Abstract
The severity of autism spectrum disorder (ASD) shows wide variations, though the reason remains unclear. Vitamin D (VitD) deficiency is considered a risk factor for ASD and its supplementation was reported to reduce symptom severity. Since VitD, either synthesized in the skin or absorbed from the food, is transported to the liver by the vitamin D binding protein (DBP), we have analyzed DBP genetic polymorphisms [rs7041 (A/C), rs4588 (G/T), and rs3755967 (C/T)] affecting DBP function [Case = 411; Control = 397], levels of plasma 25(OH)D and DBP [Case = 25; Control = 26], and DBP mRNA expression [Case = 74; Control = 44] in a group of Indo-Caucasoid ASD probands and neurotypical subjects. ASD probands with rs7041’CC’, rs4588 ‘TT’, and rs3755967 ‘TT’ genotypes exhibited higher scores for a few traits. Scores for Imitation and Listening response were also higher in the presence of the “A-T” haplotype (rs7041–rs4588). Plasma 25(OH)D and DBP levels as well as DBP mRNA expressions were significantly lower in the ASD probands as compared to the neurotypical subjects. We infer that DBP deficiency, in the presence of risk genetic variants, could be one of the reasons for the reported 25(OH)D deficiency of the ASD probands.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) is the most prevalent and complex neurodevelopmental disorder all over the world1. As per the Diagnostic and Statistical Manual of Mental Disorders -5 (DSM-5)2, the cardinal features of ASD are characterized by deficits in social communication, language, and speech along with repetitive patterns of behaviors. Genetic, nutritional, and environmental factors are believed to confer a risk for ASD3. Vitamin D (VitD) and other nutrient deficiencies, stress, low birth weight, air pollution, etc. were also studied to understand their possible role in this disorder4.
VitD, a neurosteroid, is known to have an important role in fetal brain development5 and a deficiency was reported in individuals with ASD6. VitD, synthesized in the human skin from the 7-dehydrocholesterol, is released into the bloodstream and binds with the VitD binding protein (DBP). The conjugate is then transported to the liver where the first hydroxylation takes place to form 25 hydroxy VitD [25(OH)D], the most stable circulating form of VitD7. The 2nd hydroxylation takes place in the kidney generating the biologically active form of VitD, the 1,25 dihydroxy VitD [1,25(OH)2D], which is then distributed to different organs and tissues including the brain8. Free 1,25(OH)2D has a higher affinity for the VitD receptors while having a 10–100-fold lower affinity for DBP as compared to the 25(OH)D9.
About 85–90% of circulating VitD, i.e. 25(OH)D, is transported through DBP10 also known as the Group-specific Component (GC). DBP is encoded by the GC gene residing on chromosome 4q11–q1311 and carries several polymorphic sites12,13. The two most important functional single nucleotide polymorphisms of the GC gene, rs7041 (A > C causing substitution of aspartate to glutamate) and rs4588 (G > T replacement of threonine by lysine)14, were found to be associated with variation in 25(OH)D concentration12. The combination of the two SNPs (rs4588 and rs7041) has resulted in three different GC haplotypes/isoforms: GC1f. (A allele rs7041 and G allele rs4588), GC1s (C allele rs7041 and G allele rs4588) and GC2 (A allele rs7041 and T allele rs4588)13,14. However, the link between circulating 25(OH)D level and these genetic polymorphisms has never been explored in the Indian population, more so in the ASD probands. We for the first time analyzed three DBP genetic variants (rs7041, rs4588, and rs3755967), plasma 25(OH)D and DBP levels, as well as peripheral blood DBP expression in a group of Indo-Caucasoid neurotypical children and ASD probands. We have also evaluated the association of these genetic variants with ASD trait scores. The association between GC (rs7041–rs4588) isoforms and behavioral traits were also investigated.
Result
Genetic association analyses
The genotypes of rs7041, rs4588, and rs3755967 followed the Hardy Weinberg Equilibrium (HWE) in all the subject groups. Case–control comparative analysis did not show any significant difference for the ASD probands (Supplementary Table S1).
Haplotype based analyses
Comparative analysis revealed a lower frequency of the ‘C-T-C’ haplotype (rs7041–rs4588–rs3755967) in the ASD probands (N = 411) as compared to the control (N = 397) subjects (Supplementary Table S2; χ2 = 4.06, P = 0.04; OR = 0.34, 95%CI 0.14–0.78). Analysis of GC isoform revealed slightly higher frequency in GC1s and GC2 isoform in ASD probands as compared to the control. (Data was not incorporated due to brevity).
