Abstract
Dogs diagnosed with chronic enteropathy (CE) or small-cell lymphoma (SCL) exhibit marked differences in faecal microbiota and organic acid profiles compared with healthy dogs, as well as immune abnormalities in intestinal mucosal tissue. However, few studies have analysed trace organic acids, such as succinic acid, which have been suggested to be associated with IBD in humans. Therefore, in this study, we compared the faecal microbiota and organic acid profiles as well as serum inflammatory markers between dogs with disease (n = 11; 6 with CE and 5 with SCL) and healthy controls (n = 16). We also performed machine learning and correlation analysis to obtain more detailed insights into the characteristics of affected dogs. These results revealed that dogs with CE and SCL had lower levels of Erysipelotrichaceae (e.g. Turicibacter and Allobaculum), exhibited abnormalities in the succinic acid metabolism (i.e. succinic acid accumulation and decreased levels of Phascolarctobacterium as succinic acid-utilising bacteria) and increased levels of pathobiont bacteria such as Escherichia-Shigella. Additionally, the presence of Dubosiella was significantly negatively correlated with Canine Inflammatory Bowel Disease Activity Index scores. These findings are expected to aid the development of microbiome-based medications and/or supplements, although further verification is needed.
Similar content being viewed by others
Introduction
Canine chronic enteropathy (CE) is a digestive disorder characterised by chronic digestive problems that persist for more than 3 weeks as well as primary inflammation of the intestinal mucosa1,2. The most common clinical symptoms are chronic diarrhoea, vomiting, anorexia and weight loss, and these symptoms greatly reduce a dog’s quality of life. Furthermore, CE is difficult to cure and can exhibit periodic episodes of exacerbation and remission, consequently prolonging the treatment period. Generally, first-line treatment is diet therapy involving allergy elimination diets and hydrolysed diets2,3. Multiple diet trials are reported to achieve a higher response rate; however, about 40–50% of all cases do not respond to diet therapy4,5. In such cases, treatment is switched to pharmacological therapy with corticosteroids, and if none of these treatments are successful, antibiotics may be administered5,6. In addition, for cases in which bile acid malabsorption is the cause, bile acid sequestrants may be administered. Corticosteroids are highly effective in managing severe cases and can even induce remission. However, in some cases, the extended use of corticosteroids can lead to serious adverse effects such as iatrogenic hyperadrenocorticism7. Accordingly, medication managements, such as dose reduction and/or treatment suspension, are important for CE in dogs. In addition, a small percentage of cases do not respond to corticosteroids and may even result in death8. To improve the quality of life in dogs with CE, new medications and/or supplements must be developed that improve symptoms and maintain remission.
Although the exact cause of CE remains unclear, several factors influence disease susceptibility. These factors include genetic disposition, abnormal immune response to various environmental antigens (e.g. dietary antigens) and disturbance of gut microbiota (i.e. dysbiosis)9,10,11. The gut microbiota plays an important role in the pathogenesis of CE. A previous study reported that experimental models of the development of inflammatory bowel disease (IBD), such as TCR mutant mice, were free from gut inflammation under germ-free conditions12. Moreover, in models with gut dysbiosis, the gut microbiota produced small amounts of metabolites (e.g., organic acids) through fermentation, and these metabolites are known to play crucial roles in the maintenance of intestinal homeostasis. For instance, these metabolites, including acetic acid, propionic acid and butyric acid, exhibit colonisation-resistance activity against several pathogens, strengthen the intestinal mucosal barrier functions and support the mucosal immune system. However, it has also been reported that metabolites such as phenols and indole (i.e., putrefactive products) inhibit the maintenance of intestinal homeostasis by disrupting the intestinal mucosal barrier and inhibiting immunosuppression. Accordingly, the gut microbiota is considered an important target for the treatment and improvement of symptoms in CE. This is supported by studies showing that many dogs with chronic diarrhoea and CE have markedly different intestinal bacteria and gut metabolite profiles compared with healthy dogs13,14. In recent years, faecal transplantation has gained attention as a potential new treatment modality for human IBD, and several reports have confirmed its efficacy in dogs as well15,16. These reports suggest that the gut microbiota and its metabolites play a key role in the onset and maintenance of CE and its associated symptoms; however, it is not clear which factors are responsible. Moreover, studies have shown that CE shares many characteristics with human IBD, including factors contributing to onset as well as treatment methods17. In terms of the gut microbiota, several commonalities have been reported, including an increase in Enterobacteriaceae and a decrease in Faecalibacterium and Blautia18. However, despite these similarities, studies analysing trace amounts of faecal organic acids derived from the gut microbiota such as succinic acid, which is reported to be associated with human IBD19, have not been performed in dogs with CE. Meanwhile, small-cell lymphoma (SCL) is a disease with clinical symptoms similar to those in CE. Typically, differential diagnosis of these diseases is performed using histopathology. However, due to similarities in the populations and patterns of infiltrating cells, differentiating between CE and SCL is challenging. Several diagnostic procedures have been proposed, but definitive diagnostic criteria for classifying dogs with CE and SCL have yet to be established. Therefore, it is difficult to definitively classify these two diseases according to both clinical symptoms and histopathological diagnosis20.
Therefore, the aim of this study was to better understand the characteristics of dogs with CE and SCL in order to improve symptoms and treat the condition. We compared faecal microbiota compositions, organic acid concentrations (including trace acids) and serum cytokine and chemokine concentrations between dogs with disease (n = 11; 6 with CE and 5 with SCL) and healthy controls (n = 16) and identified the characteristics of the affected dogs. Furthermore, random forest (RF) analysis and correlation analysis were performed to investigate the properties of affected dogs and the items involved in severity.
