Abstract
Plasma levels of endocannabinoids (eCBs) are very dynamic and variable in different circumstances and pathologies. The aim of the study was to determine the levels of the main eCBs and N-acylethanolamines (NAEs) in COVID-19 patients during the acute and post-acute phase of SARS-CoV-2 infection. Samples collected before December 31, 2020 were used for the determination of circulating eCB levels by liquid chromatography-tandem mass spectrometry (LC–MS/MS). The association between plasma eCB measurements and biochemical and hematological parameters, as well as serum IL-6 levels, was evaluated. Samples of 64 individuals were analysed, n = 18 healthy donors, n = 30 acute, and n = 16 post-acute patients. Plasma levels of 2-arachidonoylglycerol (2-AG), were significantly elevated in COVID-19 patients when compared to healthy individuals. Plasma N-palmitoylethanolamide (PEA) and N-arachidonoylethanolamide (AEA) levels were found to be decreased in post-acute patient samples. These results suggest that 2-AG plays an important role in the inflammatory cascade in COVID-19 disease; in addition, eCBs might be involved in the post-acute pathogenesis of COVID-19. This study provides evidence of altered levels of circulating eCBs as a consequence of SARS-CoV-2 infection.
Similar content being viewed by others
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can lead to an exacerbated systemic inflammatory response, characterized by an aberrant release of proinflammatory mediators, both cytokines and chemokines, called a cytokine storm1,2. This dysregulation of the inflammatory cascade is related to a poor prognosis and the risk of complications1,3,4,5. The relevance of this exacerbated inflammatory response has led some authors to propose coronavirus disease 2019 (COVID-19) as a multiorgan inflammatory pathology2,6,7. Currently, no specific treatments have been developed for post-acute COVID-19, due to gaps in the comprehensive knowledge of the pathogenesis; therefore, it is necessary to investigate the molecular mechanisms underlying the inflammatory cascade and its regulation to find both effective treatments and reproducible laboratory biomarkers that help improve clinical management of COVID-19 patients.
The role of the endocannabinoid system (ECS) in the regulation of the immune system has been widely described, acting mainly on the release of cytokines and chemokines, expression of adhesion molecules, cell migration, and the modulation of the phenotypic polarization of macrophages and microglia8,9,10,11. Consequently, since the pandemic outbreak, numerous authors have postulated the ECS as a potential therapeutic target for the treatment of COVID-1912,13,14,15.
The ECS consists of two receptors, cannabinoid receptor type 1 (CB1R) and type 2 (CB2R), endogenous ligands also called endocannabinoids (eCBs), and enzymes involved in the synthesis and degradation of eCBs [for review,16. eCBs are bioactive lipids derived from polyunsaturated fatty acids that are produced by different organs and tissues, including circulating immune cells. The major and best-studied eCBs are N-araquidonoylethanolamine or anandamide (AEA) and 2-araquidonoyl glycerol (2-AG). Both molecules bind to CB1R and CB2R with slight differences in their binding characteristics17. In addition, 2-AG can be metabolized by enzymes of the eicosanoid pathway, such as cyclooxygenase 2 (COX-2) and some lipooxygenasases (LOX) leading to the generation of prostaglandin E2–glycerol esters (PGE2-G)9,17,18. AEA and other N-acylethanolamines (NAEs), such as N-palmitoylethanolamine (PEA) and N-oleoylethanolamine (OEA), can also activate other receptors involved in the inflammatory response, such as transient receptor potential vanilloid 1 (TRPV1), peroxisome proliferator-activated receptor alpha (PPARα), and non-cannabinoid G-protein coupled receptors (GPRs)9,17. Circulating levels of eCBs are highly dynamic and they are altered in different pathological circumstances, in particular, in an inflammatory context [for review,19. These levels have been found to be altered in COVID-19 patients 20, as well as in other viral infections in which an inflammatory reaction is triggered, i.e., hepatitis C21. Moreover, data from in vivo and in vitro models of viral infections have shown that AEA and 2-AG have an anti-inflammatory effects22,23. Therefore, the present study aimed to analyze the circulating levels of the main eCBs in COVID-19 patients and their possible involvement in the inflammatory response after SARS-CoV-2 infection, as well as to assess their potential role as biomarkers in COVID-19 patients.
