Abstract
Triosephosphate isomerase deficiency (TPI Df) is a rare multisystem disorder with severe neuromuscular symptoms which arises exclusively from mutations within the TPI1 gene. Studies of TPI Df have been limited due to the absence of mammalian disease models and difficulties obtaining patient samples. Recently, we developed a novel murine model of TPI Df which models the most common disease-causing mutation in humans, TPI1E105D. Using our model in the present study, the underlying pathogenesis of neuromuscular symptoms has been elucidated. This is the first report detailing studies of neuromuscular pathology within a murine model of TPI Df. We identified several contributors to neuromuscular symptoms, including neurodegeneration in the brain, alterations in neurotransmission at the neuromuscular junction, and reduced muscle fiber size. TPI Df mice also exhibited signs of cardiac pathology and displayed a deficit in vascular smooth muscle functionality. Together, these findings provide insight into pathogenesis of the neuromuscular symptoms in TPI Df and can guide the future development of therapeutics.
Similar content being viewed by others
Introduction
Triosephosphate isomerase deficiency (TPI Df) is an ultra-rare metabolic disorder that was identified in the 1960’s1. While much has been learned about the disease since its discovery, this knowledge has principally been biochemical. Little is known about pathogenesis of TPI Df within individual organ systems. Human tissue for study is incredibly scarce due to the rarity and severity of the disease, and the age of typical patients. Furthermore, mammalian systems that model the disease well have not existed until recently2. Our newly developed murine model exhibits complex neuromuscular phenotypes akin to phenotypes seen in TPI Df patients and represents an opportunity to learn about the effects of TPI Df on mammals and to elucidate key details of disease pathogenesis2.
TPI Df follows an aggressive disease course characterized by hemolytic anemia, neuromuscular dysfunction, and early death. It is important to note that TPI Df can arise from a variety of mutations, though over 80% of cases reported are associated with at least one copy of the TPI1E105D mutation3. The most common disease presentation is homozygosity for the TPI1E105D mutation, responsible for ~ 45% of published TPI Df cases. Despite the variability in genetic presentation, there are some characteristic symptoms that define nearly all genetic presentations of TPI Df. The earliest signs of the disease are anemia, developmental delays, and regressions4,5. Often, patients experience recurrent infections throughout their lifespan, the basis of which is unknown but may be related to the severe anemia patients experience6. Neuromuscular dysfunction is widespread among TPI Df patients although its presentation can be variable. Most TPI Df patients will experience hypotonia and dystonia, while some may experience spasticity that results in contractures. In the limited clinical reports assessing human patients, some important findings have been noted. Findings on MRI scans in TPI Df patients have shown variable combinations of cerebral atrophy and demyelination5,7,8,9,10. Some patients display pallor of the optic discs suggestive of optic nerve atrophy5,7,11,12. Muscle biopsies from patients frequently reveal small fibers and selective type II muscle fiber atrophy5,13,14,15,16. Electromyogram (EMG) studies in patients typically show abnormalities which suggest involvement of spinal motor neurons11,13,16,17,18,19,20. Other, less frequent symptoms include cardiomyopathy and seizures7,8,13,17,18,19,21. Patients typically succumb to the disease before the age of 6, usually due to respiratory failure.
Physicians have drawn different conclusions based on their findings from individual patients. There is debate about the origin of neuromuscular symptoms, and physicians typically hone in on one or more of the following as the key driver of symptoms: peripheral denervation, central denervation, disuse atrophy, or alterations in neuronal function11,13,17,18,19,20. One particular study, which was the only documented report we could find of a full-body autopsy, showed numerous alterations in the brain structure, including neuronal loss in the hypothalamus, periaqueductal grey, olivary nuclei, cerebellum, and dentate gyrus13. Therefore, it is also possible that alterations within the brain itself could be contributing to neuromuscular symptoms. This study aims to clarify the pathological changes that underlie neuromuscular symptoms in TPI Df using a mammalian model of TPI Df.
To elucidate pathogenesis in TPI Df, we used our compound heterozygous murine model with the common Tpi1E105D allele and a null allele, Tpi1E105D/null2. Tpi1E105D/null mice recapitulate key TPI Df symptoms and display an aggressive disease course with death typically occurring ~ 40 days of age presumably from respiratory failure2. Within Tpi1E105D/null mice in the present study, alterations in structure and/or function have been evaluated at three different levels: the brain, the neuromuscular junction, and muscle. Behavioral analyses have been conducted to reveal the high-level functionality of mice modeling TPI Df. Several of these studies have been conducted at multiple timepoints including postnatal day 9 (p9), postnatal day 20 (p20) and a late timepoint near the end of the lifespan of TPI Df animals, postnatal day 39 to day 41 (p39-p41). Together, these studies help construct a temporal framework in which pathological processes in individual tissues have been identified.
Methods
Animal Care and Husbandry. All animals were maintained under supervision of the Institutional Animal Care and Use Committee (IACUC) at the University of Pittsburgh and in accordance with NIH guidelines22. All experimental protocols involving animals were approved by the IACUC committee. Mice were housed with a 12-h light/dark cycle with food and water available ad libitum. Supportive care was provided to animals as needed as symptoms progressed. Euthanasia was performed via CO2 inhalation or via isoflurane overdose. Animals were genotyped as described previously2. Experiments were completed in compliance with the ARRIVE guidelines.
Behavioral Assessment. Behavioral assays were completed on post-natal day 38 or 39. Animals were allowed to acclimate in the behavior room for 1 h prior to beginning behavior tests. The grip strength assay was performed using a grip strength meter model II-220 from World Precision Instruments (WPI, Sarasota, FL, United States), and the rotarod assay was completed with a Ugo Basile model 7650 rotarod (Ugo Basile, Varese, Italy). Grip strength assays were completed first. For each animal, 3 replicates were obtained for both the forelimb-only and all-four-limb grip strengths. Between each measurement, 5 min of rest were allowed. Rotarod assessment commenced 10 min after the completion of the grip strength measurements. An accelerating rotarod protocol was utilized with a speed gradient from 5 to 50 RPM, which gradually increases by 5 RPM every 22 s. The animals were allowed to practice before counting any trials, and three total trials were conducted per animal. A period of 5 min was allotted for rest in between rotarod trials. The rotarod was cleaned in between trials to prevent any alterations in behavior due to scents. The combination of grip strength and rotarod assays was chosen as it allows us to evaluate key neuromuscular functionalities: muscle strength and coordination/endurance.
Immunohistochemistry (IHC). Mice were perfused with ice-cold 4% PFA at days p20 and p39-41. The p9 timepoint was perfused with PBS only. Hindlimbs and brain tissue were collected and post-fixed in 4% PFA overnight at 4℃ before being transferred to PBS.
