is correlated with mitochondrial autophagy in pancreatic cancer. Lidamycin can increase the expression of Mfn2 in pancreatic cancer and affect the process of EMT, affect the level of reactive oxygen species and mitochondrial membrane potential, and increase the expression of mitochondrial autophagy marker proteins BNIP3L and Beclin1. These results demonstrate that Mfn2 affects mitophagy in pancreatic cancer cells by regulating the expression of Mfn2.
Similar content being viewed by others
Introduction
Pancreatic cancer is the 12th most common malignant tumor and the 7th leading cause of cancer death in the world, and is one of the most aggressive malignant tumors of the digestive tract1. Due to the lack of sensitive and specific tumor markers that can detect diseases early, the criteria for early diagnosis are not clear2. For pancreatic cancer diagnosed at an early stage, surgery is the best choice for cure. However, most patients have progressed to the middle and late stages when they found the disease, and may have missed the opportunity for surgical treatment. Therefore, seeking new methods to treat pancreatic cancer has become a problem that needs attention and solution in current clinical work3,4.
Autophagy is a unique phenomenon of life in eukaryotic cells, and it is one of the main pathways of programmed cell death5.Autophagy plays an important role in inhibiting tumor occurrence and development, making it a new target for cancer treatment at present6. Mitophagy is an important form of autophagy that selectively removes dysfunctional or redundant mitochondria. Dysfunction of mitophagy leads to the accumulation of damaged mitochondria and is a key influencing factor in cancer progression and tumor progression7.
Mitofusin-2 (Mfn2) is an important member of the mitochondrial fusion protein family. Mfn2 gene mediated mitophagy is a process in which cells selectively remove damaged or dysfunctional mitochondria through autophagy, maintaining a balance between the quantity and quality of mitochondria8,9. Many studies have confirmed that Mfn2 gene mediated mitophagy plays a crucial role in influencing tumor occurrence, tumor invasiveness, and drug resistance10,11,12.
Lidamycin (LDM) is a macromolecular enediyne antibiotic that has a strong killing effect on tumor cells13. LDM has been confirmed to play an anti pancreatic cancer role by affecting mitochondrial function14. At present, some drugs have been proved to play an anti-cancer role by targeting Mfn2 mediated mitophagy15.
In order to explore whether the anti-pancreatic cancer effect of LDM is related to Mfn2-mediated mitochondrial autophagy, bioinformatics and in vitro cell experiments were used to conduct experimental studies, with a view to providing new targets and new ideas for pancreatic cancer treatment.
Materials and methods
Cell culture and reagents
LDM was supplied by Professor Yongsu Zhen (Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China), with a purity of > 95.0%. Human pancreatic cancer cell lines SW1990 and AsPC-1 cells were kindly provided by Professor Yongsu Zhen. AsPC-1 was cultured in RPMI-1640 with 10% fetal bovine serum (FBS). SW1990 was cultured in DMEM with 10% fetal bovine serum (FBS). All cell lines were maintained in an incubator at 37 °C under 5% CO2 conditions. Small interfering RNAs (siRNAs) were transfected using the Lipo8000(Beyotime). Cells seeded at a density of 5 × 105 in 6-well dishes and transfected with 100 pmol siNC(control) or 100 pmol specific siRNA for 48 h in the presence of 4 μL Lipo 8000 (Beyotime) transfection reagent. siRNA was purchased from genepharma (Shanghai, China), The following antibodies were used:β-actin(ABclonal); Mfn2(ABclonal); E-cadherin(Huabio); Slug(Wanleibio); Snail(Wanleibio); LC3(Proteintech); P62(Cell signaling technolog); TOM20(Rosemont); Beclin1(Rosemont); BNIP3L(Rosemont).
Online database analysis
Through GEPIA (http://gepia.cancer-pku.cn/) analyze the expression of Mfn2 in tumors and normal tissues to preliminarily determine its expression in pan cancer. The TIME(https://cistrome.shinyapps.io/timer/) is used to analyze the correlation between Mfn2 gene and mitophagy gene in pancreatic cancer, and import the mitophagy related gene set into STRING (https://cn.string-db.org/). Construct and analyze the connectivity between Mfn2 protein and mitophagy protein through Protein–Protein Interaction Networks (PPI) on the website.
R language
Download the TCGA (https://www.cancer.gov/ccg/research/genome-sequencing/tcga) pancreatic cancer data in the database were divided into Mfn2 high expression group and Mfn2 low expression group with the median expression of Mfn2 as the boundary, and the expression of mitophagy-related genes in pancreatic cancer was explored, and then GSEA analysis was used to explore the enrichment of mitophagy pathway in Mfn2 high expression pancreatic cancer. The R language version is 4.2.2, and We used the clusterProfiler package (https://www.bioconductor.org/packages/release/bioc/html/clusterProfiler.html) for GSEA analysis and the gseavis package (https://github.com/junjunlab/GseaVis) for beautification.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
RNA was extracted using a 6-min rapid RNA extraction kit (Zsgentech, Tianjin China) and reverse-transcribed into cDNA. We used the 2−ΔΔCt method for relative quantification of mRNA and GAPDH as an internal control. The primers are shown in Table 1. The siRNA sequences are shown in Table 2.
MTT assay
The cells were inoculated into 96-well plates and cultured for 24 h, and serum-free medium containing LDM was added. The cells were continued to be cultured for 24 h or 48 h according to the experimental requirements. 100 µL 5 mg/mL MTT working solution was added to each well and then cultured for 4 h. 150 μL DMSO was added to each well, and after oscillating for 10 min, the wavelength of 570 nm was set on the enzyme marker to measure OD value, and the IC50 value (50% inhibition concentration, also known as half-inhibition concentration) of LDM on pancreatic cancer cells was calculated.
Trypan Blue experiment
Trypan Blue assay was used to detect the cell survival rate. The cells were inoculated into six-well plates at a density of 5.5 × 105 cells/well, and 10–9 mol/L serum-free medium containing LDM was added. Three multiple Wells were set up for each drug concentration (including the control group), and cultured at 37 °C and 5%CO2 for 24 h, 48 h and 72 h. All cells in the same well were centrifuged in a centrifuge tube and stained with 0.5% Trypan Blue solution for 3 min. The cell coloring was observed under an inverted microscope and counted. Finally, the cell survival rate at different time periods was calculated when the LDM concentration was 10–9 mol/L.