Results
Clinical background
The backgrounds of the dogs in this study are shown in Table 1. The average age of the dogs in the disease group was significantly higher than those in the healthy group, whereas body weight and plasma albumin concentrations were significantly lower (age, healthy: 4.79 ± 3.42, disease: 8.60 ± 3.17, P = 0.007; body weight, healthy: 15.9 ± 6.58, disease: 5.42 ± 2.19, P < 0.001; albumin, healthy: 3.26 ± 0.29, disease: 2.15 ± 0.59, P = 0.002). There was no significant difference in the sex ratio of the disease group compared with the healthy group. Medical records revealed that 5 dogs in the disease group were fed a low-fat diet, and that 4 dogs received corticosteroids (budesonide, n = 1; prednisolone, n = 3) within 4 weeks before the start of the study. Considering the possibility that these clinical backgrounds may lead to biases in comparative analyses, we evaluated the impact of these differences on the faecal microbiota and organic acid concentrations as well as serum cytokine and chemokine concentrations; however, the dogs’ clinical backgrounds had hardly any effect on these attributes (Supplementary Figure S1 and Supplementary Table S1). Consequently, for the analyses presented below, we did not consider differences in clinical backgrounds.
A difference in age was observed between the CE group (n = 6) and the small-cell lymphoma (SCL) group (n = 5) (P = 0.048). However, there were no other differences, including clinical background (Supplementary Table S2).
Comparison of faecal microbiota
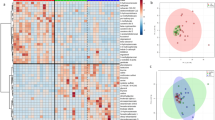
The β-diversity index (weighted UniFrac distance) of the faecal microbiota differed significantly between the disease and healthy groups (P = 0.002; Fig. 1A). Moreover, the α-diversity indices, including Faith’s PD, observed amplicon sequence variants (ASVs) and the Shannon index, were significantly lower in the disease group than in the healthy group (Faith’s PD: P = < 0.001, observed ASVs: P < 0.001, Shannon index: P < 0.001; Fig. 1B). The microbiota composition stratified by family level is shown for each dog in Fig. 2A. The microbiota composition differed between the groups, and the relative abundance of Erysipelotrichaceae, Lactobacillaceae, Fusobacteriaceae, Prevotellaceae and Ruminococcaceae were lower in the disease group than in the healthy group, while those of Lachnospiraceae and Enterobacteriaceae were higher. To perform an in-depth analysis of the differences in microbiota composition between the groups, linear discriminant analysis (LDA) of effect size (LEfSe) for the genera present in both groups was performed, and differences in the relative abundance of 35 genera were observed (α value = 0.05, LDA score > 3.0; Fig. 2B). Twenty-four genera had a low relative abundance in the disease group, including Dubosiella, Turicibacter and Allobaculum (belonging to Erysipelotrichaceae); Limosilactobacillus, Lactobacillus and Ligilactobacillus (belonging to Lactobacillaceae); Fusobacterium (belonging to Fusobacteriaceae); Alloprevotella and Prevotella 9 (belonging to Prevotellaceae); and the Ruminococcus torgues group and Faecalibacterium (belonging to Ruminococcaceae), which actively produce organic acids. In contrast, 11 genera in the disease group had a high relative abundance, including several pathogenic and/or pathobiont bacteria such as Escherichia-Shigella, Clostridioides and Staphylococcus. As shown in Table 1, there were significant differences in physical characteristics (age and body weight) between the groups. Previous studies have shown that these variables significantly affect the microbiota composition21] To test whether they were confounding factors in this study, we performed an analysis of covariance with age and body weight as covariates, but the results indicated that these factors had little effect (Supplementary Table S3).
Comparison of faecal microbiota diversity in the healthy group and disease groups. (A) Principal coordinate analysis based on the weighted UniFrac distances as β-diversity. The blue and red dots (triangle: chronic enteropathy [CE]; square: small-cell lymphoma [SCL]) correspond to healthy dogs and dogs with disease, respectively. Weighted UniFrac distances were compared using permutational multivariate analysis of variance between healthy and disease groups. (B) Three indices (Faith’s PD, observed amplicon sequence variants and Shannon index) as α-diversity (upper: main analysis; lower: subgroup analysis). The P value of the Kruskal–Wallis test is indicated by the top dashed line, while the solid line represents the P value of post hoc multiple comparisons using Dunn’s test with Bonferroni adjustment.
Comparison of faecal microbiota composition in the healthy group and disease group. (A) Taxonomic overview of the faecal microbiota at the family level. (B) Identification of significantly discriminative genera using linear discriminant analysis (LDA) effect size (α = 0.05, LDA score > 3.0). CE, chronic enteropathy; SCL, small-cell lymphoma.
Because LEfSe demonstrated that the relative abundance of pathogenic and/or pathobiont bacteria was higher in the disease group than in the healthy group, we used YIF-SCAN to quantify the bacterial counts of representative pathogenic and/or pathobiont bacteria such as Clostridioides difficile, Clostridium perfringens, Escherichia coli and Staphylococcus as well as the total bacterial counts (Table 2 and Supplementary Figure S2). Total bacterial counts were significantly lower in the disease group than in the healthy group, while bacterial counts of C. difficile, C. perfringens and E. coli were significantly higher (total bacterial counts, P = 0.014; C. difficile, P < 0.001; C. perfringens, P = 0.043; E. coli, P < 0.001). Additionally, the detection rates of C. difficile and E. coli were significantly higher in the disease group than in the healthy group (C. difficile, P < 0.001; E. coli, P = 0.001).
Comparison of faecal organic acid profile
Faecal succinic acid and formic acid concentrations were significantly higher in the disease group than in the healthy group (succinic acid: P < 0.001, formic acid: P = 0.004), while those of acetic acid and propionic acid were significantly lower (acetic acid: P < 0.001, propionic acid: P < 0.001; Table 3 and Supplementary Figure S3).