Association of genetic variants with trait scores
The Childhood Autism Rating Scale (CARS) 2-Standard Test (CARS2-ST)15, a widely used tool for quantitative assessment of behavioral traits associated with ASD, was used for the assessment of trait scores of the probands (N = 326).
Genotype-based stratified analysis showed increased CARS2-ST scores for Imitation in the presence of rs7041 ‘CC’ (P = 0.04), rs4588 ‘TT’ genotype (P = 0.004) and rs3755967 ‘TT’ genotypes (P = 0.0005) (Table 1). Probands with rs3755967 ‘TT’ genotype also exhibited higher scores for Listening response (P = 0.008) and General impression (P = 0.04) (Table 1).
Stratified analysis based on the gender of the probands revealed a positive influence of rs7041 ‘CC’ (0.05) and rs4588 ‘TT’ (0.003) on the score for CARS2-ST Imitation of the male ASD probands (Table 1). No significant influence was noticed on the scores of the female probands (data not presented for brevity).
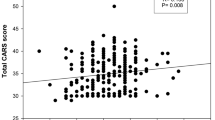
Slightly higher scores for CARS2-ST Imitation [mean score = 2.31; GC1s vs GC2 q(P) = 4.27 (0.008); F(P) = 4.88 (0.009)] and CARS2-ST Listening response [mean score = 2.41 GC1s vs GC2 q(P) = 3.50 (0.03); F(P) = 3.09 (0.05)] were detected in the ASD probands having the GC2 isoform. The data remained significant even after Post- hoc comparison was performed using the Bonferroni correction (Fig. 1; P = 0.016).
Comparative analysis on the trait scores of ASD probands stratified based on the GC isoforms. The box plot shows the values of each domain; lines within the boxes represent the median values. Post hoc comparison after one-way ANOVA was performed by Tukey’s multiple comparison test followed by Bonferroni’s correction for multiple testing (significance set at P = 0.05/3 = 0.016).
Analysis of plasma 25(OH)D and DBP levels
The total plasma 25(OH)D level was significantly lower in the ASD probands (N = 25) than in the neurotypical subjects (N = 26) (10.52 ± 1.32 and 18.48 ± 1.13 respectively; P < 0.001) (Fig. 2a).
The plasma DBP levels were also significantly lower in the ASD probands (N = 25) [24.60 ± 0.83 µg/ml; P = 0.015] in comparison to the age-matched controls (N = 26) [50.64 ± 10.57 µg/ml] (Fig. 2b).
ASD probands belonging to different ethnic groups also exhibited VitD deficiency in comparison to ethnically matched controls (Supplementary Table S3).
DBP mRNA expression in the peripheral blood
DBP mRNA expression was significantly downregulated in the ASD probands (N = 74) as compared to the age-matched controls (N = 44) (Fig. 3a; P < 0.0001); 34.48 fold down-regulated DBP expressions (2−ΔΔct; Fig. 3b) were observed in the ASD probands. Genotype-based stratified analysis revealed significantly lower DBP expression in the ASD probands having rs7041 ‘CC’ (P = 0.005), rs4588 ‘GT’ (P = 0.03), and rs3755967 ‘CT’ (P = 0.0007) genotypes as compared to age-matched controls with the same genotypes (Fig. 4); data remained significant after Post- hoc comparison using the Bonferroni correction (P = 0.016).
Discussion
The present analysis for the first time revealed a significant deficiency in circulating 25(OH)D and DBP level [Case = 25; Control = 26], as well as DBP mRNA expression [Case = 74; Control = 44] in the peripheral blood of the Indo-Caucasoid ASD probands as compared to controls matched for age and ethnicity. In the presence of the derived alleles of the functional DBP genetic variants, trait scores of the ASD probands were higher. DBP expression showed a negative correlation with trait scores.
VitD, a fat-soluble hormone, is mainly produced in the skin upon exposure to sunlight and the level of circulating 25(OH)D in the blood reflects the VitD status in the body16. 25(OH)D deficiency was found to be associated with autoimmune diseases, cardiovascular disease, diabetes, etc17,18,19,20,21. A remarkable influence of 25(OH)D was also reported on neuronal proliferation, differentiation, neurotransmission, and neuroplasticity22,23. Patients with cognitive impairment24, dementia25, Parkinson’s disease26, ASD27,28, and ADHD29,30 were found to have 25(OH)D deficiency.