Cell invasion and migration assay
A wound closure assay was used to determine cell migration. AsPC-1 and SW1990 cells (5 × 105 cells) were seeded in a 6-well plate, following treatment with NC, LDM, siRNA and siRNA + LDM. When the cells reached 90–100% confluency, wounds were created using a sterile 200-μL pipette tip and images were taken at 0 h. Then, the cells were cultured in serum-free 1640/DMEM medium and images were taken at 48 h.
In the invasion assay, transwell chamber filters(Cayman) were coated with 50 µL of Matrigel. 600 µL complete medium containing 20%FBS was added to the lower chamber of the 24-well plate, and 100 µL treated cell suspension was uniformly added to the upper chamber. The 24-well plate was cultured in a cell incubator at 37 °C and 5% CO2 for 48 h. 48 h later, discard the culture medium from Transwell chamber and well plate, gently rinse twice with PBS, add 4% paraformaldehyde to fix for 10 min, and stain in crystal violet staining solution for 10 min. Gently wipe off the upper layer of cells that have not migrated with a damp cotton swab, and observe and take photos under an inverted microscope. In the Transwell migration experiment, 600 µL of complete culture medium containing 20% FBS was added to the lower chamber of the 24 well plate. The upper chamber of the Transwell chamber was placed in a well with 20% fetal bovine serum complete culture medium added, and 100 µL of processed cell suspension was uniformly dropped into the upper chamber. The subsequent steps are the same as the Transwel invasion test.
Detection of acid vesicular organelles (AVOs)
AO (Solarbio, Beijing, China) was used to detect and quantify the formation of AVOs by fluorescence microscopy. After the cells were treated as required, they were gently washed twice with PBS buffer, diluted to 5 μg/mL of working solution according to the instructions of AO dye solution, 1 mL of AO working solution was added to each well, stained away from light for 15 min, the supernatant was discarded, and the cells were gently rinsed with PBS for 2–3 times to ensure that the excess dye solution was cleaned, and then observed under a fluorescence microscope and photographed.
Mitochondrial function assay
JC-1 solution was added to each well of the six-well plate to a final concentration of 10 µM and the plate was incubated at 37 °C in the dark for 15 min. During the incubation process, the six-well plate was shaken every 4 min or so to prevent the caking of the dye. After the incubation, the dye was removed and cleaned twice with PBS to remove the free JC-1 dye, and the solution was observed and photographed under an inverted fluorescence microscope.
Active oxygen detection was carried out according to the instructions of the reactive oxygen detection kit of Report biotech. Serum free medium was used to dilute DCFH-DA (10 mM) reagent 1000 times and configure it as working liquid. The supernant was absorbed and discarded from the six-well plate to be dyed, and 1 mL of DCFH-DA working liquid was added to each well. After staining, the supernatant was sucked up and the cells were gently rinsed with serum-free cell medium for 3 times to fully remove the free DCFH-DA that did not enter the cells. The cells were observed and photographed under an inverted fluorescence microscope. Flow cytometry selected appropriate fluorescence channels (488 nm excitation wavelength, 525 nm emission wavelength) to detect the intensity of fluorescence before and after transfection or/and drug stimulation.
GFP-LC3 fluorescent plasmid transfection
GFP-LC3 fluorescent plasmid was donated by the Institute of Medical Biotechnology, Chinese Academy of Medical Sciences. The GFP-LC3 indicator system was constructed based on the aggregation of LC3 during autophagy formation. AsPC-1 cells and SW 1990 cells were cultured in a 6-well plate at a density of 4.5 × 105 cells per well. When the cell density reached 70–90%, Biyun Tian lipo8000 transfection reagent was used for plasmid transfection. Taking plasmid transfection of one well in the 6-well plate as an example, 2.5 μg of GFP-LC3 fluorescent particles were added to an enzyme free EP tube containing 125 μL of serum-free medium. 4 μL of lipo8000 was gently blown and mixed, and then evenly dropped into the well. The 6-well plate was shaken left and right twice, and then placed in a wet cell culture incubator at 37 °C and 5% CO2 for further cultivation. Plasmid transfection was carried out. After 48 h of cultivation, discard the supernatant and add serum-free basic culture medium containing 10−9 M lidamycin to the LDM group. Add serum-free culture medium to the control group and continue cultivation for 24 h. Observe and take photos under a fluorescence microscope.
Statistical analysis
Each analysis result was displayed as the mean ± standard deviation, and the data analysis result was analyzed by one-way ANOVA or T-test, which had statistical significance when P < 0.05. SPSS 13.0 statistical analysis software was used to complete the statistical analysis result for each analysis result. It was analyzed by Graphpad prism 8.0 and Image J 1.53t software.
Results
Bioinformatics analysis showed that Mfn2 was associated with mitophagy in pancreatic cancer
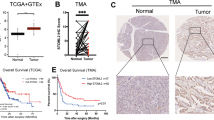
The GEPIA database compared the expression level of Mfn2 gene in normal pancreatic tissues (n = 171) and pancreatic cancer tissues (n = 179). The analysis results of GEPIA database showed that the expression level of Mfn2 gene in pancreatic cancer tissues was higher than that in normal pancreatic tissues (Fig. 1a), and the difference was statistically significant (P < 0.05). These results suggest that Mfn2 gene may be involved in the development of pancreatic cancer.
(a) GEPIA database analysis of Mfn2 expression in pancreatic cancer *P < 0.05; (b) TIMER database analysis of the correlation between Mfn2 and mitophagy related genes in pancreatic cancer; (c) Protein interaction network analysis of the correlation between Mfn2 protein and mitophagy related proteins (d) GSEA Pathway Enrichment Analysis of Autophagy Pathway and Mitophagy Pathway Expression in pancreatic cancer; € Mitophagy Gene Expression in High and Low Mfn2 Expression Groups of pancreatic cancer.
In order to explore the relationship between Mfn2 gene and mitophagy gene, gene correlation analysis was conducted through the TIMER database, and the results showed (Fig. 1b) that Mfn2 gene was moderately correlated with mitophagy genes such as ATG5, ATG12, BECN1, and BNIP3L in pancreatic cancer, suggesting that Mfn2 was correlated with mitophagy.STRING database was used to construct protein interaction network. The results showed that Mfn2 protein was closely related to most mitophagy proteins, such as TOMM20, MFN2, BNIP3L, BECN1 (Fig. 1c). The analysis results of TIMER database and STRING database preliminatively demonstrated that Mfn2 was correlated with mitophagy.