Brain samples were sent to the Pitt Biospecimen Core for processing and paraffin embedding. The brain was divided into 4 coronal chunks: frontal, proximal, medial, and posterior. For each of the 4 brain chunks, 2 slides were generated with serial 10 µM sections. One of the two slides was stained with hematoxylin and eosin, while the other was dewaxed and used for IHC. Antigen recovery was achieved with the use of Vector Laboratories Antigen Unmasking Solution (Vector Laboratories, catalog no. H-3300). Slices were incubated for an hour at room temperature in blocking buffer consisting of 5% BSA, 3% rabbit serum, and 0.5% Triton-X100. Brain slices were incubated overnight in a moistened slide box at 4℃ with either anti-GFAP (Cell Signaling Technology, 3670S, 1:500) or anti-Iba1 (Invitrogen, GT10312, 1:500). Secondary antibodies were then added along with DAPI (1:1000) and allowed to incubate for 45 min at room temperature. Slides were mounted with VectaShield mounting media (Vector Laboratories, catalog no. H100010).
Extensor digitorum longus (EDL) muscles were dissected and incubated for 2 h at room temperature in blocking buffer consisting of 5% BSA, 3% rabbit serum, and 0.5% Triton-X100. Primary antibodies anti-SV2A (SV2, DSHB, University of Iowa, 1:40) and anti-neurofilament (2H3, DSHB, University of Iowa, 1:25) were added in blocking buffer and incubated overnight with constant agitation at 4℃. Secondary antibodies were added in addition to a fluorescence conjugated α-Bungarotoxin-555 (Invitrogen, B35451, 1:500). EDLs were then divided into 4 leaflets and the largest 3 leaflets were whole mounted in VectaShield mounting media. EDLs were flattened by sandwiching the slide between metal plate and a magnet.
Tibialis anterior (TA) muscles were dissected and dehydrated in a 30% sucrose solution overnight. Muscles were then frozen down at -80℃ in optimal cutting temperature (OCT) media (Andwin Scientific, catalog no. 45831). Frozen tissue blocks were sectioned at a 16 µm thickness using a Cryostar NX50 cryostat and placed onto gelatin coated slides. Sections were incubated in blocking buffer containing 5% BSA, 3% rabbit serum, and 0.1% Triton-X100 for 2 h at room temperature. When staining for centralized nuclei, anti-laminin (Sigma, L0663, 1:500) was added to blocking buffer and allowed to incubate overnight at 4℃. When staining for muscle fiber types, the following antibodies were added to blocking buffer and allowed to incubate overnight at 4℃: anti-laminin (Sigma, L0663, 1:500), anti-MYH1 (6H1, DSHB, University of Iowa, 1:40), anti-MYH7 (BA-D5, DSHB, University of Iowa, 1:400), and anti-MYH2 (SC-71, DSHB, University of Iowa, 1:300). Secondary antibodies were added (along with DAPI 1:5000 in centralized nuclei staining) and incubated at room temperature for 3 h. Slides were mounted with VectaShield mounting media (Vector Laboratories, catalog no. H100010). Type IIB and type I fiber types were not differentiated with this fiber typing staining paradigm, however, as the TA muscle is almost completely type II fibers, unstained fibers are assumed to be type IIB23.
Single-fiber in vitro muscle electrophysiology. The ETA nerve muscle preparation was stimulated with a suction electrode at 10X threshold, and 1 µM µ-conotoxin GIIIB (Alomone Labs Ltd. Jerusalem, Israel)24 was added to block action potential-evoked muscle contraction. Borosilicate electrodes with approximately 40–60 MΩ resistance were filled with 3 M potassium acetate to perform microelectrode recordings. To assess the magnitude of transmitter release at each NMJ, spontaneous miniature endplate potentials (mEPPs) were collected for 1 min followed by 10 evoked endplate potentials (EPPs) at 0.2 Hz. The data were corrected for non-linear summation25. The quantal content (QC) was calculated by dividing the average amplitude of the EPP by the corresponding average amplitude of mEPPs recorded from the same synapse. QC was used to estimate the number of vesicles (quanta) released with each presynaptic action potential. Short-term plasticity was evaluated following 10 presynaptic action potentials at 50 Hz in each muscle fiber. Data were collected using an Axoclamp 900A and digitized at 10 kHz for data analysis with the pClamp 10. During analysis of the electrophysiology data, a 2000 Hz Gaussian low pass filter was used on both the mEPP and EPP data.
Vasoreactivity Measurements. There are well established assays for smooth muscle contractility assessments26. Our procedure used aortas from Tpi1E105D/null mice and littermate controls that were rapidly excised, cleaned of fat, and cut into 2-mm rings. Rings were placed on a 2-pin myograph (Model # 620 M, Danish Myo Technology, Denmark) filled with physiological salt solution (PSS) containing 119 mM NaCl, 4.7 mM KCl, 1.17 mM MgSO4, 1.18 mM KH2PO4, 5.5 mM D-glucose, 25 mM NaHCO3, 0.027 mM EDTA, and 2.5 mM CaCl2, pH 7.4 when bubbled with 95% O2 and 5% CO2 at 37 °C. CaCl2 was added once PSS pH reached 7.4. After a 30-min equilibration period, aortas were incrementally stretched to 500-mg initial tension. Viability was tested by 60 mM KCl exposure for 5 min. Aortas were washed three times with PSS and allowed to rest for 30 min. A final wash was performed, and aortas rested for an additional 10 min. After the last wash, rings were pre-constricted with a cumulative dose response to prostaglandin F2α (PGF2α; 1 × 10−7 to 1 × 10−5 M). After plateau was reached, a cumulative dose response curve to acetylcholine (10−8 to 10−4 M) was conducted. Ca2+-free PSS containing 1 × 10−6 sodium nitroprusside was added to determine maximal relaxation. Relaxation percentage was normalized by the change in maximal dilation via Ca2+-free PSS. Aortas and data were excluded from analyses if no constriction to KCl was observed.