Materials and Methods
Observational study
Patient samples from the COVID-19 collection from the Hospital Universitario Fundación Alcorcón (HUFA; Madrid, Spain) Biobank were used for this study. All samples used were obtained before December 31, 2020; therefore, all samples were collected in the context of the first wave of the pandemic and none of the participants had received any SARS-CoV-2 vaccine. The control samples were obtained from the collection of healthy donors from the Hospital Universitario Puerta de Hierro Majadahonda (HUPHM; Madrid, Spain) Biobank. Healthy donors had no acute disease at the moment of sample collection. We only used donor samples collected before June 30, 2019 to ensure that the control population had no contact with the virus. Besides, samples were requested from both genders and from an age range between 50 and 80 years old. Serum and plasma samples were obtained following the standard protocols of the Spanish National Biobank Network.
Samples from COVID-19 patients were classified into two groups. On the one hand, samples obtained within 6–10 days of symptoms onset and PCR-positive test (hereinafter referred as acute). On the other hand, samples from asymptomatic patients obtained between 30 and 60 days after PCR-positive test (hereinafter referred as post-acute). Post-acute patients did not show COVID-19 symptoms at follow-up visits and they had normalized their analytical tests, so they had resolved the clinical episode when the sample was collected.
Basic hematology (complete blood count), coagulation (activated partial thromboplastin time [APTT], and D-dimer), and biochemistry analyses (C-reactive protein [CRP], and lactate dehydrogenase [LDH]) data were collected from medical records. These data are from the same blood sample obtained on the day of the COVID-19 diagnostic test, which was also used for the determination of IL-6 levels.
Serum IL-6 levels quantification
Numerous publications have shown an association between elevated IL-6 levels and COVID-19 severity3,5. Therefore, IL-6 levels were determined in all samples using a non-competitive chemiluminescent immunoassay on the Advia Centaur XPT® analyzer (Siemens Healthcare GmbH, Germany).
Circulating eCBs measurement by liquid chromatography-tandem mass spectrometry (LC–MS/MS)
One milliliter of plasma was used for the extraction and analysis of eCBs. Methanol containing 1 μg/mL of N-arachidonoylethanolamine-d8 (AEA-d8), 2-arachidonoylglycerol-d8 (2-AG-d8), palmitoyethanolamide-d5 (PEA-d5) and, oleoylethanolamide-d4 (OEA-d4) (Cayman Chemical, USA) were added as internal standards. Then, samples were subjected to lipid extraction with chloroform/methanol/Tris–HCl 50 mM (2:1:1) and the organic phase was collected and dried in SpeedVac at 55 ºC (ThermoFisher Scientific, USA). Samples were reconstituted in methanol for LC–MS/MS analyses using an Acquity H class (UPLC H-Class, Waters, Spain) online QTrap 4500 system (Sciex, Spain), and Acquity HSS T3 column (1.2 × 100 mm and 1.8 µm, Waters, Spain), at 30ºC and using a mobile phase composed of formic acid 0.1% and acetonitrile (Merck, Germany). A gradient elution at a flow rate 500 µl/min was applied: 0–1.20 min, 0% B; 1.20–5 min, 0–85% B; 5–6 min, 85–90% B; maintained for 1 min at 90% B, 7–8 min, 0% B; and returned to initial conditions in 4 min. Data were acquired using Analyst software 1.6 (Sciex, Germany). The mass spectrometer was operated in positive ionization mode with a voltage of 5500 V. Parameters setting for temperature, curtain gas, ion source gas 1 and gas 2 were: 500 ºC, 20 psi, 20 psi at flow 20 L/min. Data were acquired using MRM (Multiple Reaction Monitoring): AEA (m/z 348,3–62), DP 70 V, CE 35 V, EP 10 V y CXP 4 V; 2-AG (m/z 379–287,2), DP 60 V, CE 21 V, EP 10 V y CXP 7 V; PEA (m/z 300–62), DP 60 V, CE 36 V, EP 10 V y CXP 7 V; OEA (m/z 326.3–62), DP 60 V, CE 35 V, EP 10 V y CXP 7 V; and internal standard AEA-d8 (m/z 356.4–62), DP 30 V, CE 60 V, EP 10 V y CXP 4 V; 2-AG-d8 (m/z 386,22–60), DP 116 V, CE 37 V, EP 10 V y CXP 2 V; PEA-d5 (m/z 304,26–90,83), DP 101 V, CE 59 V, EP 10 V y CXP 8 V; OEA-d4 (m/z 330.26–66), DP 111 V, CE 47 V, EP 10 V y CXP 6 V.