Based on the transcriptome data of pancreatic cancer patients in the TCGA database, the enrichment of the autophagy pathway and mitophagy pathway at different Mfn2 expression levels was analyzed by GSEA enrichment analysis. The results of GSEA enrichment analysis showed that the gene sets related to autophagy and mitophagy were highly enriched when Mfn2 was highly expressed (Fig. 1d). Next, the pancreatic cancer data were divided into two groups with high Mfn2 expression and low Mfn2 expression according to the median value of Mfn2 through R language analysis, and the differential expression of mitophagy genes between the high Mfn2 expression and low Mfn2 expression groups was compared. The results showed that the expression level of mitophagy related genes in the Mfn2 high expression group was higher than that in the Mfn2 low expression group, suggesting that the high expression of Mfn2 may be an important factor promoting the occurrence of mitophagy (Fig. 1e). The above bioinformatics analysis results demonstrated that Mfn2 is associated with mitophagy in pancreatic cancer.
Effect of lidamycin on survival of human pancreatic cancer cells
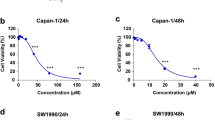
To evaluate the effect of lidamycin on the proliferation of human pancreatic cancer cells AsPC-1 and SW1990, the survival rate of pancreatic cancer cells treated with lidamycin for 24 h and 48 h was measured by MTT assay. Pancreatic cancer cells were treated with lidamycin of 10–5 mol/L, 10–7 mol/L, 10–8 mol/L, 10–9 mol/L, 10–10 mol/L, 10–11 mol/L, 10–12 mol/L, 10–13 mol/L, 10–14 mol/L and 10–15 mol/L respectively for 24 h and 48 h (Fig. 2a,b). Compared with the control group, Lidamycin inhibited the growth of pancreatic cancer cells in a time-dependent and concentration-dependent manner. The difference between the semi-inhibitory drug concentration of 24 h and 48 h (Table 3). Therefore, the concentration of Lidamycin was 10–9 mol/L for follow-up tests.
(a) MTT assay was used to detect the survival rate of AsPC-1 cells treated with different concentrations of lidamycin **P < 0.01, ***P < 0.001; (b) MTT assay was used to detect the survival rate of SW 1990 cells treated with different concentrations of lidamycin. **P < 0.01, ***P < 0.001; (c) Trypan blue test was used to detect the effect of 10–9 M lidamycin on the survival rate of pancreatic cancer AsPC-1 and SW 1990 cells at different time. ***P < 0.001; (d) Protein bands of LC3 in different groups of AsPC-1 cells; (e) Quantitative results of LC3 protein in different groups of AsPC-1 cells *P < 0.05; ***P < 0.001; (f) Protein bands of LC3 in different groups of SW 1990 cells; (g) Quantitative analysis of LC3 protein in different groups of SW 1990 cells *P < 0.05, **P < 0.01, ***P < 0.001; (h) GFP-LC3 detection of the effect of lidamycin on autophagy in AsPC-1 and SW 1990 cells (200×); (i) mRNA expression level of Mfn2 gene in AsPC-1 cells *P < 0.05; (j) The protein bands of Mfn2 protein in AsPC-1 cells; (k) Protein quantification results **P < 0.01; (l) mRNA expression level of Mfn2 gene in SW 1990 cells **P < 0.01; (m) The protein bands of Mfn2 protein in SW 1990 cells; (n) Protein quantification results ***P < 0.001.
Trypan blue staining can stain dead cells blue, while living cells are not stained. Therefore, Trypan blue assay was used to detect the survival rate of AsPC-1 and SW1990 cells treated with 10–9 mol/L lidamycin at 0 h, 24 h, 48 h and 72 h. The results showed that the survival rate of cells decreased with the increase of time at the same concentration of lidamycin (Fig. 2c). LDM had no significant effect on cell survival rate at 24 h, and the cell survival rate decreased significantly at 48 h and 72 h, but the cell survival rate at 72 h May be affected by the long-term starvation of cells in serum-free culture. Combined with the results of MTT experiment, 48 h was selected as the drug action time for follow-up tests.
Lidamycin induced autophagy level and affected Mfn2 expression in pancreatic cancer cells
After determining the optimal drug concentration and time of LDM action on pancreatic cancer cells AsPC-1 and SW1990, it was speculated that lidamycin might induce the death of pancreatic cancer cells through autophagy, which is a dynamic process and the level of autophagy varies in different time periods. Therefore, in order to better observe autophagy indicators, Western blot was used to detect the changes of LC3 at 0 h, 12 h, 24 h and 48 h. The transformation from LC3-I to LC3-II was a landmark change in the process of autophagy. The experimental results showed Fig. 2d–g that under the influence of 10–9 mol/L lidamycin, LC3-I levels in both AsPC-1 and SW1990 cells decreased, while LC3-II levels began to rise, suggesting that lidamycin could induce autophagy in AsPC-1 and SW1990 cells. LC3-I continued to transform to LC3-II during the period from 0 to 24 h, and reached a peak at 24 h and decreased at 48 h. Therefore, 24 h was selected as the best time to detect autophagy related indicators.
In order to further verify the ability of LDM to induce autophagy in pancreatic cancer cells AsPC-1 and SW1990, GFP-LC3 fluorescent plasmid transfection was performed. When autophagy did not occur in the cells, GFP-LC3 protein would be uniformly distributed in the cytoplasm, while GFP-LC3 would aggregate after autophagy occurred. Forming bright green spots that can be seen under a fluorescence microscope. The addition of LDM could promote the formation of green fluorescent spots in AsPC-1 and SW1990 cells, which further indicated that lidamycin could induce autophagy in pancreatic cancer AsPC-1 and SW1990 cells (Fig. 2h). GFP-LC3 fluorescence results and Western blot results showed that lidamycin could promote the autophagy of pancreatic cancer cells AsPC-1 and SW1990, and the best time for Lidamycin to induce autophagy was 24 h.