Echocardiography and Heart Histology. Small animal ultrasonography has been well-documented27. Briefly, our protocol was as such: echocardiography was performed using the Vevo 3100 ultrasound machine with the MX400 linear transducer capable of 40 MHz (Visualsonics). Rodents were anesthetized using 3% isoflurane mixed with 1 L/min of 100% oxygen in an induction chamber. Once anesthetized the rodent was transferred to a warming table and positioned for imaging using 1.5% isoflurane. The heart rate was maintained between 400 and 500 bpm during ultrasound imaging. B-Mode, M-mode, pulsed wave doppler and tissue doppler imaging were acquired. The parasternal long axis view was obtained and measurements of the endocardial and epicardial border were done to obtain a global longitudinal strain measurement using the Vevo Lab Strain package. The parasternal short axis at mid papillary muscle, allowed for calculations of ejection fraction, fractional shortening, cardiac output, stroke volume, left ventricular (LV) wall thickness, and LV internal diameter and volume. We then obtained a 4-chamber image to acquire mitral valve pulsed wave doppler to measure the E and A wave, IVRT, IVCT and LV MPI. Lastly, we obtained a tissue doppler sample from the septal mitral annulus to acquire the e’ and a’ velocities. All measurements and imaging were performed by a blinded sonographer.
Fresh hearts were collected from Tpi1WT and Tpi1E105D/null animals and post-fixed in 4% PFA. The hearts were then transferred to 70% ethanol and transported to the Research Histology Core at John G. Rangos Sr. Research Center (Pittsburgh, PA). Tissue was embedded in paraffin and sectioned at a thickness of 5 µm with special focus on the left ventricle. Sections were then stained with trichrome.
Image Acquisition and Analysis. Neuromuscular junction (NMJ) images were acquired on an Olympus BX51 confocal microscope (Olympus, Tokyo, Japan) with a 20 × objective. Images of brain and heart tissue were obtained on a Nikon Eclipse Ti microscope (Nikon, Tokyo, Japan), capable of both colored and fluorescent imaging with a 4 × objective. Muscle cross sections and brain IHC slides were imaged on an Olympus BX61VS slide scanning microscope (Olympus, Tokyo, Japan) with a 10 × objective. All image analyses were performed using ImageJ28.
Statistics. All statistics were performed in GraphPad Prism 10.1.2 (La Jolla, CA, United States). Normality was assessed via observation of linearity on a QQ plot before using parametric statistical approaches. Non-normal data was assessed using non-parametric statistical tests. Standard deviations between groups were compared via Bartlett’s test, and unequal standard deviations led to use of the statistical test with a Welch’s correction. A comparison between two groups was assessed via an unpaired student’s t-test with or without a Welch’s correction based on the equivalence of standard deviations. Non-parametric comparisons of two groups were executed with a Mann–Whitney U test. Comparisons of 3 or more groups were made via a one-way ANOVA with or without a Welch’s correction. Post-hoc comparisons were made with a Tukey’s or a Dunnet’s post-hoc test depending on the equivalence of standard deviations. A Kruskal–Wallis test with a Dunn’s post-hoc comparison was used in the case of a non-parametric comparison of 3 or more groups. Evaluations involving two variables were assessed with a two-way ANOVA and a Sidak’s post-hoc test. Linear response curves were assessed by linear regression, while non-linear response curves were assessed through non-linear regression.
Results
Behavioral assessment of Tpi1 E105D/null animals
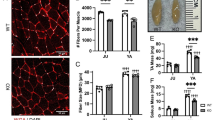
Tpi1E105D/null animals and their littermates were assessed behaviorally through grip strength and rotarod assays to identify deficits in motor function. Raw grip strength measures were generally lower in Tpi1E105D/null animals when compared to littermate control animals (Fig. 1a and b). When grip strength values were normalized to individual body weights, there was no difference in grip strength between Tpi1E105D/null animals and their littermate controls (Fig. 1c and d). A strong deficit was observed in rotarod performance, with Tpi1E105D/null animals falling with an average of 15.58 s from of the start of the assay, whereas littermate control animals had an average latency to fall between 79.21 and 94.63 s (Fig. 1e). To evaluate when pathology begins to arise, we examined animal body weights over time. Body weights in Tpi1E105D/null animals are not altered in respect to control animals at post-natal day 9 (p9; Fig. 1f and 1g). A slight difference in body weight can be seen in male Tpi1E105D/null animals relative to controls, but not females, at p20 (Fig. 1f and 1g). A drastic reduction in body weight is evident at days p39-p41 in Tpi1E105D/null animals of both sexes relative to controls (Fig. 1f and g).
Behavioral phenotype and body weights of Tpi1E105D/null mice. (a and b) Raw four-paw grip strength in male and female animals, respectively. (c and d) Body-weight standardized grip strength in male and female animals, respectively. (e) Latency to fall via Rotarod assay in seconds (s). N = 8 animals per genotype for all assays with 3 within-animal replicates and equal numbers of males and females. Rotarod data was pooled due to absence of sex differences. f and (g) Body-weight trends over development in Tpi1E105D/null animals relative to controls. N = 4 animals per group. Panels b, c and d are compared via standard one-way ANOVA with Tukey’s post-hoc comparisons. Panels a and e are compared via Welch’s ANOVA with Dunnett’s post-hoc comparisons. Panels f and g were compared via two-way ANOVA.
Evaluation of abnormalities at the neuromuscular junction
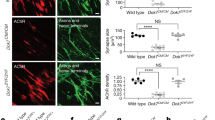
P9 timepoint: Neuromuscular junctions (NMJs) were evaluated for structural defects. At the p9 timepoint, there was a slight increase in the amount of fully innervated NMJs in Tpi1E105D/null animals relative to control animals (Fig. 2b). There were no differences in nAChR fragmentation or area, nor was there any difference in endplate area between Tpi1E105D/null and control animals (Fig. 2c–e). No sex differences were noted in any of these parameters. Representative magnified NMJ images are provided (Figure S1a).
NMJ structural analysis. Panels a, f, and k show representative NMJ images from a Tpi1WT animal (top) and a Tpi1E105D/null animal (bottom) at the timepoints of p9, p20, and p39-41, respectively. The pre-synaptic motor neuron is displayed in green and the post-synaptic nAChRs are shown in red. Scale = 50 μm. The proportion of fibers fully innervated, partially innervated, or denervated are shown in panels b, g, and l from animals at the p9, p20, and p39-41 timepoint, respectively. Fragmentation of the nAChRs is evaluated in panels c, h, and m in animals from the p9, p20, and p39-41 timepoint, respectively. The area of nAChRs from p9, p20, and p39-41 animals are evaluated in panels d, i, and n, respectively. The area of the total endplate is evaluated in panels e, j, and o, in p9, p20, and p39-41 animals, respectively. N = 8 animals per genotype with equal numbers of males and females. Sex differences were evaluated and data was pooled if there was no evidence of a sex difference. Panels b, g, l, n, and o were compared via two-way ANOVA with Sidak’s post-hoc test. Panels d, j, and m were compared via an unpaired t-test. Panels c, e, h, and i were compared with a Mann–Whitney U test.
p20 timepoint: No differences were evident in innervation status or fragmentation of nAChRs in Tpi1E105D/null animals the p20 timepoint relative to control animals (Fig. 2g and h). There was a significant decrease in the nAChR area and endplate area in Tpi1E105D/null animals relative to controls. (Fig. 2i and j). No sex differences were noted in any of these parameters. Representative magnified NMJ images are provided (Figure S1b).