Multiple proteins involved in the complex metabolic pathway of VitD play pivotal roles in the body’s inflammatory responses. It was observed that less than 2% DBP binds to VitD metabolites, while the rest is involved in the inflammatory responses13,31. The “Neurotoxic Triad”, modulating excitotoxicity, oxidative stress, and neuroinflammation, was reported to be influenced by VitD6. Glutamate dysregulation-induced excitotoxicity, leading to neuronal damage or death, was found to be altered by VitD32,33. Due to its role in the modulation of inflammatory pathways, VitD was reported to suppress neuroinflammatory processes in the CNS34 and to attenuate neuroinflammation, thereby preserving neuronal integrity and function in ASD35.
A broad range of issues, such as nutrition, family history of autoimmune diseases, air pollution, cloudy weather, cesarean delivery, birth order, gestational diabetes, and preeclampsia, were correlated with VitD deficiency and ASD36. In areas with less UVB light, a higher prevalence of ASD was speculated to be due to VitD deficiency37. As stated above, ASD probands belonging to other ethnic groups also exhibited VitD deficiency (Supplementary Table S3). Inadequate VitD level was also reported in ASD and ADHD subjects from the western part of India38. On the contrary, no significant changes in the VitD levels were detected in ASD subjects from the southern parts of India, areas having enough sunlight39.
The present study on the Indo-Caucasoid subjects from eastern India revealed significantly lower plasma 25(OH)D levels in the ASD probands, which could be due to poor dietary habits or limited exposure to sunlight owing to their lack of interest in outdoor activities, warranting further investigation in the field.
We have also observed significantly lower plasma DBP levels as well as down-regulated DBP mRNA expression in the peripheral blood of the ASD probands. At lower concentrations of DBP, more 25(OH)D remains free/unbound and is rapidly catabolized, shortening the functional half-life of 25(OH)D39. A large-scale study involving 65,589 neonates revealed a strong correlation between higher DBP levels and lower risk of 25(OH)D deficiency, multiple sclerosis, and rheumatoid arthritis39. Therefore, the observed DBP deficiency in the Indian children/adolescents diagnosed with ASD may indicate VitD malfunction which merits further extensive investigation on larger cohorts.
To find out the probable cause for the observed DBP deficiency, we analyzed three genetic variants reported to have an association with DBP40 or plasma 25(OH)D deficiencies41,42. Two of these variants, rs7041, and rs4588, also showed association with post-partum depression43 and post-traumatic stress disorder44. In South African type 1 diabetes patients, univariate analysis revealed an association of rs4588 “CC” genotype with higher serum DBP levels45. A higher frequency of the GC1f. isoform, consisting of rs4588–rs7041 A-G haplotype, was reported in the Italian ASD probands28. The other studied variant, rs3755967, was also reported to have a significant influence on plasma 25(OH)D concentration41,46. Individuals having rs7041 “C”, rs4588 “T’, rs3755967 “T” variants and GC2 isoform exhibited 25(OH)D deficiency47,48,49. Our investigation revealed a higher frequency of rs7041–rs4588–rs3755967 C-G-C haplotype in the ASD probands though the result was insignificant. CARS2-ST scores for Imitation were higher in the presence of rs7041 “CC”, rs4588 “TT”, and rs3755967 “TT” variants. CARS2-ST scores were also higher for Listening response and General impression in probands having rs3755967 “TT” genotype. Further, the male probands having rs7041 “CC” and rs4588 “TT” genotypes showed remarkably higher severity for Imitation, indicating that the studied variants may increase the ASD severity of male probands. QT analysis revealed downregulated DBP expression in the ASD probands having rs7041 “C”, rs4588 “T, and rs3755967 “T” variants. These findings indicate that the derived alleles of the studied SNPs influence the phenotypic traits, possibly through a deficiency in circulating DBP which merits further exploration.