The results of bioinformatics analysis showed that the expression level of Mfn2 in pancreatic cancer was higher than that in normal pancreatic tissue, and autophagy related genes were also highly expressed in the Mfn2 high-expression group, suggesting that Mfn2 is related to mitophagy in pancreatic cancer. Meanwhile, Western blot assay and GFP-LC3 fluorescent plasmid proved that LDM could induce autophagy of pancreatic cancer AsPC-1 and SW1990 cells. In order to explore the correlation between Mfn2 and the anti-pancreatic cancer mechanism of LDM, Western blot and qRT-PCR were used to detect the protein and mRNA expressions of Mfn2 in pancreatic cancer cells treated with 10–9 mol/L lidamycin. The results showed that lidamycin could up-regulate the mRNA and protein expression of Mfn2 in AsPC-1 and SW 1990 cells, suggesting that the anti-pancreatic cancer effect of Lidamycin may be related to Mfn2 (Fig. 2i–o).
Knocking down Mfn2 decreased the toxic effect of lidamycin on pancreatic cancer cells
Western blot and qRT-PCR results showed that lidamycin could promote the expression of Mfn2 at mRNA level and protein level in AsPC-1 and SW1990 pancreatic cancer cells. In order to explore the role of Mfn2 in inhibiting the growth of pancreatic cancer cells by lidamycin, a pancreatic cancer cell line with low expression of Mfn2 was constructed by siRNA transient transfected method. Four non-overlapping siRNA sequences of Mfn2 were designed using Gemma gene, and the siRNA with the highest efficiency was screened and knocked by Western blot and qRT-PCR. The experimental results showed that si1556 had the highest transfection efficiency in AsPC-1 and SW1990 cells, so si1556 was selected to construct pancreatic cancer AsPC-1 and SW1990 cell lines with low Mfn2 expression for subsequent experiments (Fig. 3a–f).
(a) mRNA expression level of Mfn2 gene in AsPC-1 cells. **P < 0.01, ***P < 0.001; (b) The protein bands of Mfn2 protein in AsPC-1 cells; (c) Protein quantification results *P < 0.05, **P < 0.01, ***P < 0.001; (d) mRNA expression level of Mfn2 gene in SW 1990 cells. *P < 0.05, ***P < 0.001; (e) The protein bands of Mfn2 protein in SW 1990 cells; (f) Protein quantification results. *P < 0.05, ***P < 0.001; (g) MTT assay was used to detect the survival rate of AsPC-1 cells after treatment with different concentrations of lidamycin; **P < 0.01, ***P < 0.001; (h) MTT assay was used to detect the survival rate of SW 1990 cells after treatment with different concentrations of lidamycin. *** P < 0.001.
AsPC-1 and SW1990 cells were divided into NC group and siMfn2 group, and treated with 10–7 mol/L, 10–8 mol/L, 10–9 mol/L, 10–10 mol/L, 10–11 mol/L concentration of lidamycin for 48 h, respectively. The results showed that the cell survival rate in siMfn2 group was significantly higher than that in NC group. The median inhibitory concentration in siMfn2 group was higher than that in NC group (Table 4, Fig. 3g,h), indicating that Mfn2 knockdown could reduce the toxic effect of lidamycin on pancreatic cancer cells.
Knocking down Mfn2 weakened the inhibitory effect of lidamycin on the migration and invasion of pancreatic cancer cells
The highly metastatic and invasive ability of pancreatic cancer is one of the important factors leading to poor prognosis and high mortality of pancreatic cancer patients. Lidamycin has a strong anti-tumor effect. In order to explore the influence of Lidamycin on the migration ability of human pancreatic cancer cells and to clarify the role of Mfn2 gene in it, The cells of the control group and transfection group were treated with 10–9 mol/L lidamycin for 48 h. Scratch test results showed that compared with the control group, cell mobility in both the LDM group and the siMfn2 + LDM group decreased significantly, but the cell migration ability of the siMfn2 + LDM group was stronger than that of the LDM group (Fig. 4a–c), indicating that Mfn2 knockdown weakened the inhibitory effect of LDM on the migration ability of pancreatic cancer cells.
(a) Scratch experiment to detect the migration of AsPC-1and SW 1990 cells under different conditions; (b) AsPC-1 cell migration rate. *P < 0.05, ***P < 0.001; (c) SW 1990 cell migration rate. *P < 0.05, ***P < 0.001; (d) Transwell migration experiment was used to detect the migration of AsPC-1 cells under different conditions; (e) AsPC-1 cell migration quantity. *P < 0.05, ***P < 0.001; (f) Transwell invasion assay was used to detect the migration of AsPC-1 cells under different conditions; (g) AsPC-1 cell invasion quantity. **P < 0.01, ***P < 0.001; (h,i) EMT related protein bands in AsPC-1 and SW 1990 cells; (j–l) Quantitative results of EMT related proteins *P < 0.05, ***P < 0.001.
Based on the results of the scratch test, the Transwell migration test was applied to verify that 10–9 mol/L LDM was applied to AsPC-1 and SW1990 cells transfected or not transfected with siRNA for 48 h. As can be seen from (Fig. 4d,e), compared with the control group, the number of cells passing through the Transwell chamber in the LDM group decreased significantly, while the number of cells in the siMfn2 + LDM group increased compared with the LDM group. The results of Transwell migration test were consistent with those of scratch test, indicating that LDM can inhibit the migration ability of pancreatic cancer cells, and knocking down Mfn2 can weaken the inhibitory effect of lidamycin on the migration of human pancreatic cancer cells.
In order to further verify the effect of LDM on pancreatic cancer invasion and the role of Mfn2 gene, 10–9 mol/L LDM was applied to AsPC-1 and SW1990 cells transfected or not transfected with siRNA for 48 h, and Transwell cell invasion assay was performed. As can be seen from Fig. 4f–g, compared with the control group, the number of cells passing through the Transwell chamber in the LDM group decreased significantly, while the number of cells in the siMfn2 + LDM group increased compared with the LDM group. The experimental results showed that LDM could inhibit the invasion ability of AsPC-1 and SW1990 cells. Knocking down Mfn2 can weaken the inhibitory effect of lidamycin on pancreatic cancer cell invasion.
Epithelial-mesenchymal transition (EMT) plays an important role in the metastasis of pancreatic cancer. The scratch test and Transwell migration and invasion test have preliminatively confirmed that lidamycin can inhibit the migration and invasion of human pancreatic cancer cells AsPC-1 and SW 1990, and the inhibitory effect of LDM on the migration and invasion of pancreatic cancer cells can be partially reversed after the Mfn2 gene is down-regulated. Next, the molecular mechanism of LDM affecting the migration and invasion ability of pancreatic cancer AsPC-1 and SW1990 cells and the role of Mfn2 were explored.. After 10–9 mol/L lidamycin treatment for 48 h, the expression of E-cadherin protein was up-regulated in AsPC-1 and SW 1990 cells, while the expression of Slug and Snail proteins was down-regulated. The above protein changes caused by Lidamycin were weakened after Mfn2 was down-regulated (Fig. 4h–l). Combined with the results of the scratch test and Transwell migration and invasion test, Lidamycin can inhibit the migration and invasion ability of AsPC-1 and SW1990 pancreatic cancer cells through EMT pathway, and knock down Mfn2 can reduce the effect of lidamycin on EMT process of pancreatic cancer cells.