For eCB quantification calibration curves were performed using commercial standards. Individual signals were normalized based on total protein account (BCA assay kit; ThermoFisher Scientific, Spain) for observed sample variability and, peak areas were normalized using internal standard (MultiQuant software 2.1, Sciex, Germany). Calibration range, linearity, limit of detection, limit of quantification, carryover, accuracy and precision, selectivity, recovery and matrix effect were evaluated.
Statistical analyses
Data were analysed using STATA17 software (StataCorp.2021. Stata Statistical Software: Release 17. StataCorp LLC, USA). Univariate analysis was performed to compare demographics and biochemical and hematological data. Fisher’s exact test was used for the analysis of qualitative data. Quantitative data were analysed using the Kruskall-Wallis test for the analysis of three independent samples, or the Mann Whitney U-test in the case of two independent samples. Levels of IL-6 and circulating eCBs distribution were described as geometric mean (GM), median, and interquartile range (IQR). Kruskal–Wallis test was performed to analyse differences by group and, Dunn test with Bonferroni methods were used to adjust for pairwise comparisons. To estimate differences by group adjusted for age and sex, data were log-transformed, performing linear regression models. The arithmetic mean of log-transformed values corresponds to the log GM of the original values. Therefore, the exponentiated regression coefficient estimated in the model can be interpreted as a relative change in the GM of eCB values in the original scale. Finally, eCB levels, biochemical and hematological parameters association was explored using the Spearman rank correlation coefficient. Statistical significance was defined as *p < 0.05 and **p < 0.001.
Ethical standards
The study was conducted in accordance with the Declaration of Helsinki, and approved by the HUFA Comité de Ética en la Investigación con Medicamentos (CEIm; Protocol code: 21/60; Date of approval: March 25, 2021).
Informed consent
Informed consent was obtained from all subjects involved in the study.
Results
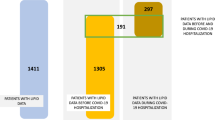
A total of 64 participants were included in the analysis. Samples of 64 subjects were analysed, including n = 18 healthy donors, n = 30 acute and, n = 16 post-acute patients. Before analyzing the samples, it was considered whether the participants were obese, as this is a factor that can affect eCB levels. Of all the participants involved, only one acute patient was obese, so we have no data to analyse the effect of obesity on the eCB measurements. The median age was 61 (IQR 51–71) years and 50% were women. No significant differences in sex and age were found among the 3 groups (Table 1).
Blood cell counts revealed smaller values of neutrophils in post-acute patients (p = 0.023). Regarding biochemical parameters, higher levels of CRP and IL-6 were observed in acute patients (Fig. 1, p < 0.001); as well as in LDH but with no statistically significant difference. APTT and D-dimer values were elevated, but there were no differences between patient groups (Table 1, p = 0.862 and p = 0.122, respectively).
On the other hand, Fig. 2 shows plasma measurements of eCBs. Noteworthy, 2-AG levels were higher in COVID-19 patients compared to those obtained in healthy donors (Fig. 2A), with the highest concentration found in the acute group (Table 2, p < 0.001). When compared by group, AEA values (Fig. 2B) were significantly lower in the post-acute group than in acute patient samples (Table 2, p = 0.016). However, no differences were found when compared to controls. Something similar was observed with PEA levels (Fig. 2C), which are lower in post-acute patients, but this difference only reached statistical significance when compared with subjects in the control group (Table 2, p = 0.022). Healthy donors and acute patients showed similar values of PEA.
Circulating eCB levels per milligram of total protein in COVID-19 patients (acute n = 30; post-acute n = 16) compared to healthy donors (control n = 16). Univariate analysis was performed using the Kruskall-Wallis test and Dunn test was used to calculate adjusted p-values: *p < 0.05; **p < 0.001. 2-AG: 2-arachidonoylglycerol, AEA: N-araquidonoylethanolamine, PEA: N-palmitoylethanolamine, OEA: N-oleoylethanolamine.
There were no statistically significant differences in OEA levels (Fig. 2D); but higher levels were observed in post-acute samples (Table 2, p = 0.236). The results described above were maintained in the multivariate analysis adjusted for age and gender (Table 3). However, the magnitude of the effect is very high in 2-AG levels, estimating a relative difference of 10.23 and 5.52 times more in post-acute and acute respectively versus control samples.