In the Italian ASD subjects, the GC1f. isoform (rs7041–rs4588 A-G haplotype) showed a significant association with the total Childhood Autism Rating Scale score28. On the contrary, in the eastern Indian ASD subjects, we have observed higher scores for Imitation and Listening response in the presence of the GC2 isoform (A-T haplotype). This difference in the association of traits could be due to the number of probands recruited for the analysis; we have analyzed the trait scores of 326 ASD probands while the previous authors have performed the association analysis only on 91 ASD probands28. Additionally, our observed association of the GC2 isoform (rs7041–rs4588 A-T haplotype) with higher trait scores should be validated in a larger cohort, since independent QT analysis showed an association of rs7041 “C” and rs4588 “T” with increased trait severity and association of these derived alleles with downregulated DBP expression.
Analysis of the association between the GC isoforms, DBP expression, 25(OH)D, and DBP levels failed to show any significant information (data not presented for brevity). Further investigation, using a larger cohort belonging to different ethnic groups, would be required to confirm the role of DBP genetic variants in ASD. Based on the observations of the present study we postulated that the presence of genetic variants and deficiency in DBP mRNA expression may induce vitamin D deficiency, since lower DBP levels reduce Vit D transport and availability of 25(OH)D, even in the presence of adequate Vit D in the circulation.
The major limitations of the present study are (1) evaluation of 25(OH)D and DBP levels in a limited number of subjects; (2) use of only CARS-2 ST for assessment of ASD trait scores; assessment using other tools, like Autism Diagnostic Observation Schedule (ADOS) may have strengthened our observation.
However, the observed correlation between the risk genetic variants, higher trait scores, and altered DBP expression, indicates that the genetic variants may affect ASD severity by a deficiency in circulating DBP, leading to VitD malfunction in the ASD probands. Ergocalciferol or cholecalciferol supplementation (1000 IU/day) was reported to significantly increase serum DBP levels in patients with hip fractures50. An acute increase in serum DBP and 25(OH)D concentration was also observed following oral supplementation of 50,000 IU ergocalciferol over two weeks51. We infer from these observations that ergocalciferol or cholecalciferol supplementation may help the ASD probands carrying the risk DBP genetic variants or exhibiting inadequate 25(OH)D or DBP levels, thereby reducing the trait severities.
Materials and methods
Recruitment of study subjects
ASD probands (N = 411; 333 males: 78 females; age range 2.7–19 years; male: female ratio 4.27:1) were recruited following the Diagnostic and Statistical Manual of Mental Disorders criteria2. An ethnically matched group of neurotypical subjects (N = 397; female: male ratio 1.2:1; age 5–40 years), devoid of any history of developmental delay, neurological problems, and psychiatric disorders in the family, was recruited for population-based genetic association analysis. All the recruited subjects were from the state of West Bengal in Eastern India (23° N, 87° E), belonging to the Indo-Caucasoid ethnic category. Age was not considered while recruiting subjects for genetic association analysis since genomic constitution may not change with increasing age.
All the ASD probands were urban, unemployed and belonged to medium/high socioeconomic status. In the control group, all adults were professionals and belonged to moderate/high socioeconomic status. Age-matched controls were regular school-going children belonging to moderate socioeconomic status residing in urban areas.
Exclusion criteria
Subjects with only Pervasive developmental disorders, Intellectual disability, Fragile X syndrome, Tourette syndrome, Down’s syndrome, Klinefelter’s syndrome, Turner syndrome, Prader-Willi syndrome, Rett syndrome, and Childhood disintegrative disorders were excluded from this study.
Assessment of traits
Childhood Autism Rating Scale (CARS) 2-Standard Test (CARS2-ST)15 was administered to assess the behavioral attributes of 326 ASD probands. CARS-2 is a widely used tool for quantitative assessment of 15 behavioral domains that help to categorize autistic patients into mild-moderate (scores 30–36.5) and severe (scores 37–60) groups. The 15 domains include (1) Relating to People, (2) Imitation, (3) Emotional response, (4) Body use, (5) Object use, (6) Adaption to change, (7) Visual response, (8) Listening response, (9) Taste, smell and touch response and use, (10) Fear or nervousness, (11) Verbal communication, (12) Nonverbal communication, (13) Activity level, (14) Level and consistency of intellectual response, and (15) General impression. Each of the phenotypes was rated by a score range of 1–4 with 0.5 intervals. Out of the recruited ASD probands, 243 probands belong to the mild-moderate category of CARS (mean CARS score = 33.02), and 83 probands belong to the severe category (mean CARS score = 39.11).