Knocking down Mfn2 decreased the autophagy level of lidamycin-induced pancreatic cancer cells
To investigate the relationship between Mfn2 and the autophagy of human pancreatic cancer cells AsPC-1 and SW1990 induced by lidamycin, AsPC-1 and SW1990 cells in the control group and transfection group were treated with 10–9 M of lidamycin for 24 h, and AO staining was performed. Due to its sensitivity to PH value, AO is often used as a fluorescent indicator to observe autophagy indicators. AO dyes cytoplasm and DNA green and acidic autophagy bubbles orange red, so the level of autophagy can be judged according to the staining results.AO staining results showed that the number of cells emitting orange fluorescence was significantly increased in the LDM group compared with the control group (Fig. 5a). However, the number of cells with orange fluorescence in siMfn2 + LDM group was less than that in LDM group, indicating that lidamycin can induce autophagy in pancreatic cancer AsPC-1 and SW1990 cells, and knocking down Mfn2 can reduce the autophagy level of human pancreatic cancer cells induced by lidamycin. The expression levels of autophagy marker proteins P62 and LC3-II/I were detected by Western blot assay. P62 is the substrate required for autophagy. With the occurrence of autophagy, the level of P62 will gradually decrease, and LC3-I will change to LC3-II. compared with the control group, the expression level of P62 protein in the LDM group was significantly decreased, while the expression level of LC3-II/I was increased, with statistical significance (P < 0.05); however, the P62 protein level in siMfn2 + LDM group was higher than that in the LDM group, and the expression level of LC3-II/I was lower than that in the LDM group (P < 0.05) (Fig. 5b–f). The results of Western blot and AO fluorescence staining were consistent, suggesting that lidamycin can induce autophagy in AsPC-1 and SW1990 pancreatic cancer cells, and knocking down Mfn2 can reduce the level of LDM-induced autophagy.
Knocking down Mfn2 reduces mitochondrial damage induced by lidamycin in pancreatic cancer cells
DCFH is an oxidative stress indicator often used to detect the level of reactive oxygen species. Dcfh-da does not have the ability to emit fluorescence, but reactive oxygen species in cells can oxidize non-fluorescent DCFH to generate fluorescent DCF. The level of reactive oxygen species in cells can be evaluated by detecting the fluorescence level of DCF. Cells in the control group and transfection group were treated with 10–9 mol/L lidamycin for 24 h, and the level of reactive oxygen species produced in the cells could be evaluated by observing the fluorescence color changes after DCFH-DA staining. Compared with the control group, the number of cells emitting green fluorescence was significantly increased in the LDM group and the siMfn2 + LDM group, but the number of cells emitting green fluorescence in the siMfn2 + LDM group was less than that in the LDM group (Fig. 6a), indicating that Mfn2 depletion could reduce the accumulation of mitochondrial reactive oxygen species in human pancreatic cancer cells by lidamycin.
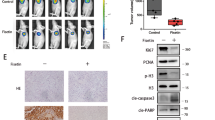
(a) DCFH-DA fluorescence detection of changes in cellular reactive oxygen species (× 100); (b,c) Detection of changes in cellular reactive oxygen species by flow cytometry. (d) JC-1 fluorescence detection of changes in mitochondrial membrane potential of cells (× 100). (e,f) TOM20 protein bands in AsPC-1 and SW 1990; (f) DCFH-DA average fluorescence intensity *P < 0.05, ***P < 0.001; (g) Quantification results of TOM20 protein. **P < 0.01, ***P < 0.001; (h,i) Beclin1 ang BNIP3L protein bands in AsPC-1 and SW 1990; (j,k) Quantification results of TOM20 protein. *P < 0.05, ***P < 0.001.
On the basis of the above results, flow cytometry was used to further verify the level of reactive oxygen species. The fluorescence intensity of each group was detected by flow cytometry, and its average value was calculated for statistical analysis. Compared with the control group, the peak pattern in the LDM group shifted significantly to the right, indicating an increase in fluorescence intensity, while the fluorescence intensity in the siMfn2 + LDM group was weaker than that in the LDM group (Fig. 6b), demonstrating once again that Mfn2 depletion can reduce the accumulation of mitochondrial reactive oxygen species in human pancreatic cancer cells by lidamycin.
The mitochondrial health status could be assessed by observing the fluorescence color change of JC-1 after the control and transfection cells were treated with 10–9 mol/L lidamycin for 24 h. Normal and healthy mitochondria usually show red fluorescence, while damaged or dysfunctional mitochondria show green fluorescence. The results showed that the number of cells emitting green fluorescence was significantly increased in the LDM and siMfn2 + LDM groups compared with the control group (Fig. 6c). However, the number of cells that emitted green fluorescence in siMfn2 + LDM group was less than that in LDM group. These results indicated that lidamycin could damage the mitochondrial membrane potential of AsPC-1 and SW1990 cells, and the damage of lidamycin on mitochondrial membrane potential of human pancreatic cancer cells could be weakened by Mfn2 reduction. The expression of mitochondrial protein TOM20 was detected by Western blot. The results showed that the mitochondrial protein TOM20 level in the LDM group was significantly higher than that in the control group, which further indicated that LDM had an impact on the mitochondrial function of pancreatic cancer, while the mitochondrial protein TOM20 level in the siMfn2 + LDM group was lower than that in the LDM group (Fig. 6d,e). Combined with the results of mitochondrial membrane potential and reactive oxygen species detection, the mitochondrial protein TOM20 level in the SIMFN2 + LDM group was lower than that in the LDM group. It is suggested that LDM can affect mitochondrial function and cause mitochondrial damage in pancreatic cancer cells. Mfn2 knockdown can reduce mitochondrial dysfunction and mitochondrial damage in human pancreatic cancer cells induced by lidamycin.