Finally, Tables 4 and 5 summarize Spearman's rank correlation analyses between circulating eCBs and the main biochemical and hematological parameters in acute and post-acute patients respectively. Despite finding a significant increase in 2-AG levels in COVID-19 acute patients, these values did not correlate with any of the routine biochemical/hematological parameters analyzed in the laboratory, nor with IL-6 levels (Table 4). However, both AEA and PEA showed a negative correlation with the lymphocyte count in these patients. In post-acute patients, the negative correlation between AEA and PEA levels and the number of lymphocytes was persistent (Table 5). Furthermore, in post-acute patients, a positive correlation was observed between 2-AG and D-dimer which, coupled with a positive correlation calculated between OEA levels and CRP values, seem to indicate that alterations in eCB tone are maintained in post-acute patients.
Discussion
SARS-CoV-2 infection is characterized by the activation of a systemic inflammatory response that, in severe cases, is associated with the so-called cytokine storm. Given the relevant role of the ECS on the function of the immune system, since the onset of the pandemic, some authors proposed to consider this system as a possible therapeutic target12,14,24,25. However, to our knowledge, there is so far only one study where alterations in two eCBs have been found, mainly related to glucocorticoid treatment20. The purpose of our work was then to find alterations in the main circulating eCBs related to COVID-19 disease in acute and post-acute patients.
eCBs are bioactive lipids that are synthesized “on demand” and act as hydrophobic cellular messengers. These lipid messengers bind mainly to cannabinoid receptors, CB1R and CB2R. CB1R has a very broad distribution pattern, especially in the central nervous system, although it has also been described in peripheral tissues, such as liver, kidney and lung. However, CB2R is mostly expressed in cells of the immune system, as well as in related peripheral tissues, such as the spleen9,26. Several in vitro and animal model studies have shown that CB2R activation regulates leukocyte migration by decreasing the expression of endothelial adhesion molecules as well as decreasing the release of pro-inflammatory cytokines, while promoting the release of anti-inflammatory cytokines9. Thus, CB2R activation seems to play a relevant role in the response to viral infections and, especially, in the inflammatory response27. Moreover, an association has been described between CB2R-Q63R polymorphism with more severe inflammation in chronic hepatitis C virus infection28 and, more recently, with viral infections of the respiratory tract such as respiratory syncytial virus and even an increased risk of developing severe COVID-19 following SARS-CoV-2 infection29,30.
The eCB circulating levels are dependent on the synthesis/degradation ratio in the different tissues and cells in which the eCB-related enzymes are expressed. This ratio can be altered both in physiological (i.e. exercise, dietary intake, circadian rhythm)19,31 and in pathological conditions such as depression, anxiety, cancer, neurological diseases and particularly, in inflammatory processes32,33,34,35,36,37. In the context of COVID-19, Palmos et al.38 have published a study where more than 3000 blood proteins have been analyzed to find potential biomarkers to evaluate the prognosis and evolution of patients. Interestingly, they found a strong association between elevated levels of the enzyme Fatty Acid Amide Hydrolase isoform-2 (FAAH2) and a higher risk of hospitalization. FAAH is the main enzyme involved in the hydrolysis and inactivation of AEA and other NAEs, such as PEA and OEA39. Furthermore, the work of de Carvalho and co-workers has shown alterations in the expression of enzymes involved in the synthesis but not in the degradation of AEA and 2-AG in leukocytes from COVID-19 patients20. Therefore, it could be expected that eCB levels may be altered in SARS-CoV-2 infection.
Our study was raised in the context of the first wave of the pandemic, when no treatment or vaccine was yet available. The fact that the population had not developed immunity or had access to antivirals that alter the course of the disease, more accurately reflects the initial inflammatory response. As subsequent waves occurred and vaccination efforts intensified, the focus shifted, but monitoring inflammation remains relevant to refine our response to COVID-19. Additionally, the information gathered during the first wave could serve as a starting point for identifying therapeutic targets or prognostic markers in future research.
When analyzing the eCB blood levels, we first observed that AEA, PEA and OEA were not significantly modified in acute patients. However, decreases in AEA and PEA levels were found in post-acute patients. This could be due to an increase in the expression of the FAAH enzyme described previously38, which would be inactivating these endogenous ligands therefore, their circulating levels were reduced. Furthermore, in both groups of patients, a negative correlation was noted between AEA and PEA levels and the lymphocytes count. Despite the fact that both NAEs have anti-inflammatory properties, we found no correlation with inflammatory markers. Interestingly, although no significant changes in OEA levels were observed in any of the patient groups, a positive correlation with CRP values was obtained in post-acute patients. OEA has traditionally been linked to the regulation of energy metabolism, mainly via PPAR receptors40; however, in recent years controversial effects on the regulation of the immune system have been described. In some cases, anti-inflammatory effects have been reported41,42, but in other studies, it appeared to have a pro-inflammatory effect43. Based on the results of a previous clinical trial and despite the non-statistically significant results, further studies would be necessary to assess the long-term evolution of OEA levels and even to evaluate the possible role of OEA in long COVID44.