Comparison of serum cytokine and chemokine profiles
As shown in Table 4, the disease group showed significantly higher levels of inflammatory cytokines and chemokines, including IFN-γ, KC-like, IL-8, MCP-1, IL-12 (p40) and the anti-inflammatory cytokine IL-10, compared with the healthy group (IFN-γ, P = 0.009; KC-like, P < 0.001; IL-8, P = 0.023; MCP-1, P = 0.001; IL-12 (p40), P < 0.001; IL-10, P = 0.007).
Differences between disease subtypes
The β-diversity index (weighted UniFrac distance) of the CE group showed a significant difference compared with the healthy group, while there was only a marginal difference compared with the SCL group (Fig. 1A; Healthy vs CE, P = 0.002; Healthy vs SCL, P = 0.064). There were no significant differences between the two subtypes (CE vs. SCL, P = 0.481). For items that showed significant differences between the healthy and disease groups in terms of α-diversity, β-diversity, faecal organic acids, and serum cytokine and chemokine concentrations, there were also significant differences by three-group analyses (Kruskal–Wallis test; healthy group, CE group and SCL group). However, there was no significant difference between the CE and SCL groups (Fig. 1B, Tables 3 and 4).
Random forest analysis
The input data for the RF analysis were the relative abundance of 35 genera and the 4 types of organic acid concentrations, for which differences were observed between the disease and healthy groups. Using the RF algorithm, a model to classify each group was constructed.
Serum cytokine and chemokine concentrations also showed group differences in 6 types, but 2 samples in the disease group were missing, slightly reducing the reliability of the model; therefore, these data were excluded from the input data. To obtain more accurate results, the model was run 10 times, and the mean decrease in accuracy (MDA) was calculated for each input. The mean out-of-bag estimate for the error rate of 10 models was 1.85 ± 1.95%, indicating that the models had high accuracy. Figure 3 shows the top 10 items with a high mean of MDA, which were considered useful for classifying the groups. Eight of the 10 selected items were relative abundance of genus (Turicibacter, Allobaculum, Escherichia-Shigella, Phascolarctobacterium, Parasutterella, Romboutsia, Alloprevotella and Muribaculaceae unidentified), and the other 2 items were organic acid concentration (propionic acid and succinic acid); the highest mean of MDA was the relative abundance of Turicibacter.
Prediction of outcomes using a random forest classifier trained to differentiate between the healthy group and disease groups. Ten important features were selected from the combined metagenome at the genus level and faecal organic acids, using a random forest classification model. The features were ranked based on the average mean decrease in accuracy, which was determined using 10 model constructions. Data are expressed as means ± SD.
Correlation analysis
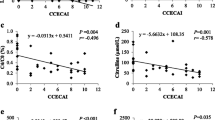
We performed a correlation analysis of 45 items, including 39 items used as input data for the RF analysis and the serum cytokine or chemokine concentration of 6 types for which differences were observed between the disease and healthy groups, as well as the canine inflammatory bowel disease activity index (CIBDAI) score. This analysis revealed a significant correlation only between the relative abundance of Dubosiella and CIBDAI (Rho = − 0.883, P = 0.002; Fig. 4 and Supplementary Table S4). Moreover, Dubosiella showed a strong correlation with 9 of the 10 items, except succinic acid, selected by RF (Supplementary Table S5).
Discussion
The objective of this study was to understand the pathophysiological properties of dogs with CE and SCL by analysing serum inflammatory markers as well as the faecal microbiota and its metabolite profiles. Then, we compared the faecal microbiota, organic acids, and serum cytokine and chemokine concentrations in dogs with disease, sub-grouped into those with CE or SCL, with healthy controls. Based on the items in which differences were observed between the groups, the characteristics of dogs with CE and SCL were further investigated using machine learning and correlation analyses.
The composition of the faecal microbiota (β-diversity) in dogs with disease differed from that of healthy dogs, and the total bacterial counts and α-diversity were significantly lower. A detailed analysis of the microbiota composition revealed that dogs with disease had significantly higher counts of C. difficile, C. perfringens and E. coli, which are pathobiont bacteria typically associated with diarrhoea22,24,24. Additionally, the relative abundance of various bacterial taxa known to produce organic acids were significantly lower in dogs with disease. In addition, the concentrations of short-chain fatty acids in dogs with disease were lower than those in healthy controls. These results suggest that the gut microbiota in dogs with disease investigated in this study differed markedly from that of healthy dogs. However, to clarify these findings, it will be necessary to increase the number of cases in future studies. Faecal acetic acid and propionic acid concentrations were significantly lower in dogs with disease than in healthy controls. Acetic acid and propionic acid are the major components of the short-chain fatty acids produced in the gut that exhibit biological activities such as colonisation-resistance activity against pathogens and the maintenance of the mucosal immune system26,25, 27. Thus, a decline in these factors indicates worsening of intestinal homeostasis. Previous studies have reported that dogs with acute diarrhoea and CE not only exhibit dysbiosis but also have reduced levels of the bacterial taxa known to produce organic acids24,25. AlShawaqfeh et al. proposed a “dysbiosis index” comprising total bacteria and 7 bacterial counts (Faecalibacterium, Turicibacter, Streptococcus, E. coli, Blautia, Fusobacterium, and C. hiranonis) deemed to be characteristic of the gut microbiota of dogs with CE (i.e. Streptococcus and E. coli are higher in dogs with CE while others are higher in healthy dogs)30. Our results are largely consistent with this “dysbiosis index”, although there is a distinction between the methods of qPCR and 16S rRNA amplicon sequencing (i.e. absolute quantification vs. relative abundance). However, C. hiranonis could not be assessed using 16S rRNA amplicon sequencing in this study. C. hiranonis plays an important role in the conversion of primary bile acids to secondary bile acids via antimicrobial activity in dogs and has been reported to show a negative correlation with C. difficile31. In the future, C. hiranonis should be evaluated using PCR methods. Moreover, Minamoto et al. reported that faecal acetic acid and propionic acid concentrations in dogs with CE were significantly lower than those in the healthy dogs and that a decrease in the concentrations of these organic acids was caused by changes in intestinal bacteria32. In this study, we also analysed trace organic acids in faeces. Faecal succinic and formic acid concentrations were significantly higher in dogs with disease than in healthy dogs. Succinic acid is an intermediate metabolite produced by intestinal bacteria that is barely detectable in the faeces of healthy humans. However, its accumulation has been observed in patients with CE such as IBD19. In humans with Crohn’s disease, the concentration of succinate and the expression of its receptor, SUCNR1, are upregulated in the intestinal mucosa33. In addition, succinic acid induced inflammation and fibrosis of the intestinal mucosa in an experimental model of Crohn’s disease33. Considering these findings, succinic acid and its signalling pathway have been proposed as potential treatment targets for canine CE and SCL. To our knowledge, the present study is the first to report an accumulation of succinic acid in dogs with CE and SCL. Although not significantly different, the concentration of valeric acid, which has been suggested to be related to dysbiosis-mediated metabolic chronic enteritis and necrotising enteritis28,29, was lower in dogs with disease than in healthy controls. Therefore, we consider that these trace organic acids may serve as useful markers for the diagnosis and treatment of CE and SCL, although a long-term follow-up study is needed to verify whether this is indeed the case.