Late timepoint: The proportion of fully innervated NMJs was significantly reduced in Tpi1E105D/null animals relative to control NMJs (Fig. 2l). Fragmentation of the nicotinic acetylcholine receptors (nAChRs) was unaffected (Fig. 2m). Endplate area was reduced in both male and female Tpi1E105D/null animals relative to control animals, while nAChR area was only reduced from controls in males (Fig. 2n and o). Representative magnified NMJ images are provided (Figure S1c).
In vitro single fiber electrophysiology: Tpi1E105D/null animals and control animals between the ages of p35 and p39 were utilized for single-fiber recordings. Interestingly, sex differences became apparent within the electrophysiological data. Male Tpi1E105D/null animals displayed a normal miniature end plate potential (mEPP), a decrease in the end plate potential (EPP), and a reduction in quantal content (QC) relative to age-matched control animals (Fig. 3a–c). In contrast, female Tpi1E105D/null animals did not display any alterations in mEPP, EPP, or QC relative to controls (Fig. 3d–f). Intriguingly, application of a stimulus train resulted in synaptic depression in female Tpi1E105D/null animals but not male Tpi1E105D/null animals, when compared to age and sex matched control animals (Fig. 3g, h, j, and k). Each data set was fitted to a line and slopes for each line were compared. Female Tpi1E105D/null animals showed a significantly reduced relative amplitude over the course of a train of stimuli when compared with Tpi1WT animals (Fig. 3k). Comparison of the relative amplitude of the 10th stimuli in the train showed a significantly reduced amplitude in female Tpi1E105D/null animals when compared to control animals (Fig. 3l), that was not observed in Tpi1E105D/null males when compared to age-matched controls (Fig. 3i).
Single-fiber electrophysiology. Panels a–c show mEPP amplitude, EPP amplitude, and quantal content, respectively, in male animals. Panels d–f show the same parameters as a–c in female animals. Panels g – i show the individual fiber relative amplitudes from 10-stimulus train stimulation, the linear regression of relative response across stimulations, and the amplitude of the 10th stimulation in the train, respectively, in male animals. Panels j–l show the same parameters as g–i in female animals. N = 6 animals per genotype with equal numbers of males and females, 10–15 fibers were recorded from in each animal. Panels a and l were compared via unpaired t-test. Panels b, c, d, e, f, and i were compared via Mann–Whitney U test. Panels h and k were compared via simple linear regression and an F-test to compare slopes.
Skeletal muscle abnormalities
P9 timepoint: Early in development, at the p9 timepoint, there are no evident alterations in muscle fiber size in the tibialis anterior (TA) muscle between Tpi1E105D/null animals and Tpi1WT animals for any of the type II fiber subtypes (Fig. 4b–d). Additionally, there is no change in the prevalence of centralized nuclei in the TA in Tpi1E105D/null animals relative to controls (Figure S2b). There were no differences in the distribution of individual fiber types (Figure S3a).
Fiber-typing and fiber-size analyses. Representative images of p9, p20, and p39-41 tibialis anterior muscles are shown in panel a, with Tpi1WT animals on the left of each panel and Tpi1E105D/null animals on the right of each panel. Type IIA fibers are stained in red/fuchsia, type IIX fibers are stained in blue, and type I/IIB fibers are unstained and assumed to be type IIB fibers. Panels b – d show quantifications of muscle fiber size by the respective fiber type, with panel b showing type IIA fibers, panel c showing type IIX fibers, and panel d showing type IIB fibers. N = 8 animals per genotype with equal numbers of males and females. All groups were evaluated for sex differences before pooling. Only the p39-41 timepoint showed sex differences. All comparisons at the p9 and p20 timepoint were made using an unpaired t-test, except for the comparison of type IIA fibers at p20 which was compared using a Mann–Whitney U test. The late timepoint areas were compared using 2-way ANOVAs with Sidak’s post-hoc comparisons.
p20 timepoint: At weaning age, slight reductions in muscle fiber size could be seen within type IIX and type IIB fibers in Tpi1E105D/null animals relative to control animals (Fig. 4b–d). Like the p9 timepoint, there is no difference in the proportion of fibers with centralized nuclei between Tpi1E105D/null and Tpi1WT animals (Figure S2c). There were no differences in the distribution of individual fiber types (Figure S3b).
Late timepoint: At the late timepoint in Tpi1E105D/null animals, alterations to the size of muscle fibers in the TA muscle were evident relative to control animals. While all three type II fiber subtypes were significantly reduced in area in Tpi1E105D/null animals, the more glycolysis-dependent type IIX and type IIB fibers were more severely affected relative to controls (Fig. 4b–d). There was no difference in the prevalence of centralized nuclei between Tpi1E105D/null and control animals (Figure S2d). There was a modest decrease in the prevalence of Type IIX fibers with a corresponding increase in the prevalence of Type I/IIB fibers in Tpi1E105D/null animals relative to controls (Figure S3c).
Assessment of neurodegeneration in the CNS
An overview of the broad structure of the brain does not reveal any observable differences in Tpi1E105D/null animals at any timepoint when compared to Tpi1WT control animals (Fig. 5a). Closer examination of late timepoint Tpi1E105D/null brain tissue revealed some indications of pathology relative to control animals. Visually, there was an increased prevalence of shrunken, pyknotic neurons that were observed in Tpi1E105D/null animals in both the cortex (Fig. 5b) and the dentate gyrus (Fig. 5c) that were not observed in age-matched control tissues. Areas of potential vacuolar pathology were assessed, and individual events were delineated as a blood vessel or a likely lesion by the presence or absence of an endothelial wall, respectively (Fig. 5d). The area of blood vessels was not altered in Tpi1E105D/null animals relative to controls (Fig. 5e). Interestingly, the prevalence of blood vessels was increased in Tpi1E105D/null animals relative to controls, suggesting some level of hypervascularization (Fig. 5f). The size of suspected lesions was not altered in Tpi1E105D/null animals relative to controls (Fig. 5g). However, the prevalence of lesions was elevated in Tpi1E105D/null animals relative to controls (Fig. 5h). Visualization of the size of individual lesions from Tpi1E105D/null and Tpi1WT animals revealed that the largest lesions were from Tpi1E105D/null animals (Fig. 5i–k).