On the other hand, 2-AG followed a different profile to the NAEs analyzed, showing an increase in its circulating levels in acute patients and remaining elevated in post-acute patients. This could suggest that NAEs and 2-AG might be involved in different stages of the immune response. In fact, contradictory effects of 2-AG have been described in different models of inflammatory diseases. On the one hand, it has been seen that increased circulating levels of 2-AG can alleviate inflammation in an animal model of osteoarthritis45. On the other hand, elevated levels of 2-AG showed detrimental effects either by potentiating the release of IL-6 or by being metabolized by COX-2-generating prostaglandins in inflammatory models in vitro46,47.
In the COVID-19 patient samples analyzed, a sustained increase in 2-AG, even 60 days post-infection, was observed. This finding could be consistent with the increased expression of one of the isoforms of phospholipase C-β involved in the 2-AG synthesis pathway20. Since 2-AG is a substrate of COX-2, which is also upregulated in these patients, this increase could be related to elevated prostaglandin E2 (PGE2) levels described in COVID-19 patients at the time of hospital admission48. However, no association was found between 2-AG and some of the inflammatory parameters measured, such as IL-6 or CRP. Interestingly, we did find a positive correlation between 2-AG and D-dimer, which reached statistical significance in the post-acute group. During the post-acute phase of COVID-19, patients often exhibit elevated levels of D-dimer, along with other coagulation abnormalities. These changes may be related to increased mortality rates and have been observed even in recovering or post-hospital discharge patients. The relationship between D-dimer and 2-AG levels can provide valuable insights into the inflammatory response during the post-acute phase.
Based on the results of this study, we cannot rule out 2-AG as a possible biomarker of inflammation, or even as an indicator of the severity of SARS-CoV-2 infection, supporting the findings of previous studies20,38, due to limitations primarily related to the context of sample collection during the first wave of COVID-19. Furthermore, it would be also valuable to assess the possible role of OEA in the pathogenesis of COVID-19.
The context for this study has determined both the recruitment and patient sample collection, limiting both the study population size and the volume of sample available from each participant. Moreover, given that our objective was to determine that the ECS, and specifically the eCBs, could be altered because of SARS-CoV-2 infection and we could not know the potential impact of vaccines, it was essential to use samples collected before the start of the vaccination program in Spain. Therefore, neither gene expression nor enzyme activity studies have been performed to support the idea that eCB levels could be altered as a consequence of the alteration of ECS synthesis and/or degradation enzymes. These circumstances also affected the selection of healthy donor samples, as those prior to June 2019 had to be selected to ensure zero contact of these donors with the virus. Furthermore, given that our study population was in the age range of 49–75 years old, this was also a limitation of the study. Healthy donors are usually younger, so our control population was very limited in size. Another important limitation is the interindividual variability of the immune response, which has meant that the results obtained also showed some variability. In addition, it should be noted that samples from infected patients correspond to the first pandemic wave and inflammatory response was higher with this original virus. Similar variability has also been observed in other studies and does not invalidate the results obtained. Finally, another limitation of the study was the impossibility to analyze the association between eCB levels and severity of pneumonia, due to the fact that we had very few severe cases among our study population. In any event, our results are consistent with a role of the ECS in the pathogenesis of COVID-19, both in the acute and the post-acute phases. However, further studies are needed to strengthen these findings and to fully determine the role of this system in the SARS-CoV-2 inflammatory response.
Conclusions
This study shows that circulating eCBs have been altered following SARS-CoV-2 infection. These variations mainly concern 2-AG that showed increased levels that persisted even 30–60 days post-infection. Further studies are needed to address the potential role of the ECS in the SARS-CoV-2 inflammatory response and its potential role in long COVID development.
Data availability
Data used in this study are stored by corresponding authors and are only available by request, which can be submitted to M. Velasco and will be evaluated by the Hospital Universitario Fundación Alcorcón (HUFA) research committee.
References
Gómez-Escobar, L. G. et al. Cytokine signatures of end organ injury in COVID-19. Sci. Rep. 11(1), 12606. https://doi.org/10.1038/s41598-021-91859-z (2021).