The top 10 items selected by RF analysis included mainly the bacterial taxa belonging to the family Erysipelotrichaceae (Turicibacter, Allobaculum), items related succinic acid metabolism (succinic acid, propionic acid, Phascolarctobacterium), and pathobiont bacteria and bacteria involved in their growth (Escherichia-Shigella, Muribaculaceae unidentified). Bacteria in the family Erysipelotrichaceae are reported to have a lower faecal relative abundance in dogs with acute haemorrhagic diarrhoea and CE compared with healthy dogs26,15, 24. Moreover, Bermingham et al. investigated the relationship of physiological parameters such as the intake of key nutrients and their digestibility with the gut microbiota and found that the Erysipelotrichaceae family may play a central role in the link between gut microbiota and their health 37. The present study revealed that the relative abundances of Turicibacter and Allobaculum were significantly lower in dogs with disease and that these genera are associated with intestinal health. As described above, succinic acid is correlated with human CE. Succinic acid is generally metabolised to propionic acid in the intestine by succinic acid-utilising bacteria such as Phascolarctobacterium38. Dogs with disease showed significantly higher concentrations of faecal succinic acid and significantly lower concentrations of propionic acid and relative abundance of Phascolarctobacterium compared with healthy dogs. In addition, a significant correlation was observed between both organic acids and the relative abundance of Phascolarctobacterium (succinic acid: Rho = − 0.644, P < 0.001) and propionic acid (Rho = 0.678, P < 0.001). These results indicate that an increase in succinic acid and a decrease in propionic acid are associated with a decrease in Phascolarctobacterium in the gut. Propionic acid not only possesses anti-inflammatory effects, but is also involved in immunomodulation via Treg cell regulation39,40. Pathways involved in the metabolism of succinic acid may be good candidates for the treatment of CE and SCL. Supporting this concept, Nagao-Kitamoto et al. reported that supplementation with Phascolarctobacterium genera into mice improved symptoms related to C. difficile infection along with a reduction in succinate concentration in the gut41. It has been reported that pathobiont bacteria such as Escherichia-Shigella (grouped together because differentiating E.coli from Shigella based on 16S rRNA sequencing is difficult), including E. coli, are detected at higher concentrations and relative abundances in the faeces of dogs with CE than in healthy dogs13. The present study also produced similar results: dogs with disease had significantly higher counts of C. difficile, C. perfringens and E. coli compared with healthy controls. Some pathobiont bacteria such as E. coli and C. difficile proliferate using polysaccharides derived from mucin present in the intestinal mucosa36,37. Using a mouse model, Pereira et al. demonstrated that the administration of 5 bacterial species with high utilisation of polysaccharides derived from mucin, including Muribaculaceae, competed with C. difficile and inhibited its growth, and that Muribaculaceae, in particular, played a key role44. In the present study, dogs with disease had significantly lower levels of Muribaculaceae compared with healthy dogs. Muribaculaceae may serve as a potential target for alleviating diarrhoea symptoms caused by the proliferation of pathobiont bacteria such as C. difficile.
Dubosiella, a recently discovered bacteria belonging to the Erysipelotrichaceae family45, showed a significant negative correlation with CIBDAI scores. Although there are no reports on its relationship with human or canine intestinal diseases, several recent studies using a mouse model of dextran sulphate sodium salt (DSS)-induced colitis have indicated the involvement of Dubosiella46,47. In those studies, the relative abundance of Dubosiella decreased after the onset of colitis. Li et al. reported that treatment with 5-aminosalicylic acid, which is a medicine for ulcerative colitis in humans, improved the disease activity index score and the relative abundance of Dubosiella increased46. Li et al. reported that compared with general DSS mice, those fed a high dose of lean meat in order to trigger inflammation were associated with higher disease activity index scores and a lower relative abundance of Dubosiella47. This suggests an association between Dubosiella and the severity of inflammatory bowel disease. Dubosiella was not selected by the RF but did demonstrate a strong correlation with several items that were selected. These results suggest that Dubosiella affects the severity of CE and SCL but does not play a role in their onsets, and its relative abundance may be controlled by improving the items selected by the RF.