Whole brain H&E staining. (a) Cross-sections of p9, p20, and p39-41 brains with a representative Tpi1WT (left) and Tpi1E105D/null (right) animal. (b) High magnification of cortex in a p39-41 timepoint Tpi1WT (left) animal and a Tpi1E105D/null (right) animal showing shrunken, pyknotic neurons (black arrows). (c) High magnification of the dentate gyrus in a p39-41 timepoint Tpi1WT animal (top) and a Tpi1E105D/null (bottom) animal showing shrunken, pyknotic neurons. (d) High magnification of a blood vessel (top) versus a potential degenerative vacuole (bottom) in a p39-41 timepoint animal. BV = blood vessel, D = degeneration. (e) Quantification of blood vessel area in the brain in p39-41 animals, represented as the average vessel size per animal. (f) Quantification of vessel prevalence between Tpi1E105D/null animals in comparison to Tpi1WT animals, expressed as number of vessels per mm2. (g) Quantification of the area of suspected lesions in p39-41 animals, represented as average lesion size per animal. Regions were denoted as lesions is there was no visible endothelial wall. h) Quantification of lesion prevalence between Tpi1E105D/null animals in comparison to Tpi1WT animals, expressed as number of lesions per mm2. (i–k) Areas of individual lesions broken down into small lesions (i, < 500 μm2), medium-sized lesions (j, 500–1000 μm2) and large lesions (k, > 1000 μm2). N = 8 animals per group. Panels e and g were compared using a Mann–Whitney U test. Panel f was compared via unpaired t-test. Panel h was compared with a Welch’s t-test.
To further evaluate the potential for neurodegeneration in TPI Df mice, we stained the tissue for glial fibrillary acidic protein (GFAP) to look for signs of astrogliosis as well as ionized calcium binding adaptor molecule 1 (Iba1) to look for microgliosis. In most cases, instances of neurodegeneration are accompanied by reactive astrogliosis and microgliosis29,30. This assessment can identify alterations in the activation of astrocytes or microglia which could suggest pathological processes that may not have manifested as neuronal loss. At a global level, late timepoint Tpi1E105D/null animals qualitatively appear to have increased GFAP signal suggestive of reactive astrocytosis when compared to age-matched Tpi1WT animals (Fig. 6a). A higher magnification of the dentate gyrus revealed alterations in the morphology of both astrocytes and microglia in aged Tpi1E105D/null animal tissues relative to that of age-matched control animals (Fig. 6b). Astrocytes showed thickening of the processes while microglia displayed a “rounded-up” morphology which both suggest a shift to a more reactive nature in Tpi1E105D/null brain tissues relative to age-matched controls. The intensity of GFAP staining and the total area occupied by GFAP staining were elevated in Tpi1E105D/null animals relative to controls (Fig. 6c and d). No differences were noted between the sexes. Interestingly, there seemed to be some level of lateralization of GFAP staining, suggesting that pathology may begin on one side of the brain (Fig. 6a).
Assessment of glial activation in Tpi1E105D/null animals at the p39-41 timepoint. (a) Representative images of astrocyte (GFAP) staining in three Tpi1WT animals (top) and three Tpi1E105D/null animals (bottom). (b) High magnification images of the dentate gyrus in the same animals as a and b. Top two rows show Tpi1WT animals with GFAP staining on the top row and Iba1 staining on the second row. The bottom two rows show Tpi1E105D/null animals with GFAP staining on the third row and Iba1 staining on the bottom row. The last image on each row of panel b includes an inset showing an even closer look at cell morphology. Inset scale = 50 μm. (c) Total area occupied by GFAP staining in Tpi1E105D/null animals relative to controls. (d) Intensity of GFAP signal in Tpi1E105D/null animals relative to controls. N = 4 animals per group.
Cardiac deficits in TPI Df mice
The weight of dissected hearts in late timepoint TPI Df animals was not significantly different from littermate controls (Fig. 7a), despite a large decrease in overall body weight (Fig. 7b). When expressing the heart weight as a ratio to body weight, the heart weighed significantly more than expected in Tpi1E105D/null animals compared to wild-type littermate controls (Fig. 7c). Base functional cardiac parameters including heart rate, cardiac output, and stroke volume were unchanged in Tpi1E105D/null mice relative to control animals (Fig. 7d-f). Interestingly, both the ejection fraction and fractional shortening were significantly increased in Tpi1E105D/null animals compared to control animals (Fig. 7g and 7h). The left ventricle showed signs of hypertrophy, namely increased wall thicknesses, a decreased ventricular diameter, and a decreased ventricular volume, particularly in the systolic phase relative to controls (Fig. 7i–k). Surprisingly, myocardial performance index (MPI) was significantly reduced in Tpi1E105D/null mice, suggesting improved function of the LV relative to control animals (Fig. 7l). Relaxation time between phases was significantly reduced in Tpi1E105D/null animals when compared to controls (Fig. 7m). The peak velocity of the mitral valve in the atrial wave was significantly increased in Tpi1E105D/null animals, while the early wave was unaffected relative to controls (Fig. 7n and o). The ratio between the peak velocities of the early to atrial waves was significantly reduced in Tpi1E105D/null animals when compared to controls (Fig. 7p). Alternatively, no differences were seen in the peak tissue motion velocities at the mitral valve in either the atrial or early wave between mutants and controls (Fig. 7r and s). The ratio between the fluid motion velocity and the tissue motion velocity in the early phase at the mitral valve was not altered relative to controls (Fig. 7t). There was a trend, though not significant, towards decreased strain in Tpi1E105D/null animals relative to controls (Fig. 7q). Assessment of broad cardiac structure, particularly focusing on the left ventricle, did not reveal any signs of fibrosis (Figure S3a and b).
Echocardiogram analysis of Tpi1E105D/null animals. Echocardiogram analyses of TPI deficient mice. General animal information is displayed in panels (a) Heart weights, (b) Sex-separated body weights, and (c) Ratio of heart to body weight. High-level functional parameters of the heart are shown in panels (d) Heart rate, (e) Cardiac output, (f) Stroke volume, (g) Ejection fraction, and (h) Fractional shortening. Structural measurements are depicted in (i) Thicknesses of the left ventricular walls, (j) Left ventricular diameter, and (k) Left ventricular volume. Panel l) displays the myocardial performance index. (m) shows the isovolumic change times (IVRT and IVCT). Mitral valve characteristics relating to the peak atrial wave velocity, peak early wave velocity, and ratio of the E/A wave velocities are shown in panels n, o, and p, respectively. Global longitudinal strain is represented in panel q. Panels r and s) show the peak tissue motion velocities for the mitral valve in the atrial wave and early wave, respectively. The ratio between the E and E’ ratio is shown in panel t. N = 8 animals per genotype with equal numbers of males and females. Data was evaluated for sex differences, and if none were present data was pooled. Panel a was evaluated using an unpaired t-test with a Welch’s correction. Panels b, e, g, h, i, j, k, m, o, q and r were evaluated by 2-way ANOVA with Sidak’s post-hoc test. Panels c, d, l, n, p, s, and t were evaluated via an ordinary unpaired t-test. Panel f was evaluated with a Mann–Whitney U test.