Ethical considerations
All the methods were performed following the relevant guidelines and regulations. Informed written consent was obtained from the studied participants or their legal guardians/caregivers for participation in the study. The protocol was approved by the institutional Ethics Committee on Human Subjects, including Scientists, Psychiatrists, Psychologists, Advocates, and Social workers (PR-006-19).
Selection of SNPs and genotyping
Three DBP missense variants, rs7041, rs4588, and rs3755967, were selected based on previous reports of association14,28,41,42.
Peripheral blood was collected from the participants in anticoagulant-treated vials and genomic DNA was isolated following the phenol/chloroform method52. The oligonucleotide sequences used for Polymerase Chain Reaction were designed in the lab using the Primer3 software and the rs7041, rs4588, and rs3755967 were amplified in Thermal cycler (Proflex PCR system, Thermo Fisher Scientific), followed by Restricted Fragment Length Polymorphism analysis; details are provided in the Supplementary Table S4.
Estimation of plasma 25(OH)D and DBP level
Peripheral blood was collected from ASD probands (N = 25, mean age 7.68 years; male: female ratio 1.78:1) as well as age-matched control subjects (N = 26, mean age 9.3 years; male: female ratio 2.33:1) in pre-cooled vacutainers. The plasma was separated by centrifugation (3000g for 10 min at 4 °C) and stored at − 80 °C until analysis. Plasma 25(OH)D was measured by competitive ELISA following the manufacturer’s guidelines (My Biosource, 25(OH)D ELISA kit, Cat No: MBS722906). Human DBP was measured by quantitative sandwich ELISA (My Biosource, DBP ELISA Kit, Cat No: MBS021936). The optical density of the end products was measured at 450 nm using an ELISA reader (Genetix, Biotech Asia. Pvt. Ltd., India).
Quantification of DBP mRNA expression
DBP mRNA expression was quantified in ASD probands (N = 74, mean age 6.05 years; male: female ratio 3.35:1) as well as age-matched control subjects (N = 44, mean age 8.8 years; male: female ratio 2.14:1). Total RNA was isolated from the peripheral blood using TRIzol reagent followed by DNAse treatment. The concentration of isolated RNA was measured using a Qubit 4 Fluorometer. The cDNA was synthesized from total RNA (700 ng) using a high-capacity cDNA Reverse-transcription kit (Applied Biosystems). Quantitative PCR was carried out in QuantStudio3 (Applied Biosystems by Thermo Fisher Scientific) using PowerUp SYBR green master mix (Applied Biosystems); the primer sequence used was sense 5’ GCCATGTTAAGTGGAGGGTTA3’ and antisense 5’ TGGTTTTTCAGACTGGCAGA 3’ designed using Primer-Blast (https://www.ncbi.nlm.nih.gov/tools/primer-blast). The cycle threshold (ct) value for each sample was obtained, and the data were normalized against an endogenous reference i.e. glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Statistical analyses
Genetic association analyses
Genotypes obtained for the three DBP sites were tested for the HWE using a web platform (http://apps.biocompute.org.uk/hwe-mr-calc.html)53 Case–control comparative analyses on the allelic, genotypic, and haplotypic frequencies were performed using the UNPHASED program v3.1.754 using 1000 iterations to take care of testing for multiple corrections. The odds ratio (OR) was calculated using an online calculator (http://www.hutchon.net/ConfidORnulhanypo.htm)55.
Quantitative trait (QT) analysis
QT analysis was performed using the UNPHASED program v3.1.7, which uses the generalized linear model for quantitative traits54. The association of the studied markers with the behavioral phenotypes of ASD subjects was investigated by applying 1000 permutation tests to take care of multiple corrections. The association of haplotypes with trait scores was analyzed using One-way ANOVA test on GraphPad Prism 9.5.1 after checking the normality of the data with the D’Agostino and Pearson test. Post hoc comparison after one-way ANOVA was performed by Tukey’s multiple comparison test followed by Bonferroni’s correction for multiple testing (significance set at P = 0.05/3 = 0.016).
Analysis of plasma 25(OH)D and DBP level
Case–control comparative analysis on the plasma 25(OH)D and DBP levels was performed using the non-parametric Mann–Whitney test under GraphPad Prism 9.5.1 followed by the generation of a box plot graph. The data obtained are presented as Mean ± Standard error of the mean (SEM).