One approach for treating CE and improving its symptoms is the use of probiotics and/or prebiotics. Bifidobacterium and lactobacilli, which are common probiotics, appear to improve the gut microbiota and intestinal environment by increasing the concentration of organic acids and reducing the abundance of pathogenic and/or pathobiont bacteria; these effects are similar in both dogs and humans48. Several interventional studies using probiotics for dogs with CE have been conducted. The multi-probiotic VSL#3, which consists of 4 strains of lactobacilli, 3 strains of Bifidobacterium and 1 strain of Streptococcus and is used to prevent and treat human ulcerative colitis, is reported to enhance immune function as well as improve dysbiosis and overall clinical symptoms in dogs49. In contrast, the use of prebiotics led to increased levels of Bifidobacterium and organic acids, as in humans; therefore, prebiotics are also considered to contribute to intestinal health in dogs50. In humans, it has been reported that prebiotic intervention improved clinical symptoms, although this was not definitively demonstrated51. There are few reports on dogs, and thus more validation is needed in the future.
This study has several limitations. First, the sample size was small and the statistical power was limited. Specifically, the small sample size may have influenced the results of the correlation analysis and the RF algorithm. Second, the characteristics of the dogs included in this study were not normalised. All dogs with disease were observed to have chronic digestive symptoms and intestinal mucosal inflammatory findings, but they received pharmacological and/or diet therapy. Additionally, the healthy dogs were kept in the same facility, and there were biases in terms of dog breeds, ages and body weights compared with the disease group. It has been reported that these characteristics can significantly affect study parameters, especially gut microbiota17,47. It was confirmed that differences in some characteristics, including clinical background, age and body weight, had almost no influence on the results of this study. However, for a more accurate analysis, it will be necessary to normalise the clinical background and perform group matching for characteristics. Third, high levels of inflammatory cytokines and chemokines, including GM-CSF, KC-like, IL-8 and MCP-1, have been observed in some healthy dogs. These may indicate the occurrence of an inflammatory response in the body, which might have introduced some noise into the study. In addition, approximately half of the dogs with disease analysed in this study exhibited signs of hypoalbuminemia, indicating that these dogs may have had more severe intestinal disease. Further analysis, including stratified analysis based on hypoalbuminemia status, would provide more detailed information on the characteristics of the dogs with disease. Finally, the analysis of the gut microbiota in this study was based solely on the 16S rRNA amplicon sequencing, which provides a relative value, but no data validation was conducted. These data may complicate comparisons with other studies and diminish the reliability of the data. To strengthen our arguments, validation data, such as those obtained through quantitative PCR methods that provide an absolute value, are needed.
In this study, we demonstrated that the gut microbiota of dogs with CE and SCL was in a state of dysbiosis, the intestinal environment was poor, and there was severe inflammation. Moreover, we reported the faecal microbiota and metabolite characteristics of the affected dogs. Specifically, this study is the first to demonstrate an abnormality in the metabolism of succinic acid involving Phascolarctobacterium as well as a relationship between Dubosiella and CE and SCL. In the future, it will be necessary to investigate whether the findings obtained in this study might contribute to the treatment of this disease and the improvement of its symptoms, and whether probiotics and/or prebiotics may be an effective approach.
Methods
Sample collection
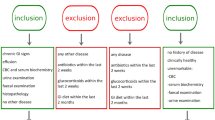
In this study, we evaluated dogs that were brought to the Veterinary Medical Center of the University of Tokyo because of chronic gastrointestinal symptoms (e.g. vomiting, diarrhoea, anorexia, weight loss) that had continued for ≥ 3 weeks or hypoalbuminemia (< 2.6 g/dL). Based on medical history, blood test, X-ray and ultrasound examinations, and gastrointestinal endoscopy, we screened and enrolled the dogs with CE and SCL. The breed, sex, age, body weight, clinical symptoms, diet, medical history and clinicopathological data of dogs were extracted, and those that received antibiotic treatment during the 2 weeks before the sample collection were excluded from the study. The severity of clinical symptoms was assessed using the CIBDAI according to six factors: activity, appetite, vomiting, faecal characteristics, frequency and weight loss53. Intestinal tissue collected by gastrointestinal endoscopy was fixed using 10% neutral buffered formalin solution and embedded in paraffin after clipping. The samples were sliced, stained with haematoxylin and eosin, and histopathologically analysed under an optical microscope. SCL was diagnosed based on the high-density monomorphic clustering of tumour-infiltrating lymphocytes, which have a small nucleus less than the diameter of 1.5 red blood cells, as well as symptoms of malignancy, including invasion into the muscularis mucosa, structural distortion, and abnormal cell size20. Inflammatory infiltration of diffuse lymphoplasmacytic lymphoma was observed in all specimens. Healthy dogs raised at the Veterinary Medical Center were enrolled as controls. Faecal and serum samples of dogs with disease and healthy dogs were collected during endoscopic and physical examinations, respectively.
Treatment of faecal samples
After collection from the rectum, faecal samples were immediately frozen and preserved at − 80 °C. Frozen samples were weighed after thawing and diluted tenfold using phosphate-buffered saline (PBS) (faeces:PBS = 1:9). After adding zirconia beads, the faecal diluent was suspended for 10 min at room temperature, using ShakeMaster® (Bio Medical Science, Tokyo, Japan). The suspension was divided into three tubes of 200 µL each. One tube was washed with PBS and centrifuged at 13,000 × g for 5 min. The supernatant was discarded and the faecal pellet was preserved at − 80 °C for DNA extraction. Another tube was further diluted fivefold using PBS, achieving a 50-fold dilution. Following the same protocol, 200 µL was isolated, and the obtained faecal pellet was preserved at − 80 °C for RNA extraction. The third tube was preserved at − 80 ℃ for organic acid analysis.