Knocking down Mfn2 attenuated lidamycin-induced mitophagy in human pancreatic cancer cells
Lidamycin induces autophagy and mitochondrial dysfunction in pancreatic cancer cells, and Mfn2 can reduce the autophagy and mitochondrial dysfunction induced by LDM. In order to explore whether LDM induces pancreatic cancer autophagy through mitochondrial pathway and the role of Mfn2 in this process, combined with the previous bioinformatics analysis results, BNIP3L and Beclin1 (BECN1) proteins were selected as targets for Western blot analysis to further verify. The results showed that compared with the control group, the expression levels of BNIP3L and Beclin1 proteins in pancreatic cancer cells in the LDM group were significantly increased, while the levels of BNIP3L and Beclin1 proteins in siMfn2 + LDM group were lower than those in the LDM group, with statistical significance (P < 0.05) (Fig. 6f–k). LDM activates mitophagy by inducing the BNIP3L pathway in pancreatic cancer cells, and knocking down Mfn2 weakens mitophagy in the BNIP3L pathway induced by lidamycin, suggesting that LDM may play an anti-pancreatic cancer role through the MFN2-BNIP3L pathway.
Discussion
Pancreatic cancer is one of the malignant tumors with hidden onset and the worst prognosis. Due to the lack of obvious early symptoms and early diagnostic markers of pancreatic cancer, most patients with pancreatic cancer are already in the advanced stage of pancreatic cancer when diagnosed, and only less than 20% of patients can be diagnosed and surgically resected in the early stage16,17. Although the nucleotide analogue gemcitabine is used as a standard chemotherapy agent for Pancreatic ductal adenocarcinoma (PDAC), there has been no significant increase in survival due to chemotherapy resistance2,18. Current studies have shown that the growth and metastasis of pancreatic ductal adenocarcoma are dependent on the energy produced by mitochondrial Oxidative phosphorylation (OXPHOS), and mitochondrial fusion induces mitophagy in pancreatic cancer cells, suggesting that mitophagy is expected to become a new therapeutic approach for pancreatic cancer19.
Mitofusin2 (Mfn2) is located in the outer membrane of mitochondria and plays an indispensable role in regulating mitochondrial fusion, mitochondrial function and mitophagy20. Genetic studies have shown that Mfn2 is not only a key protein regulating mitochondrial fusion, but also an important factor affecting the occurrence and development of tumors. Mfn2 has a bidirectional regulatory effect, and the role of Mfn2 in tumors is currently controversial21. In this study, the expression of Mfn2 in pancreatic cancer was analyzed through GEPIA database, and the results showed that Mfn2 was highly expressed in pancreatic cancer, suggesting that Mfn2 gene may be related to the occurrence and development of pancreatic cancer. In the current study, Mfn2 is believed to play a role in cancer inhibition in pancreatic cancer. Sun et al. established Mfn2 overexpression Bxpc-3 and L3.6pl cell lines and found that Mfn2 overexpression significantly inhibited the invasion and migration of tumor cells22. Yu et al. found that leflunomide can play an anti-pancreatic cancer role by up-regulating Mfn2 expression and promoting mitochondrial fusion, and the anticancer mechanism of mitochondrial fusion is mainly caused by its initiation of mitophagy. Leflunomide originally used in the treatment of arthritis is therefore used as a chemotherapy drug, suggesting that Mfn2 gene may be an important target for enhancing the effect of anticancer drugs15.
Lidamycin (LDM) is an enediyne antibiotic, also known as C1027, which is extracted from streptomycin metabolites and has a strong tumor killing effect23,24. Tumor metastasis is the main cause of death in cancer patients, and more than 90% of cancer patients' deaths are related to tumor metastasis, and more than 3/4 of pancreatic cancer patients have tumor metastasis, which is also one of the important factors leading to the high mortality of pancreatic cancer25. Lidamycin can inhibit the growth of pancreatic cancer cells, and reduce the migration and invasion ability of pancreatic cancer cells by affecting the expression of VEGF and MMP proteins14. Mfn2 also known as proliferation suppressor gene, can affect the proliferation, migration and invasion ability of cancer cells. In prostate cancer, overexpression of Mfn2 gene can inhibit the proliferation, migration and invasion ability of PC3 cells and DU145 cells26. The results showed that LDM can increase the expression of Mfn2 in pancreatic cancer cells, LDM can reduce the migration and invasion ability of pancreatic cancer cells, and knocking down Mfn2 gene can weaken the inhibition effect of LDM on the migration and invasion ability of pancreatic cancer cells. Epithelial–mesenchymal transition (EMT) is considered a key step in cancer metastasis, in which cancer cells lose the characteristics of Epithelial cells and gain the characteristics of mesenchymal cells in order to facilitate their separation from the primary site and gain the ability to move27. E-cadherin is often used as a marker for epithelial cells, and its loss, when combined with alterations in expression of other EMT marker genes, signals the occurrence of EMT processes. Snail (SNAI1) and Slug (SNAI2) are the core transcription factors that activate EMT, and they are both inhibitors of epithelial factors such as E-cadherin28. Western blot analysis showed that lidamycin resulted in increased expression of E-cadherin protein and decreased expression of Slug and Snail proteins, while Mfn2 knockout reduced the above protein changes caused by LDM, demonstrating that LDM inhibited the migration and invasion ability of AsPC-1 and SW1990 pancreatic cancer cells by regulating EMT pathway. Knocking out Mfn2 attenuates the effect of LDM on EMT progression, demonstrating that Mfn2 is an important factor in inhibiting the migration and invasion of pancreatic cancer cells by LDM.