Comparative analysis of gene expression
Comparative analysis on the ΔCt values obtained from the DBP gene expression pattern was performed by the Mann–Whitney (M–W) test using the GraphPad Prism 9.5.1 and generation of a box plot. The relative changes in the gene expression pattern are expressed as the 2−ΔΔct. The association of genotypes with DBP mRNA expression (Δct) was analyzed using Kruskal–Wallis (K–W) statistics of GraphPad Prism 9.5.1 after checking the normality of the data by D’Agostino and Pearson test. Post hoc comparison after Kruskal–Wallis (K–W) statistics was performed by Dunn’s multiple comparison test followed by Bonferroni’s correction for multiple testing (significance set at P = 0.05/3 = 0.016).
Data availability
Data generated for the study are presented in tabular format as Supplementary Tables and Additional files. Further details on data will be available from the corresponding author upon reasonable request.
References
Hodges, H., Fealko, C. & Soares, N. Autism spectrum disorder: definition, epidemiology, causes, and clinical evaluation. Transl. Pediatr. 9(Suppl 1), S55–S65 (2020).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th edn. Washington (2013).
Grabrucker, A. M. Environmental factors in autism. Front Psychiatry. 3, 118 (2013).
Ng, M., de Montigny, J. G., Ofner, M. & Do, M. T. Environmental factors associated with autism spectrum disorder: A scoping review for the years 2003–2013. Health Promot. Chronic Dis. Prev. Can. 37, 1–23 (2017).
Arshad, R. et al. Impact of vitamin D on maternal and fetal health: A review. Food Sci. Nutr. 10(10), 3230–3240 (2022).
Wang, Z., Ding, R. & Wang, J. The association between vitamin D status and autism spectrum disorder (ASD): A systematic review and meta-analysis. Nutrients. 13, 86 (2020).
Ramasamy, I. & Vitamin, D. Metabolism and guidelines for vitamin D supplementation. Clin. Biochem. Rev. 41(3), 103–126 (2020).
Bikle, D. D. et al. Vitamin D: Production, Metabolism and Mechanisms of Action. In: (eds. Feingold, K. R. et al.) Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000 (2021).
Tsuprykov, O. et al. Opposite correlation of 25-hydroxy-vitamin D- and 1,25-dihydroxy-vitamin D-metabolites with gestational age, bone- and lipid-biomarkers in pregnant women. Sci. Rep. 11(1), 1923 (2021).
Al-Daghri, N. M. et al. Efficacy of vitamin D supplementation according to vitamin D-binding protein polymorphisms. Nutrition. 63–64, 148–154 (2019).
Braithwaite, V. S. et al. Vitamin D binding protein genotype is associated with plasma 25OHD concentration in West African children. Bone. 74, 166–170 (2015).
Enlund-Cerullo, M. et al. Genetic variation of the Vitamin D binding protein affects Vitamin D status and response to supplementation in infants. J. Clin. Endocrinol. Metab. 104, 5483–5498 (2019).
Bouillon, R., Schuit, F., Antonio, L. & Rastinejad, F. Vitamin D binding protein: A historic overview. Front. Endocrinol. 10, 910 (2020).
Gozdzik, A. et al. Association of vitamin D binding protein (VDBP) polymorphisms and serum 25(OH)D concentrations in a sample of young Canadian adults of different ancestry. J. Steroid Biochem. Mol. Biol. 127, 405–412 (2011).
Schopler, E., Van Bourgondien, M. E., Wellman, G. J. & Love, S. R. Childhood autism rating scale 2nd edn. (Western Psychological Services, 2010).
Amrein, K. et al. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 74, 1498–1513 (2020).
Yang, C. Y., Leung, P. S., Adamopoulos, I. E. & Gershwin, M. E. The implication of vitamin D and autoimmunity: a comprehensive review. Clin. Rev. Allergy Immunol. 45, 217–226 (2013).
Cosentino, N. et al. Vitamin D and cardiovascular disease: Current evidence and future perspectives. Nutrients. 13, 3603 (2021).
McCarthy, K. et al. Association between vitamin D deficiency and the risk of prevalent type 2 diabetes and incident prediabetes: A prospective cohort study using data from The Irish Longitudinal Study on Ageing (TILDA). E. Clin. Med. 53, 101654 (2022).