Investigation of vascular function
To assess the possibility that the alterations in cardiac function were associated with alterations in smooth muscle function, the contractility of smooth muscle in the vasculature was assessed. Aortic rings were assessed for their contractile and relaxation responses. Neither male nor female Tpi1E105D/null animals showed any deficit in their maximal response to KCl stimulation when compared to control animals (Fig. 8a and d). Male Tpi1E105D/null animals showed deficits in Ach-stimulated relaxation when compared to Tpi1WT animals (Fig. 8b). Female Tpi1E105D/null animals, alternatively, displayed no difference in Ach-mediated relaxation when compared to Tpi1WT animals (Fig. 8e). Neither male nor female Tpi1E105D/null animals showed any difference in contractile responses relative to controls when stimulated with PGF2α (Fig. 8c and f).
Smooth muscle contractility in Tpi1E105D/null animals. (a and d) Maximal KCl response to assess viability of the aortic ring in male and female animals, respectively. (b and e) Relaxation of aortic rings in response ACh stimulation in male and female animals, respectively. (c and f) Constrictive response of aortic rings in response to PGF2α stimulation in male and female animals, respectively. N = 6 animals per genotype with equal numbers of males and females. 4–6 aortic rings were collected per animal. Panels a and d were compared via an unpaired t-test. Panels b, c, e, and f were fitted to a non-linear curve and an F-test was performed to determine whether one curve could fit both sets of data. Panel B demonstrated significantly different curve fits between Tpi1WT and Tpi1E105D/null animals and the fitted line for each data set is demonstrated by the dotted lines within the graph.
Discussion
Using our murine model of TPI Df, the pathogenesis of neuromuscular symptoms has been thoroughly evaluated. On a behavioral level, mice modeling TPI Df exhibit strong deficits in motor coordination and endurance as identified in the rotarod assay. Interestingly, however, TPI Df animals seem to retain normal muscle strength. A deficit in coordination suggests neuronal involvement, while poor endurance could be associated with either neuronal or primary muscle involvement. Meanwhile, the absence of a deficit in grip strength suggests that skeletal muscle is structurally sound. It is important to note that the poor performance on the rotarod assay could also be due to a lack of motivation/attention. Contextualizing the results of these behavioral assays with structural findings allows for a more concrete framework of the neuromuscular deficits in TPI Df.
The integrity of skeletal muscle was evaluated through structural analyses. Alterations in skeletal muscle structure were not evident early in development but became prominent as animals aged. Remarkably, the more glycolysis-dependent type IIX and IIB fibers showed an approximate 50% reduction in fiber size at the end of the lifespan, which aligns with the deficiency of a glycolytic enzyme such as TPI. As TPI Df animals are smaller than their littermates, a reduction in muscle fiber size is not unexpected. While these muscle fibers are smaller, they seem to retain appropriate contractile properties, as grip strength was unaffected in TPI Df animals when standardized to the body weight. As changes in skeletal muscle structure can only be seen close to the end of the lifespan, these data suggest that TPI Df mice develop normally and then undergo some form of muscle atrophy whether due to a degenerative process or disuse. However, the muscle did not seem to be undergoing cycles of de- and re- generation, as there was no increase in the presence of centrally located nuclei in Tpi1E105D/null animals at any timepoint31. Together, this suggests that disuse atrophy may be the primary driver of the structural changes in skeletal muscle as TPI Df animals become less capable of moving around their environment.
Cardiac muscle was assessed through structural and functional studies. Cardiomyopathy is occasionally observed in TPI Df, though many patients die very young and have a negative clinical history for this feature. Interestingly, in our TPI Df mice, there seemed to be convincing evidence for a cardiac hypertrophy phenotype. It is known that ejection fractions higher than 75% can be indicative of hypertrophic cardiomyopathy, and 3 out of 8 of the TPI Df mice met this cutoff, while 0 out of 8 of the WT mice met this cutoff32. Other indications of hypertrophic cardiomyopathy that are relevant are increased left ventricular (LV) wall thicknesses and increased heart weight relative to body weight. Many alterations were only apparent in the systolic phase of the heart. Interestingly, overall functional parameters such as heart rate, cardiac output, stroke volume, myocardial performance index, and global longitudinal strain were either unchanged or altered in such a way that they suggested improved cardiac function in TPI Df animals. The changes in cardiac function overall suggest some level of hypertrophy and systolic dysfunction. Evaluation of the structure of the LV through tissue histology did not suggest fibrosis as a driver of hypertrophy, indicating that some other form of pathology must be contributing to the cardiac phenotype.
To investigate whether cardiac function was compensating for changes in vascular tone, smooth muscle contractility was additionally assessed. Interestingly, the vascular smooth muscle displayed a decreased relaxation response, particularly in male mice modeling TPI Df. This suggests that there is some level of smooth muscle dysfunction, particularly in males, but this does not seem to align with the alterations we see in cardiac muscle. Follow up studies should further evaluate this deficit to determine whether NO production in endothelial cells is driving this alteration, or if soluble guanylate cyclase in smooth muscle may be contributing to the phenotype33. Additionally, looking at smaller vessels located distally from the heart may be informative as they can function quite differently.
The neuromuscular junction (NMJ) was assessed through both structural and functional assays. Alterations in NMJ structure become more prominent as animals aged and consisted of decreases in the size of the endplate and a modest decrease in NMJ innervation in TPI Df animals. Likely, the reduction in endplate size is not functionally significant and rather reflects the reduced muscle fiber size of TPI Df animals. While the reduction in NMJ innervation was modest, peripheral denervation may be a minor contributor to neuromuscular phenotypes. The clustering of nAChRs is seemingly unaffected by TPI Df.
Functionally, evaluation of neurotransmission at the NMJ in TPI Df animals revealed sexually dimorphic alterations. The synaptic depression observed in females could result from alterations in the probability of neurotransmitter release. Meanwhile, the decreased quantal content in males could be the result of a decreased readily releasable pool of synaptic vesicles, a reduction in the number of active zones, or a loss of functional calcium channels. Follow-up studies should evaluate the mechanisms responsible for the sexually dimorphic electrophysiological changes seen in Tpi1E105D/null animals.