Autophagy is a catabolic process, which is the core molecular pathway for maintaining homeostasis in cells and organisms. Autophagy can play a role by limiting the development of tumors, but it also has a primary role in tumor, promoting tumor growth and metastasis and spread29. The autophagy system targets damaged mitochondria and passes them on to lysosome for degradation. This catabolic process, known as mitophagy, helps maintain mitochondrial quality control and mitochondrial count in a variety of cell types30. All modes of mitophagy follow the core mechanism of autophagy, and the important steps include the formation of mitochondria-coated autophagosomes, the fusion of autophagosomes with lysosomes, and the degradation of functional damaged mitochondria31. Mitophagy is a basic process necessary to maintain tumor homeostasis, which can degrade dysfunctional mitochondria targeting autophagosomes32,33. Bioinformatics results showed that Mfn2 was correlated with mitophagy genes ATG5, ATG12, BECN1, BNIP3L and MFN1. GSEA enrichment analysis results showed that the expression level of Mfn2 was correlated with the mitophagy pathway. In the highly expressed Mfn2 group, mitophagy genes showed a high level of expression. These results all suggest that Mfn2 may play an important role in the mitophagy pathway of pancreatic cancer. In this study, various methods were used to evaluate the level of LDM-induced autophagy. Firstly, the expression level of LC3 protein, which is known to be specifically located in the structure of autophagy and participate in the entire process of autophagy, will be transformed from LC3-I protein to LC3-II protein when autophagy occurs34. The results of this study showed that AsPC-1 and SW1990 cells promoted the conversion of LC3-I to LC3-II after lidamycin stimulation, and LC3-II/I reached a peak value at 24 h. LDM promotes the aggregation of GFP-LC3 green fluorescent dots, suggesting that LDM can induce autophagy in pancreatic cancer cells, and the best time to observe autophagy indexes is 24 h. P62(SQSTM1) is an autophagy adaptor protein located in the autophagosome membrane and is often used as a marker to measure autophagy activity35,36. P62 acts as a receptor in autophagy by interacting with LC3 proteins and ubiquitination substrates37. With the occurrence of autophagy, P62 itself will be continuously degraded by ubiquitination substrates, and its expression level will decrease when autophagy occurs, while it can increase when autophagy is damaged38,39. The experimental results showed that LDM reduced the expression of P62 protein, further confirming that LDM can induce autophagy in AsPC-1 and SW1990 cells. In pancreatic cancer cells that knocked down Mfn2, the changes of autophagy protein induced by LDM were weakened, suggesting that lidamycin may induce autophagy through Mfn2 pathway.
At present, it has been proved that LDM can reduce the mitochondrial membrane potential of pancreatic cancer cells, cause mitochondrial damage, and induce apoptosis of pancreatic cancer cells through Caspase-3 pathway40. Combined with the previous bioinformatics and cell experiments, we hypothesized that LDM-induced autophagy is related to Mfn2-mediated mitophagy. The destruction of mitochondrial membrane potential is an effective trigger for mitophagy41. The experimental results show that LDM can reduce mitochondrial membrane potential. Reactive oxygen species cause oxidative damage to mitochondrial proteins, lipids and DNA, thus leading to mitochondrial damage, which stimulates mitophagy43,44. It has been demonstrated that ROS accumulation triggered by oxidative phosphorylation promotes BNIP3L-mediated mitophagy through recruitment of LC3 to mitochondria42. The experimental results demonstrated that LDM can promote the production of reactive oxygen species in pancreatic cancer cells, and the expression level of mitochondrial membrane protein TOM20 was significantly increased under the action of LDM, indicating that LDM can damage mitochondrial function in pancreatic cancer cells, and the effect of LDM on mitochondrial membrane potential and reactive oxygen species level was reduced after Mfn2 was knocked down. The up-regulation effect of LDM on TOM20 protein was also partially reversed, suggesting that Mfn2 knock down can reduce mitochondrial damage caused by lidamycin (Fig. 7).
Excessive or persistent mitophagy will disrupt the health of mitochondria during tumorigenesis and metastasis, eventually leading to autophagy cell death43,44. BNIP3L destroys the BCL2-BECN1 complex and releases BECN1 (Beclin1), thus promoting the formation of autophagosomes45,46. In this study, we investigated whether mitophagy is involved in LDM-mediated mitochondrial damage of pancreatic cancer cells, and explored the role of Mfn2 in the process of mitophagy, and detected the protein expressions of mitophagy proteins BNIP3L and Beclin1. The results showed that, compared with the control group, Lidamycin promoted the expression of BNIP3L protein and Beclin1 protein, and the down-knocked Mfn2 decreased the up-regulation of BNIP3L protein and Beclin1 protein expression by LDM, suggesting that LDM activated Mfn2-mediated mitophagy of BNIP3L pathway by up-regulating Mfn2 expression in pancreatic cancer cells.
Conclusion
In conclusion, lidamycin induces mitophagy of pancreatic cancer cells by up regulating the expression of Mfn2, and knockdown Mfn2 can attenuate mitochondrial dysfunction and autophagy of BNIP3L pathway of pancreatic cancer cells induced by LDM. In this study, Mfn2 mediated mitochondrial autophagy was the focus of the research on the pharmacological mechanism of LDM in the treatment of cancer for the first time. These findings suggest that Mfn2 and mitochondrial autophagy pathway can be used as gene therapy targets for pancreatic cancer, providing new ideas for the application of LDM in the future.
Data availability
The datasets generated and/or analyzed during this study are available from the corresponding author on reasonable request.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021).
Kamisawa, T. et al. Pancreatic cancer. Lancet 388(10039), 73–85 (2016).
Chen, H. et al. Organoid model: A new hope for pancreatic cancer treatment?. Biochim. Biophys. Acta Rev. Cancer 1875(1), 188466 (2021).
Duan, H., Li, L. & He, S. Advances and prospects in the treatment of pancreatic cancer. Int. J. Nanomed. 18, 3973–3988 (2023).
Yamamoto, H., Zhang, S. & Mizushima, N. Autophagy genes in biology and disease. Nat. Rev. Genet. 24(6), 382–400 (2023).
Miller, D. R. & Thorburn, A. Autophagy and organelle homeostasis in cancer. Dev. Cell 56(7), 906–918 (2021).
Panigrahi, D. P. et al. The emerging, multifaceted role of mitophagy in cancer and cancer therapeutics. Semin. Cancer Biol. 66, 45–58 (2020).
Benischke, A. S. et al. Activation of mitophagy leads to decline in Mfn2 and loss of mitochondrial mass in Fuchs endothelial corneal dystrophy. Sci. Rep. 7(1), 6656 (2017).
Sebastián, D. et al. Mfn2 deficiency links age-related sarcopenia and impaired autophagy to activation of an adaptive mitophagy pathway. Embo J. 35(15), 1677–1693 (2016).
Li, Y. et al. The anti-tumor effects of Mfn2 in breast cancer are dependent on promoter DNA methylation, the P21(Ras) motif and PKA phosphorylation site. Oncol. Lett. 15(5), 8011–8018 (2018).
Hu, Y. et al. Mammalian STE20-like kinase 1 regulates pancreatic cancer cell survival and migration through Mfn2-mediated mitophagy. Mol. Med. Rep. 22(1), 398–404 (2020).