16S rRNA amplicon sequencing
The faecal microbiota was characterised through amplicon sequencing of the 16S rRNA gene, following a previously reported method54. Briefly, DNA was extracted from the faecal samples, and the V1–V2 region of the 16S rRNA gene was amplified with an ABI 7500 Real-Time PCR system (Life Technologies Japan, Ltd., Tokyo, Japan), using the primers Forward: 27Fmod2-MiSeqV2 and Reverse: 338RMiSeqV2-001. Amplified DNA was purified using the AMPure XP kit (Beckman Coulter, Brea, CA). After quantitation using the Quant-iT PicoGreen double-stranded DNA assay kit (Life Technologies Japan, Ltd.), the gene was sequenced on MiSeq system (Illumina, Inc., San Diego, CA) using MiSeq Reagent Kitsv2 (Illumina, Inc.). Each read was processed using the software Quantitative Insights Into Microbial Ecology 2 (ver. 2021.04) and then assigned using the SILVA 138 database. Weighted UniFrac distance and α-diversity indices (Faith’s PD, observed ASVs, and Shannon index) were calculated at a sequencing depth of 10,000.
Quantification of faecal bacteria
The total bacteria in faeces was quantified by quantitative real-time PCR (qPCR), using an ABI PRISM 7900HT Sequence Detection System (Life Technologies Japan, Ltd.) and following a previously reported method 55. Four kinds of pathogenic and/or pathobiont faecal bacteria (C. difficile, C. perfringens, E. coli and Staphylococcus) were quantified by real-time (RT)-qPCR, using the Yakult Intestinal Flora Scan system56. RT-qPCR was performed with an ABI PRISM 7900HT Sequence Detection System (Life Technologies Japan, Ltd.), using RNA extracted according to a previously reported method as well as bacteria-specific primer sets55.
Organic acid analysis
The concentration of organic acids in the faeces was analysed following a previously reported method57 with some modifications. Briefly, the faecal diluent was diluted tenfold with 10% perchloric acid and allowed to stand at 4 ℃ for 12 h. After centrifugation at 20,400 × g for 15 min, the supernatant was treated with a 0.45-μm filter (Millipore Japan, Tokyo, Japan) for a high-performance chromatography system (HPLC: e2695; Waters Corp., Milford, MA). A Shodex Rspack KC-811 (Showa Denko, Tokyo, Japan) column and a 2432 Conductivity Detector (Waters Corp.) were used. Hydrogen peroxide (1 mM) including 7% acetonitrile was used as an eluent.
Serum cytokine and chemokine analysis
The serum was separated immediately after sampling and preserved at − 80 °C. GM-CSF, IFN-γ, KC-like, IP-10, IL-2, IL-6, IL-7, IL-8, IL-10, IL-15, IL-18, MCP-1 and TNF-α were analysed using the Milliplex MAP canine Cytokine/Chemokine Premixed Magnetic Beads Kit (Millipore, Burlington, MA) according to the manufacturer’s instructions. These measurements were performed by Filgen Inc. (Nagoya, Japan). TGF-β1 and IL-12 (p40) were quantified using a Transforming Growth Factor β 1 (Cloud-Clone Corp., Katy, TX) and Interleukin 12 p40 ELISA kits (MyBioSource Inc., San Diego, CA), respectively, according to the manufacturer’s instructions. Two disease samples (CE and SCL) with inadequate serum were designated as missing values.
Statistical analysis
Data are presented as mean ± standard deviation or the median value. R ver 4.0.5 was used for the statistical analysis (R Foundation, Vienna, Austria). As for the clinical characteristics, differences in sex ratio were assessed using Fisher’s exact test, while age, body weight, and albumin concentration were compared using Welch’s t-test. The Mann–Whitney U test was used to compare the α-diversity index and bacterial count among the microbiota data obtained through 16S rRNA amplicon sequencing, qPCR, and RT-qPCR. The detection rates of the 4 kinds of pathogenic and/or pathobiont bacteria were compared using Fisher’s exact test. The composition of the microbiota was determined using principal coordinate analysis based on weighted UniFrac distance and compared using the permutational multivariate analysis of variance. LEfSe (ver. 1.0; http://huttenhower.sph.harvard.edu/galaxy/)58 was used to compare the relative abundance of bacterial taxa at the genus level, and the cut-off level was defined as α value = 0.05 and LDA score > 3.0. Among the genera detected through 16S rRNA amplicon sequencing, only those with a mean relative abundance of > 0.1% were analysed using LEfSe. The Mann–Whitney U test was used to compare organic acid concentrations and immune parameters. The Kruskal–Wallis test was used to compare all variables among the three groups, and Dunn’s test with Bonferroni correction was used for post hoc analysis. Spearman’s rank correlation coefficient was used for correlation analysis and P < 0.05 was considered statistically significant. RF59, a machine learning algorithm, was used to construct the model used to classify dogs with disease and healthy dogs.
Ethics approval
The study protocol was approved by the Ethics Committee on Animal Care of The University of Tokyo (P21-052) and the study was conducted in accordance with the ARRIVE guidelines; all experiments were conducted in compliance with the appropriate guidelines and regulations. Informed consent was obtained from the owners of the dogs evaluated in this study.
Data availability
Faecal 16S rRNA gene amplicon sequencing data have been deposited in the Sequence Read Archive of the National Center for Biotechnology Information under accession number PRJNA1012802. The authors declare that all other data supporting the finding of this study are available in this article and its Supplementary file, or from the corresponding author upon request.
References
Sacoor, C., Barros, L. M. & Montezinho, L. What are the potential biomarkers that should be considered in diagnosing and managing canine chronic inflammatory enteropathies?. Open Vet. J. 10, 412–430 (2020).
Makielski, K., Cullen, J., O’Connor, A. & Jergens, A. E. Narrative review of therapies for chronic enteropathies in dogs and cats. J. Vet. Intern. Med. 33, 11–22 (2019).
Simpson, K. W. et al. Randomized controlled trial of hydrolyzed fish diets in dogs with chronic enteropathy. J. Vet. Intern. Med. 37, 2334–2343 (2023).
Schramm, A. & Kook, P. H. A descriptive study on the extent of dietary information obtained during consultations at a veterinary teaching hospital. Animals 12, (2022).