Central nervous system abnormalities were first assessed through the evaluation of any possible regions of neurodegeneration. Histological assessment of late timepoint Tpi1E105D/null animals revealed an increased prevalence of suspected lesions and an increase in vascularization. Additional suggestions of neuropathology came from the detection of numerous pyknotic neurons in Tpi1E105D/null animals that were not present in control animals. These changes were accompanied by apparent increases in reactive astrocytosis. There was some level of lateralization in the staining pattern of GFAP which could suggest that pathology begins on one side of the brain. Future studies should further investigate this possibility. Interestingly, the hippocampus and specifically the dentate gyrus seemed to show extremely high levels of astrocyte and microglia activation, suggestive of neurodegeneration of this region. Importantly, there is evidence of neuropathology in TPI Df patients within the dentate gyrus, though this hasn’t been investigated in a significant number of patients13. Aside from the hippocampus, the substantia nigra, primary motor area, cerebellar peduncles, anterior commissure, primary sensory area, fornix, caudoputamen, and subiculum showed relatively high levels of reactive astrocytosis when compared to control animals. Together, these findings suggest that there are inflammatory and likely degenerative processes within the central nervous system (CNS) of Tpi1E105D/null animals which could contribute to disease pathogenesis.
This work has highlighted several considerations in relation to the development of TPI Df therapies. First, TPI Df animals seem to develop normally up to a point. The eventual decline in their condition may be due to a rapid neurodegenerative process or could simply be caused by increased bioenergetic demands as the animal’s mass increases. Regardless, this work highlights the cruciality of early diagnosis and intervention for therapies to have the greatest possibility of success. Second, cardiac hypertrophy may be an underreported consequence of TPI Df. Individuals with TPI Df should be monitored for cardiac involvement, especially if a theray were to be developed that extends the lifespan of patients. Importantly, while fibrosis was not detected in our animals in association with hypertrophy, it may arise over time in less severe disease presentations and should be closely monitored34. Third, there are numerous organ systems that must be considered when developing TPI Df therapies. From previous studies, we know bone marrow transplants are an effective way to treat anemia. In the current study, however, we elucidate that the central nervous system, upper and lower motor neurons, and all three types of muscle are also important targets. This presents a challenge for an individual therapy. For now, efforts for therapy development in TPI Df are focused on the development of small molecules that can broadly stabilize functional TPIE105D protein35.
In summary, this work identifies broad multi-system dysfunction resulting from TPI Df in mammals. Pathology has been identified in the CNS, the NMJ, and in all three types of muscle. These studies also revealed sexually dimorphic dysfunction in multiple systems that could not have been predicted from clinical reports and that will require further study to fully understand. Most importantly, the Tpi1E105D/null mouse seems to recapitulate numerous aspects of pathology seen in human patients and will likely prove to be a highly useful model for the development and assessment of potential therapeutics for TPI Df.
Limitations and future directions
A considerable limitation of this study is the use of staining to evaluate denervation at the neuromuscular junction. Axonal and presynaptic staining was somewhat inconsistent. Due to this staining inconsistency, it was difficult to definitively determine whether an innervating motor nerve was absent. As a result, only images with sufficient staining were used to evaluate denervation. This could lead to over- or under- estimations of the prevalence of denervation. Future studies should utilize a genetic technique, such as YFP expression in motor neurons using the Thy1 promoter, to overcome this limitation. Staining for the muscle fiber types is also limited by the inability to delineate type I and IIB fibers. As the TA muscle is predominately made up of type II fibers, we assume that unstained fibers are type IIB versus type I fibers. With a four-channel microscope, these fiber types could be better delineated. An additional limitation is the lack of spinal cord analysis. It is possible that there are structural defects and denervation occurring between upper and lower motor neurons within the spinal cord, that these studies would have missed. Finally, demyelination was not examined and should be assessed in the future.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Schneider, A. S., Valentine, W. N., Hattori, M. & Heins, H. L. Jr. Hereditary hemolytic anemia with triosephosphate isomerase deficiency. N Engl. J. Med. 272, 229–235. https://doi.org/10.1056/NEJM196502042720503 (1965).
Myers, T. D., Ferguson, C., Gliniak, E., Homanics, G. E. & Palladino, M. J. Murine model of triosephosphate isomerase deficiency with anemia and severe neuromuscular dysfunction. Curr. Res. Neurobiol. 3, 100062. https://doi.org/10.1016/j.crneur.2022.100062 (2022).
Arya, R., Lalloz, M. R., Bellingham, A. J. & Layton, D. M. Evidence for founder effect of the Glu104Asp substitution and identification of new mutations in triosephosphate isomerase deficiency. Hum. Mutat. 10, 290–294. https://doi.org/10.1002/(SICI)1098-1004(1997)10:4%3c290::AID-HUMU4%3e3.0.CO;2-L (1997).
Schneider, A. S. Triosephosphate isomerase deficiency: historical perspectives and molecular aspects. Baillieres. Best Pract. Res. Clin. Haematol. 13, 119–140. https://doi.org/10.1053/beha.2000.0061 (2000).
Selamioglu, A. et al. Triosephosphate isomerase deficiency: E105D mutation in unrelated patients and review of the literature. Mol. Syndromol. 14, 231–238. https://doi.org/10.1159/000528192 (2023).
Jonker, F. A. & Boele van Hensbroek, M. Anaemia, iron deficiency and susceptibility to infections. J. Infect 69, S23-27. https://doi.org/10.1016/j.jinf.2014.08.007 (2014).
Schneider, A. et al. Triosephosphate isomerase deficiency: repetitive occurrence of point mutation in amino acid 104 in multiple apparently unrelated families. Am. J. Hematol. 50, 263–268. https://doi.org/10.1002/ajh.2830500407 (1995).
Vafaie, M., Mahram, M. & Farshadmoghadam, H. Triosephosphate isomerase deficiency: The first case report from I.R. Iran. Iran J. Pediatr. 32, 4 (2022).
Roland, B. P. et al. Missense variant in TPI1 (Arg189Gln) causes neurologic deficits through structural changes in the triosephosphate isomerase catalytic site and reduced enzyme levels in vivo. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 2257–2266. https://doi.org/10.1016/j.bbadis.2019.05.002 (2019).
Fermo, E. et al. Triose phosphate isomerase deficiency associated with two novel mutations in TPI1 gene. Eur. J. Haematol. 85, 170–173. https://doi.org/10.1111/j.1600-0609.2010.01451.x (2010).