Varghese, E. J. et al. Role of vitamin D3 on apoptosis and inflammatory-associated gene in colorectal cancer: An in vitro approach. J. King Saud Univ. Sci. 32(6), 2786–2789 (2020).
Rozmus, D., Płomiński, J., Augustyn, K. & Cieślińska, A. rs7041 and rs4588 polymorphisms in Vitamin D binding protein gene (VDBP) and the risk of diseases. Int. J. Mol. Sci. 23, 933 (2022).
Anjum, I., Jaffery, S. S., Fayyaz, M., Samoo, Z. & Anjum, S. The role of Vitamin D in brain health: A mini literature review. Cureus. 10, e2960 (2018).
Mayne, P. E. & Burne, T. H. J. Vitamin D in synaptic plasticity, cognitive function, and neuropsychiatric illness. Trends Neurosci. 42, 293–306 (2019).
Maddock, J. et al. Vitamin D and cognitive function: A mendelian randomisation study. Sci Rep. 7, 13230 (2017).
Di Somma, C. et al. Vitamin D and neurological diseases: An endocrine view. Int. J. Mol. Sci. 18, 2482 (2017).
Pignolo, A. et al. Vitamin D and Parkinson’s disease. Nutrients. 14, 1220 (2022).
Bener, A., Khattab, A. O. & Al-Dabbagh, M. M. Is high prevalence of Vitamin D deficiency evidence for autism disorder?: In a highly endogamous population. J. Pediatr. Neurosci. 9, 227–233 (2014).
Bolognesi, E. et al. GC1f Vitamin D binding protein isoform as a marker of severity in autism spectrum disorders. Nutrients. 14, 5153 (2022).
Garipardic, M. et al. Association of attention deficit hyperactivity disorder and autism spectrum disorders with mean platelet volume and vitamin D. Med. Sci. Monit. 23, 1378–1384 (2017).
Naeini, A. A., Fasihi, F., Najafi, M., Ghazvini, R. M. & Hasanzadeh, A. The effects of vitamin D supplementation on ADHD (Attention Deficit Hyperactivity Disorder) in 6–13 year-old students: A randomized, double-blind, placebo-controlled study. Eur. J. Integr. Med. 25, 28–33 (2019).
Kew, R. R. The Vitamin D binding protein and inflammatory injury: A mediator or sentinel of tissue damage?. Front Endocrinol. (Lausanne) 10, 470 (2019).
Martami, F. & Holton, K. F. Targeting glutamate neurotoxicity through dietary manipulation: Potential treatment for migraine. Nutrients. 15(18), 3952 (2023).
Taniura, H. et al. Chronic vitamin D3 treatment protects against neurotoxicity by glutamate in association with upregulation of vitamin D receptor mRNA expression in cultured rat cortical neurons. J. Neurosci. Res. 83, 1179–1189 (2006).
Wimalawansa, S. J. Vitamin D deficiency: Effects on oxidative stress, epigenetics, gene regulation, and aging. Biology (Basel). 8(2), 30 (2019).
Cannell, J. J. Vitamin D and autism, what’s new?. Rev. Endocr. Metab. Disord. 18, 183–193 (2017).
Kalra, R., Kaur, D., Verma, R. & Ghildiyal, R. Vitamin D deficiency in children and adolescents with Autism and ADHD-a retrospective study. Int J. Ind. Psychol. 8, 342–348 (2020).
Cannell, J. J. & Hollis, B. W. Use of vitamin D in clinical practice. Altern. Med. Rev. 13, 6–20 (2008).
Basheer, S. et al. Vitamin D status of children with autism spectrum disorder: Case-control study from India. Asian J. Psychiatr. 30, 200–201 (2017).
Albiñana, C. et al. Genetic correlates of vitamin D-binding protein and 25-hydroxyvitamin D in neonatal dried blood spots. Nat. Commun. 14, 852 (2023).
Rivera-Paredez, B. et al. Association of GC variants with bone mineral density and serum VDBP concentrations in Mexican population. Genes. 12, 1176 (2021).
Shao, B. et al. Vitamin D pathway gene polymorphisms influenced vitamin D level among pregnant women. Clin. Nutr. 37, 2230–2237 (2018).