Jergens, A. E. & Heilmann, R. M. Canine chronic enteropathy—Current state-of-the-art and emerging concepts. Front. Vet. Sci. 9, (2022).
Cerquetella, M. et al. Proposal for rational antibacterial use in the diagnosis and treatment of dogs with chronic diarrhoea. J. Small Anim. Pract. 61, 211–215 (2020).
Angelucci, E., Malesci, A. & Danese, S. Budesonide: teaching an old dog new tricks for inflammatory bowel disease treatment. Curr. Med. Chem. 15, 2527–2535 (2008).
Benvenuti, E. et al. Immunosuppressant‐responsive enteropathy and non‐responsive enteropathy in dogs: prognostic factors, short‐ and long‐term follow up. Animals 11, (2021).
Isaacs, K. L., Lewis, J. D., Sandborn, W. J., Sands, B. E. & Targan, S. R. State of the art: IBD therapy and clinical trials in IBD. Inflamm. Bowel Dis. 11, S3–S12 (2005).
Simpson, K. W. & Jergens, A. E. Pitfalls and progress in the diagnosis and management of canine inflammatory bowel disease. Vet. Clin. North Am. Small Anim. Pract. 41, 381–398 (2011).
Eissa, N., Kittana, H., Gomes-Neto, J. C. & Hussein, H. Mucosal immunity and gut microbiota in dogs with chronic enteropathy. Res. Vet. Sci. 122, 156–164 (2019).
Bhan, A. K., Mizoguchi, E., Smith, R. N. & Mizoguchi, A. Spontaneous chronic colitis in TCR α-mutant mice; an experimental model of human ulcerative colitis. Int. Rev. Immunol. 19, 123–138 (2000).
Honneffer, J. B., Minamoto, Y. & Suchodolski, J. S. Microbiota alterations in acute and chronic gastrointestinal inflammation of cats and dogs. World J. Gastroenterol. 20, 16489–16497 (2014).
Galler, A. I., Klavins, K. & Burgener, I. A. A preliminary metabolomic study of yorkshire terrier enteropathy. Metabolites 12, (2022).
Niina, A. et al. Fecal microbiota transplantation as a new treatment for canine inflammatory bowel disease. Biosci. Microbiota, Food Heal. 40, 98–104 (2021).
Sugita, K. et al. Successful outcome after a single endoscopic fecal microbiota transplantation in a Shiba dog with non-responsive enteropathy during the treatment with chlorambucil. J. Vet. Med. Sci. 83, 984–989 (2021).
Cerquetella, M. et al. Inflammatory bowel disease in the dog: Differences and similarities with humans. World J. Gastroenterol. 16, 1050–1056 (2010).
Vázquez-Baeza, Y., Hyde, E. R., Suchodolski, J. S. & Knight, R. Dog and human inflammatory bowel disease rely on overlapping yet distinct dysbiosis networks. Nat. Microbiol. 1, (2016).
Connors, J., Dawe, N. & Van Limbergen, J. The role of succinate in the regulation of intestinal inflammation. Nutrients 11, 1–12 (2019).
Carrasco, V. et al. Distinguishing intestinal lymphoma from inflammatory bowel disease in canine duodenal endoscopic biopsy samples. Vet. Pathol. 52, 668–675 (2015).
You, I. W. & Kim, M. J. Comparison of gut microbiota of 96 healthy dogs by individual traits: Breed, age, and body condition score. Animals 11, 2432 (2021).
Beutin, L. Escherichia coli as a pathogen in dogs and cats. Vet. Res. 2–3, 285–298 (1999).
Weese, J. S. et al. The roles of clostridium difficile and enterotoxigenic clostridium perfringens in diarrhea in dogs. J. Vet. Intern. Med. 15, 374–378 (2001).
Cave, N. J., Marks, S. L., Kass, P. H., Melli, A. C. & Brophy, M. A. Evaluation of a routine diagnostic fecal panel for dogs with diarrhea. J. Am. Vet. Med. Assoc. 221, 52–59 (2002).
Al-Lahham, S. H., Peppelenbosch, M. P., Roelofsen, H., Vonk, R. J. & Venema, K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 1801, 1175–1183 (2010).
Havenaar, R. Intestinal health functions of colonic microbial metabolites: a review. Benef. Microbes 2, 103–114 (2011).
Fukuda, S. et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469, 543–549 (2011).
Suchodolski, J. S. et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS One 7, e51907 (2012).
Minamoto, Y. et al. Alteration of the fecal microbiota and serum metabolite profiles in dogs with idiopathic inflammatory bowel disease. Gut Microbes 6, 33–47 (2015).
AlShawaqfeh, M. K. et al. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 93, 1–8 (2017).
Werner, M. et al. Prevalence of Clostridioides difficile in canine feces and its association with intestinal dysbiosis. Animals 13, 1–13 (2023).
Minamoto, Y. et al. Fecal short-chain fatty acid concentrations and dysbiosis in dogs with chronic enteropathy. J. Vet. Intern. Med. 33, 1608–1618 (2019).
Macias-Ceja, D. C. et al. Succinate receptor mediates intestinal inflammation and fibrosis. Mucosal Immunol. 12, 178–187 (2019).
Liang, Y. et al. Oral administration of compound probiotics ameliorates HFD-induced gut microbe dysbiosis and chronic metabolic inflammation via the G protein-coupled receptor 43 in non-alcoholic fatty liver disease rats. Probiotics Antimicrob. Proteins 11, 175–185 (2019).
Onrust, L. et al. Valeric acid glyceride esters in feed promote broiler performance and reduce the incidence of necrotic enteritis. Poult. Sci. 97, 2303–2311 (2018).
Vázquez-Baeza, Y., Hyde, E. R., Suchodolski, J. S. & Knight, R. Dog and human inflammatory bowel disease rely on overlapping yet distinct dysbiosis networks. Nat. Microbiol. 1, 16177 (2016).