Zanza, C. et al. Cytokine storm in COVID-19: Immunopathogenesis and therapy. Medicina 58(2), 144. https://doi.org/10.3390/medicina58020144 (2022).
Del Valle, D. M. et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 26(10), 1636–1643. https://doi.org/10.1038/s41591-020-1051-9 (2020).
Zhu, Z. et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int. J. Infect. Dis. 95, 332–339. https://doi.org/10.1016/j.ijid.2020.04.041 (2020).
Han, H. et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 9(1), 1123–1130. https://doi.org/10.1080/22221751.2020.1770129 (2020).
Lamers, M. M. & Haagmans, B. L. SARS-CoV-2 pathogenesis. Nat Rev Microbiol 20(5), 270–284. https://doi.org/10.1038/s41579-022-00713-0 (2022).
Meeran, M. F., Sharma, C., Goyal, S. N., Kumar, S. & Ojha, S. CB2 receptor-selective agonists as candidates for targeting infection, inflammation, and immunity in SARS-CoV-2 infections. Drug Dev. Res. 82(1), 7–11. https://doi.org/10.1002/ddr.21752 (2021).
Sacerdote, P., Massi, P., Panerai, A. E. & Parolaro, D. In Vivo and in vitro treatment with the synthetic cannabinoid CP55,940 decreases the in vitro migration of macrophages in the rat: Involvement of both CB1 and CB2 receptors. J. Neuroimmunol. 109(2), 155–163. https://doi.org/10.1016/S0165-5728(00)00307-6 (2000).
Turcotte, C., Chouinard, F., Lefebvre, J. S. & Flamand, N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J. Leukoc. Biol. 97(6), 1049–1070. https://doi.org/10.1189/jlb.3RU0115-021R (2015).
Mecha, M. et al. Endocannabinoids drive the acquisition of an alternative phenotype in Microglia. Brain Behav. Immun. 49, 233–245. https://doi.org/10.1016/j.bbi.2015.06.002 (2015).
Franklin, A. & Stella, N. Arachidonylcyclopropylamide increases microglial cell migration through cannabinoid CB2 and abnormal-cannabidiol-sensitive receptors. Eur. J. Pharmacol. 474(2–3), 195–198. https://doi.org/10.1016/S0014-2999(03)02074-0 (2003).
Rossi, F., Tortora, C., Argenziano, M., Di Paola, A. & Punzo, F. Cannabinoid receptor Type 2: A possible target in SARS-CoV-2 (CoV-19) infection?. IJMS 21(11), 3809. https://doi.org/10.3390/ijms21113809 (2020).
Esposito, G. et al. The potential of cannabidiol in the COVID-19 pandemic. Br. J. Pharmacol. 177(21), 4967–4970. https://doi.org/10.1111/bph.15157 (2020).
Paland, N. et al. The immunopathology of COVID-19 and the cannabis paradigm. Front. Immunol. 12, 631233. https://doi.org/10.3389/fimmu.2021.631233 (2021).
Rastegar, M. et al. Functional Variation (Q63R) in the cannabinoid CB2 receptor may affect the severity of COVID-19: A human study and molecular docking. Arch. Virol. 166(11), 3117–3126. https://doi.org/10.1007/s00705-021-05223-7 (2021).
Zou, S. & Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. IJMS 19(3), 833. https://doi.org/10.3390/ijms19030833 (2018).
Pertwee, R. G. et al. International union of basic and clinical pharmacology. LXXIX cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol. Rev. 62(4), 588–631. https://doi.org/10.1124/pr.110.003004 (2010).
Kozak, K. R., Rowlinson, S. W. & Marnett, L. J. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J. Biol. Chem. 275(43), 33744–33749. https://doi.org/10.1074/jbc.M007088200 (2000).
Hillard, C. J. Circulating endocannabinoids: From whence do they come and where are they going?. Neuropsychopharmacol. 43(1), 155–172. https://doi.org/10.1038/npp.2017.130 (2018).
De Carvalho, J. C. S. et al. The interplay among glucocorticoid therapy, platelet-activating factor and endocannabinoid release influences the inflammatory response to COVID-19. Viruses 15(2), 573. https://doi.org/10.3390/v15020573 (2023).
Patsenker, E. et al. Elevated levels of endocannabinoids in chronic hepatitis C may modulate cellular immune response and hepatic stellate cell activation. IJMS 16(12), 7057–7076. https://doi.org/10.3390/ijms16047057 (2015).