Lee, S. S. et al. Influence of vitamin D binding protein polymorphism, demographics and lifestyle factors on vitamin D status of healthy Malaysian pregnant women. BMC Pregnancy Childbirth. 20, 714 (2020).
Pillai, R. R. et al. Association of VDBP (rs4588 and rs7041) gene polymorphisms with susceptibility to postpartum depression in South Indian population: A cross-sectional study. Psychiatry Res. 316, 114713 (2022).
Terock, J. et al. Posttraumatic stress disorder is associated with reduced vitamin D levels and functional polymorphisms of the vitamin D binding-protein in a population-based sample. Prog. Neuropsychopharmacol. Biol. Psychiatry. 96, 109760 (2020).
Cave, E. M. et al. The association of vitamin D binding protein levels and genotypes with type 1 diabetes in the black South African population. BMC Endocr. Disord. 22, 182 (2022).
Monge-Rodríguez, S. L., Vindas-Smith, R. & Gómez, G. Plasma 25-hydroxyvitamin D levels, Vitamin D-related genes and risk of type 2 diabetes: Case-control study in a Costa Rican population. Preprint at https://doi.org/10.2139/ssrn.4180035 (2022)
Zhou, J. C. et al. The GC2 haplotype of the vitamin D binding protein is a risk factor for a low plasma 25-hydroxyvitamin D concentration in a Han Chinese population. Nutr. Metab. (Lond.) 16, 5 (2019).
Zhang, Y., Wang, Z. & Ma, T. Associations of genetic polymorphisms relevant to metabolic pathway of Vitamin D3 with development and prognosis of childhood bronchial asthma. DNA Cell Biol. 36, 682–692 (2017).
Ordóñez-Mena, J. M. et al. Genetic variants in the Vitamin D pathway, 25(OH)D Levels, and mortality in a large population-based cohort study. J. Clin. Endocrinol. Metab. 102, 470–477 (2017).
Glendenning, P., Chew, G. T., Inderjeeth, C. A., Taranto, M. & Fraser, W. D. Calculated free and bioavailable vitamin D metabolite concentrations in vitamin D-deficient hip fracture patients after supplementation with cholecalciferol and ergocalciferol. Bone 56, 271–275 (2013).
Berg, A. H. et al. Acute homeostatic changes following Vitamin D2 supplementation. J. Endocr. Soc. 1, 1135–1149 (2017).
Miller, S. A., Dykes, D. D. & Polesky, H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucl. Acids Res. 16, 1215 (1988).
Rodriguez, S., Gaunt, T. R. & Day, I. N. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am. J. Epidemiol. 169(4), 505–514 (2009).
Dudbridge, F. Likelihood-based association analysis for nuclear families and unrelated subjects with missing genotype data. Hum. Hered. 66, 87–98 (2008).
Bland, J. M. & Altman, D. G. Statistics notes. The odds ratio. BMJ. 320(7247), 1468 (2000).
Petruzzelli, M. G. et al. Vitamin D deficiency in autism spectrum disorder: A cross-sectional study. Dis. Markers. 2020, 9292560 (2020).
Şengenç, E., Kıykım, E. & Saltik, S. Vitamin D levels in children and adolescents with autism. J. Int. Med. Res. 48(7), 300060520934638 (2020).
Arastoo, A. A. et al. Evaluation of serum 25-Hydroxy vitamin D levels in children with autism Spectrum disorder. Ital. J. Pediatr. 44(1), 150 (2018).
Saad, K. et al. Vitamin D status in autism spectrum disorders and the efficacy of vitamin D supplementation in autistic children. Nutr. Neurosci. 19(8), 346–351 (2015).
Acknowledgements
The authors are thankful to the participants for volunteering in the study. The authors are also thankful to the Ganapati Sugar Industries for partial financial support for the research work.
Author information
Authors and Affiliations
Contributions
Genotyping, data collection, and analysis, as well as manuscript draft preparation, were performed by SShom. SSaha and MC assisted in data analysis. SSinha recruited the subjects and performed psychological evaluations. KM conceptualized the study and supervised the design, execution, interpretation, and manuscript editing. All the authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shom, S., Saha, S., Chatterjee, M. et al. Indian ASD probands with 25(OH)D and vitamin D binding protein deficiency exhibited higher severity. Sci Rep 14, 19242 (2024). https://doi.org/10.1038/s41598-024-70188-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70188-x
- Springer Nature Limited