Bermingham, E. N., Maclean, P., Thomas, D. G., Cave, N. J. & Young, W. Key bacterial families (Clostridiaceae, Erysipelotrichaceae and Bacteroidaceae) are related to the digestion of protein and energy in dogs. PeerJ 5, e3019 (2017).
Watanabe, Y., Nagai, F. & Morotomi, M. Characterization of Phascolarctobacterium succinatutens sp. Nov., an asaccharolytic, succinate-utilizing bacterium isolated from human feces. Appl. Environ. Microbiol. 78, 511–518 (2012).
Louis, P. & Flint, H. J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 19, 29–41 (2017).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 341, 569–573 (2013).
Nagao-Kitamoto, H. et al. Interleukin-22-mediated host glycosylation prevents Clostridioides difficile infection by modulating the metabolic activity of the gut microbiota. Nat. Med. 26, 608–617 (2020).
Chang, D. E. et al. Carbon nutrition of Escherichia coli in the mouse intestine. Proc. Natl. Acad. Sci. U. S. A. 101, 7427–7432 (2004).
Janoir, C. et al. Adaptive strategies and pathogenesis of clostridium difficile from In vivo transcriptomics. Infect. Immun. 81, 3757–3769 (2013).
Pereira, F. C. et al. Rational design of a microbial consortium of mucosal sugar utilizers reduces Clostridiodes difficile colonization. Nat. Commun. 11, 5104 (2020).
Cox, L. M. et al. Description of two novel members of the family erysipelotrichaceae: Ileibacterium valens gen. nov., sp. nov. and Dubosiella newyorkensis, gen. nov., sp. nov., from the murine intestine, and emendation to the description of Faecalibacterium rodentium. Int. J. Syst. Evol. Microbiol. 67, 1247–1254 (2017).
Li, Q. et al. Effects of dietary phosphatidylcholine and sphingomyelin on DSS-induced colitis by regulating metabolism and gut microbiota in mice. J. Nutr. Biochem. 105, 109004 (2022).
Li, D. P., Cui, M., Tan, F., Liu, X. Y. & Yao, P. High red meat intake exacerbates dextran sulfate-induced colitis by altering gut microbiota in mice. Front. Nutr. 8, 646819 (2021).
Lee, D. et al. Perspectives and advances in probiotics and the gut microbiome in companion animals. J. Anim. Sci. Technol. 64, 197–217 (2022).
Rossi, G. et al. Comparison of microbiological, histological, and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL#3 strains in dogs with idiopathic inflammatory bowel disease. PLoS One 9, e94699 (2014).
Pilla, R. & Suchodolski, J. S. The gut microbiome of dogs and cats, and the influence of diet. Vet. Clin. North Am. - Small Anim. Pract. 51, 605–621 (2021).
Martyniak, A., Medyńska-Przęczek, A., Wędrychowicz, A., Skoczeń, S. & Tomasik, P. J. Prebiotics, probiotics, synbiotics, paraprobiotics and postbiotic compounds in IBD. Biomolecules 11, 1903 (2021).
Mizukami, K. et al. Age-related analysis of the gut microbiome in a purebred dog colony. FEMS Microbiol. Lett. 366, 1–7 (2019).
Jergens, A. E. et al. A scoring index for disease activity in canine inflammatory bowel disease. J. Vet. Intern. Med. 17, 291–297 (2003).
Kato-Kataoka, A. et al. Fermented milk containing Lactobacillus casei strain Shirota preserves the diversity of the gut microbiota and relieves abdominal dysfunction in healthy medical students exposed to academic stress. Appl. Environ. Microbiol. 82, 3649–3658 (2016).
Shima, T. et al. Association of life habits and fermented milk intake with stool frequency, defecatory symptoms and intestinal microbiota in healthy Japanese adults. Benef. Microbes 10, 841–854 (2019).
Matsuda, K., Tsuji, H., Asahara, T., Kado, Y. & Nomoto, K. Sensitive quantitative detection of commensal bacteria by rRNA-targeted reverse transcription-PCR. Appl. Environ. Microbiol. 73, 32–39 (2007).
Asahara, T. et al. Protective effect of a synbiotic against multidrug-resistant Acinetobacter baumannii in a murine infection model. Antimicrob. Agents Chemother. 60, 3041–3050 (2016).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60 (2011).
Breiman, L. Random forests. Mach. Learn. 45, 5–32 (2001).
Acknowledgements
The authors thank Dr. Mitsuyoshi Kano and Mr. Ryousuke Kamiya for assistance with administrative procedures in this study. We also thank ThinkSCIENCE Inc. (Tokyo, Japan) for English language editing. This study was supported by Grants from the Yakult Honsha Co., Ltd.
Author information
Authors and Affiliations
Contributions
C.K.: study concept and design, assay of faecal microbiota, analysis data, preparation of manuscript. S.K., Y.O.: assay of faecal microbiota. A.H.: assay of faecal organic acids. J.S.: ELISA assay for TGF-β1 and IL-12(p40). Tak.N., M.N., Tai.N.: acquisition of dogs and, collect the samples. J.K.C., K.U.: pathological classification of cases. Ha.T.: study concept and design. S.M., T.K. and N.M.: study concept and design, interpretation of data. Hi.T.: interpretation of data, preparation of manuscript. N.M.: study concept and design, interpretation of data, preparation of manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.K., S.K., H.A., Y.O, J.S., S.M., T.K. and N.M. are affiliated with the Yakult Honsha Co., Ltd.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kaga, C., Kakiyama, S., Hokkyo, A. et al. Characterization of faecal microbiota and serum inflammatory markers in dogs diagnosed with chronic enteropathy or small-cell lymphoma: a pilot study. Sci Rep 14, 19387 (2024). https://doi.org/10.1038/s41598-024-69923-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69923-1
- Springer Nature Limited