Mestre, L. et al. Anandamide inhibits theiler’s virus induced VCAM-1 in brain endothelial cells and reduces leukocyte transmigration in a model of blood brain barrier by activation of CB1 receptors. J. Neuroinflamm. 8(1), 102. https://doi.org/10.1186/1742-2094-8-102 (2011).
Krishnan, G. & Chatterjee, N. Endocannabinoids affect innate immunity of muller glia during HIV-1 tat cytotoxicity. Mol. Cell. Neurosci. 59, 10–23. https://doi.org/10.1016/j.mcn.2014.01.001 (2014).
Lucaciu, O. et al. In quest of a new therapeutic approach in COVID-19: The endocannabinoid system. Drug Metab. Rev. 53(4), 478–490. https://doi.org/10.1080/03602532.2021.1895204 (2021).
Schiano Moriello, A. et al. First evidence of the protective effects of 2-pentadecyl-2-oxazoline (PEA-OXA) in In vitro models of acute lung injury. Biomolecules 13(1), 33. https://doi.org/10.3390/biom13010033 (2023).
Lu, H.-C. & Mackie, K. Review of the endocannabinoid system. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 6(6), 607–615. https://doi.org/10.1016/j.bpsc.2020.07.016 (2021).
Tahamtan, A., Tavakoli-Yaraki, M., Rygiel, T. P., Mokhtari-Azad, T. & Salimi, V. Effects of cannabinoids and their receptors on viral infections. J. Med. Virol. 88(1), 1–12. https://doi.org/10.1002/jmv.24292 (2016).
Coppola, N. et al. Association between a polymorphism in cannabinoid receptor 2 and severe necroinflammation in patients with chronic hepatitis C. Clin. Gastroenterol. Hepatol. 12(2), 334–340. https://doi.org/10.1016/j.cgh.2013.05.008 (2014).
Tahamtan, A. et al. Effects of cannabinoid receptor type 2 in respiratory syncytial virus infection in human subjects and mice. Virulence 9(1), 217–230. https://doi.org/10.1080/21505594.2017.1389369 (2018).
Rastgar, M., Samadizadeh, S., Yasaghi, M., Moradi, A., Tabarraei, A., Salimi, V., Tahamtan, A. Cannabinoid CB2 Receptor Functional Variation (Q63R) Is Associated with COVID-19 Severity: From Human Study to Molecular Docking; preprint, In review (2021). https://doi.org/10.21203/rs.3.rs-144850/v1.
Watkins, B. A. Endocannabinoids, exercise, pain, and a path to health with aging. Mol. Aspects Med. 64, 68–78. https://doi.org/10.1016/j.mam.2018.10.001 (2018).
Gouveia-Figueira, S., Späth, J., Zivkovic, A. M. & Nording, M. L. Profiling the oxylipin and endocannabinoid metabolome by UPLC-ESI-MS/MS in human plasma to monitor postprandial inflammation. PLoS ONE 10(7), e0132042. https://doi.org/10.1371/journal.pone.0132042 (2015).
Altamura, C. et al. Elevation of plasma 2-arachidonoylglycerol levels in alzheimer’s disease patients as a potential protective mechanism against neurodegenerative decline. JAD 46(2), 497–506. https://doi.org/10.3233/JAD-142349 (2015).
Harfmann, E. J. et al. Circulating endocannabinoid concentrations in grieving adults. Psychoneuroendocrinology 120, 104801. https://doi.org/10.1016/j.psyneuen.2020.104801 (2020).
Carter, G. T. et al. Endocannabinoids and related lipids in serum from patients with amyotrophic lateral sclerosis. Muscle and Nerve 63(1), 120–126. https://doi.org/10.1002/mus.27096 (2021).
Forte, N. et al. Positive association between plasmatic levels of orexin A and the endocannabinoid-derived 2-arachidonoyl lysophosphatidic acid in alzheimer’s disease. Front. Aging Neurosci. 14, 1004002. https://doi.org/10.3389/fnagi.2022.1004002 (2022).
Ota, K. et al. The association of circulating endocannabinoids with cancer cachexia: A cross-sectional study. Clin. Nutr. ESPEN 55, 20–29. https://doi.org/10.1016/j.clnesp.2023.02.020 (2023).
Palmos, A. B. et al. Proteome-wide mendelian randomization identifies causal links between blood proteins and severe COVID-19. PLoS Genet 18(3), e1010042. https://doi.org/10.1371/journal.pgen.1010042 (2022).