Sun, Q. B., Hui, H. X. & Zhi, X. L. Progress of mitofusion 2 and its relationship with digestive tract tumors. Bachu Med. J. 4(03), 108–111 (2021).
Su, Y. Z. Antitumor antibiotics and targeted cancer therapy. Chin. J. Antibiot. 02, 65–68 (2006).
Chen, J. et al. Down-regulation of the nuclear factor-kappaB by lidamycin in association with inducing apoptosis in human pancreatic cancer cells and inhibiting xenograft growth. Oncol. Rep. 17(6), 1445–1451 (2007).
Yu, M., Nguyen, N. D., Huang, Y. et al. Mitochondrial fusion exploits a therapeutic vulnerability of pancreatic cancer. JCI Insight. 5(16) (2019).
Wood, L. D. et al. Pancreatic cancer: Pathogenesis, screening, diagnosis, and treatment. Gastroenterology 163(2), 386-402.e1 (2022).
Yang, J. et al. Early screening and diagnosis strategies of pancreatic cancer: A comprehensive review. Cancer Commun. (Lond.) 41(12), 1257–1274 (2021).
O’Reilly, E. M. & Abou-Alfa, G. K. Cytotoxic therapy for advanced pancreatic adenocarcinoma. Semin. Oncol. 34(4), 347–353 (2007).
Viale, A. et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 514(7524), 628–632 (2014).
Schrepfer, E. & Scorrano, L. Mitofusins, from mitochondria to metabolism. Mol. Cell 61(5), 683–694 (2016).
Filadi, R., Pendin, D. & Pizzo, P. Mitofusin 2: From functions to disease. Cell Death Dis. 9(3), 330 (2018).
Sun, Q. & Wang, W. MFN2 provides antitumor efficacy in pancreatic cancer cells. Pancreatology 16(4 Supplement), S171 (2016).
Hu, J. L. et al. A new macromolecular antitumor antibiotic, C-1027. I. Discovery, taxonomy of producing organism, fermentation and biological activity. J. Antibiot. (Tokyo) 41(11), 1575–1579 (1988).
Wei, Y. S., Dong, D. L. & Su, Y. Z. Advances in antitumor research of C1027, an enediyne macromolecula antitumor antibiotic. Pharm. Biotechnol. 04, 252–256 (1998).
Li, Y. J. et al. Emerging nanomedicine-based strategies for preventing metastasis of pancreatic cancer. J. Control Release 320, 105–111 (2020).
Jun, L. J. et al. Mfn2 mRNA expression in PCa tissues and observation of proliferation migration, invasion and apoptosis of human PCa cell line transfected wi Mfn2 overexpression plasmid. Shandong Med. J. 63(12), 6–11 (2023).
Lu, W. & Kang, Y. Epithelial–mesenchymal plasticity in cancer progression and metastasis. Dev. Cell 49(3), 361–374 (2019).
Brabletz, S. et al. Dynamic EMT: A multi-tool for tumor progression. EMBO J. 40(18), e108647 (2021).
Amaravadi, R., Kimmelman, A. C. & White, E. Recent insights into the function of autophagy in cancer. Genes Dev. 30(17), 1913–1930 (2016).
Pickles, S., Vigié, P. & Youle, R. J. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. 28(4), R170–R185 (2018).
Lu, Y. et al. Cellular mitophagy: Mechanism, roles in diseases and small molecule pharmacological regulation. Theranostics 13(2), 736–766 (2023).
Wai, T. & Langer, T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol. Metab. 27(2), 105–117 (2016).
Doblado, L. et al. Mitophagy in human diseases. Int. J. Mol. Sci. 22(8), 3903 (2021).
Nakatogawa, H. et al. Dynamics and diversity in autophagy mechanisms: Lessons from yeast. Nat. Rev. Mol. Cell Biol. 10(7), 458–467 (2009).
Komatsu, M. et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131(6), 1149–1163 (2007).
Yoshii, S. R. & Mizushima, N. Monitoring and measuring autophagy. Int. J. Mol. Sci. 18(9), 1865 (2017).
Lamark, T., Svenning, S. & Johansen, T. Regulation of selective autophagy: The p62/SQSTM1 paradigm. Essays Biochem. 61(6), 609–624 (2017).
Rogov, V. et al. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol. Cell 53(2), 167–178 (2014).
Puissant, A., Fenouille, N. & Auberger, P. When autophagy meets cancer through p62/SQSTM1. Am. J. Cancer Res. 2(4), 397–413 (2012).
Chen, J. et al. Synergy of gemcitabine and lidamycin associated with NF-kappaB downregulation in pancreatic carcinoma cells. Acta Pharmacol. Sin. 29(5), 614–619 (2008).
Eisner, V., Picard, M. & Hajnóczky, G. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat. Cell Biol. 20(7), 755–765 (2018).
Melser, S. et al. Rheb regulates mitophagy induced by mitochondrial energetic status. Cell Metab. 17(5), 719–730 (2013).
Zhang, J. et al. Mechanisms of autophagy and relevant small-molecule compounds for targeted cancer therapy. Cell Mol. Life Sci. 75(10), 1803–1826 (2018).
Poole, L. P. & Macleod, K. F. Mitophagy in tumorigenesis and metastasis. Cell Mol. Life Sci. 78(8), 3817–3851 (2021).
Bellot, G. et al. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol. Cell Biol. 29(10), 2570–2581 (2009).
Mazure, N. M. & Pouysségur, J. Atypical BH3-domains of BNIP3 and BNIP3L lead to autophagy in hypoxia. Autophagy 5(6), 868–869 (2009).
Acknowledgements
This work was supported by the Natural Science Foundation of Hebei Province (H2021209004) and Key Scientific Research Project of North China University of Science and Technology (ZD-YG-202312).
Author information
Authors and Affiliations
Contributions
J.C. designed the experiments. B.W. performed the experiments. Bioinformatics analysis was performed by B.W. and B.Q. B.Q. and L.D analyzed and interpreted the data. B.W., B.Q., L.D. and J.C. reviewed the manuscript and wrote the paper. All authors read and approved the final paper. The participant has consented to the submission of the paper to the journal.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, B., Qi, B., Duan, L. et al. Lidamycin induces mitophagy in pancreatic cancer cells by regulating the expression of Mfn2. Sci Rep 14, 20713 (2024). https://doi.org/10.1038/s41598-024-71377-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71377-4
- Springer Nature Limited