Van Egmond, N., Straub, V. M. & Van Der Stelt, M. Targeting endocannabinoid signaling: FAAH and MAG lipase inhibitors. Annu. Rev. Pharmacol. Toxicol. 61(1), 441–463. https://doi.org/10.1146/annurev-pharmtox-030220-112741 (2021).
Matias, I. et al. Role and regulation of acylethanolamides in energy balance: Focus on adipocytes and β-cells. Br. J. Pharmacol. 152(5), 676–690. https://doi.org/10.1038/sj.bjp.0707424 (2007).
Yao, E. et al. Immunomodulatory effect of oleoylethanolamide in dendritic cells via TRPV1/AMPK activation. J. Cell. Physiol. 234(10), 18392–18407. https://doi.org/10.1002/jcp.28474 (2019).
Santa-María, C. et al. Update on anti-inflammatory molecular mechanisms induced by oleic acid. Nutrients 15(1), 224. https://doi.org/10.3390/nu15010224 (2023).
Kwon, E.-K. et al. Oleoylethanolamide induces eosinophilic airway inflammation in bronchial asthma. Exp. Mol. Med. 53(6), 1036–1045. https://doi.org/10.1038/s12276-021-00622-x (2021).
Akbari, N. et al. Possible therapeutic effects of boron citrate and oleoylethanolamide supplementation in patients with COVID-19: A pilot randomized, double-blind, clinical trial. J. Trace Elem. Med. Biol. 71, 126945. https://doi.org/10.1016/j.jtemb.2022.126945 (2022).
Philpott, H. T. & McDougall, J. J. Combatting joint pain and inflammation by dual inhibition of monoacylglycerol lipase and cyclooxygenase-2 in a rat model of osteoarthritis. Arthritis Res. Ther. 22(1), 9. https://doi.org/10.1186/s13075-020-2096-3 (2020).
Valdeolivas, S. et al. The inhibition of 2-arachidonoyl-glycerol (2-AG) biosynthesis, rather than enhancing striatal damage, protects striatal neurons from malonate-induced death: A potential role of cyclooxygenase-2-dependent metabolism of 2-AG. Cell Death Dis 4(10), e862–e862. https://doi.org/10.1038/cddis.2013.387 (2013).
Özdemir, B. et al. Endocannabinoids and inflammatory response in periodontal ligament cells. PLoS ONE 9(9), e107407. https://doi.org/10.1371/journal.pone.0107407 (2014).
Rocha Santos Passos, F., Heimfarth, L., Souza Monteiro, B., Bani Corrêa, C., Rodrigues De Moura, T., Antunes De Souza Araújo, A., Ricardo Martins-Filho, P., Quintans-Júnior, L. J., De Souza Siqueira Quintans, J. Oxidative Stress and Inflammatory Markers in Patients with COVID-19: Potential Role of RAGE, HMGB1, GFAP and COX-2 in Disease Severity. Int. Immunopharmacol. 2022, 104, 108502. https://doi.org/10.1016/j.intimp.2021.108502.
Acknowledgements
The authors are indebted to Hospital Universitario Fundación Alcorcón and Hospital Universitario Puerta de Hierro Majadahonda Biobank staffs. Graphical abstract was created with Biorender.com (https://www.biorender.com).
Funding
This work was supported by the Hospital Universitario Fundación Alcorcón (HUFA) Award for research support and, Ministerio de Ciencia e Innovación – Agencia Estatal de Investigación and Fondo Europeo de Desarrollo Regional (Proyecto PID2022-138461OB-I00, supported by MCIN/AEI/10.13039/501100011033/FEDER, UE).
Author information
Authors and Affiliations
Contributions
Conceptualization: M.V. and M.R.P.; Statistical analysis: E.P.; Funding acquisition: J.R.P, M.V. and M.R.P.; Methodology: F.L, M.A., M.P.A., E.J. and J.M.R.; Resources: M.V. and M.R.P.; Writing—original draft: E.P., M.V, and M.R.P.; Writing—review and editing: J.R., C.G., F.L., M.V. and M.R.P. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Velasco, M., Posada-Ayala, M., Pérez-Fernández, E. et al. Circulating endocannabinoid levels in SARS-CoV-2 infection and their potential role in the inflammatory response. Sci Rep 14, 19558 (2024). https://doi.org/10.1038/s41598-024-70172-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70172-5
- Springer Nature Limited