Poll-The, B. T., Aicardi, J., Girot, R. & Rosa, R. Neurological findings in triosephosphate isomerase deficiency. Ann. Neurol. 17, 439–443. https://doi.org/10.1002/ana.410170504 (1985).
Osaguona, V. B. Differential diagnoses of the pale/white/atrophic disc. Commun. Eye Health 29, 71–74 (2016).
Clay, S. A., Shore, N. A. & Landing, B. H. Triosephosphate isomerase deficiency. A case report with neuropathological findings. Am. J. Dis. Child 136, 800–802 (1982).
Serdaroglu, G., Aydinok, Y., Yilmaz, S., Manco, L. & Ozer, E. Triosephosphate isomerase deficiency: a patient with Val231Met mutation. Pediatr. Neurol. 44, 139–142. https://doi.org/10.1016/j.pediatrneurol.2010.08.016 (2011).
Bardosi, A., Eber, S. W., Hendrys, M. & Pekrun, A. Myopathy with altered mitochondria due to a triosephosphate isomerase (TPI) deficiency. Acta Neuropathol. 79, 387–394. https://doi.org/10.1007/BF00308714 (1990).
Linarello, R. E., Shetty, A. K., Thomas, T. & Warrier, R. P. Triosephosphate isomerase deficiency in a child with congenital hemolytic anemia and severe hypotonia. Pediatr. Hematol. Oncol. 15, 553–556. https://doi.org/10.3109/08880019809018318 (1998).
Valentine, W. N., Schneider, A., Baughan, M. A., Paglia, D. E. & Heins, H. L. Jr. Hereditary hemolytic anemia with triosephosphate isomerase deficiency: studies in kindreds with coexistent sickle cell trait and erythrocyte glucose-6-phosphate dehydrogenase deficiency. Am. J. Med. 41, 15 (1966).
Clark, A. C. L. & Szobolotzky, M. A. Triose phosphate isomerase deficiency - report of a family. Aust. Paediatr. J. 22, 135–137 (1986).
Valentin, C. et al. Triose phosphate isomerase deficiency in 3 French families: two novel null alleles, a frameshift mutation (TPI Alfortville) and an alteration in the initiation codon (TPI Paris). Blood 96, 1130–1135 (2000).
Eber, S. W. et al. Triosephosphate isomerase deficiency: haemolytic anaemia, myopathy with altered mitochondria and mental retardation due to a new variant with accelerated enzyme catabolism and diminished specific activity. Eur. J. Pediatr. 150, 761–766. https://doi.org/10.1007/BF02026706 (1991).
Rosa, R. et al. Hereditary triose phosphate isomerase deficiency: seven new homozygous cases. Hum. Genet. 71, 235–240. https://doi.org/10.1007/BF00284582 (1985).
National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.) & National Academies Press (U.S.). xxv, 220 p (National Academies Press,, Washington, D.C., 2011).
Augusto, V., Padovani, C. R., Eduardo, G. & Campos, R. Skeletal Muscle Fiber Types in C57BL6J Mice. J. Morphol. Sci. 21 (2004).
Hong, S. J. & Chang, C. C. Use of geographutoxin II (mu-conotoxin) for the study of neuromuscular transmission in mouse. Br. J. Pharmacol. 97, 934–940. https://doi.org/10.1111/j.1476-5381.1989.tb12034.x (1989).
McLachlan, E. M. & Martin, A. R. Non-linear summation of end-plate potentials in the frog and mouse. J. Physiol. 311, 307–324. https://doi.org/10.1113/jphysiol.1981.sp013586 (1981).
Wenceslau, C. F. et al. Guidelines for the measurement of vascular function and structure in isolated arteries and veins. Am. J. Physiol. Heart Circ. Physiol. 321, H77–H111. https://doi.org/10.1152/ajpheart.01021.2020 (2021).
Grune, J. et al. Evaluation of a commercial multi-dimensional echocardiography technique for ventricular volumetry in small animals. Cardiovasc. Ultrasound 16, 10. https://doi.org/10.1186/s12947-018-0128-9 (2018).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. https://doi.org/10.1038/nmeth.2089 (2012).
Li, K., Li, J., Zheng, J. & Qin, S. Reactive astrocytes in neurodegenerative diseases. Aging Dis. 10, 664–675. https://doi.org/10.14336/AD.2018.0720 (2019).
Xu, Y., Jin, M. Z., Yang, Z. Y. & Jin, W. L. Microglia in neurodegenerative diseases. Neural Regen. Res. 16, 270–280. https://doi.org/10.4103/1673-5374.290881 (2021).
Roman, W. & Gomes, E. R. Nuclear positioning in skeletal muscle. Semin. Cell Dev. Biol. 82, 51–56. https://doi.org/10.1016/j.semcdb.2017.11.005 (2018).
American Heart Association - Ejection Fraction Heart Failure Measurement, <https://www.heart.org/en/health-topics/heart-failure/diagnosing-heart-failure/ejection-fraction-heart-failure-measurement> (2023).
Galley, J. C. & Straub, A. C. Redox control of vascular function. Arterioscler Thromb. Vasc. Biol. 37, e178–e184. https://doi.org/10.1161/ATVBAHA.117.309945 (2017).
Conrad, C. H. et al. Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circulation 91, 161–170. https://doi.org/10.1161/01.cir.91.1.161 (1995).
Vogt, A. et al. A high-content screening assay for small molecules that stabilize mutant triose phosphate isomerase (TPI) as treatments for TPI deficiency. SLAS Discov. 26, 1029–1039. https://doi.org/10.1177/24725552211018198 (2021).
Acknowledgements
This project used the UPMC Hillman Cancer Center and Tissue and Research Pathology/Pitt Biospecimen Core shared resource which is supported in part by award P30CA047904. Additional work was completed by the Pitt Histology Core located in the UPMC Children's Hospital of Pittsburgh in the John G. Rangos Sr. Research Center. Echocardiograms were completed through the small-animal ultrasonography core at the University of Pittsburgh and supported by award NIH 1S10OD023684; Advanced High Resolution Rodent Ultrasound Imaging System. We are grateful to Brenda McMahon for conducting echocardiography experiments. We gratefully acknowledge grant support for this project via R03 NS119664 (MJP), R01 HD105311 (MJP), R01 HD104346 (MJP), U01 AA020889 (GEH), R35HL 161177 (ACS), T32 GM133332 (TDM), the William C. DeGroat Neuropharmacology fellowship (TDM), and the Save JT Borofka research fund. This work was supported through shared resources within the Pittsburgh Institute for Neurodegenerative Diseases (PIND) and the Department of Pharmacology & Chemical Biology at the University of Pittsburgh. We are thankful to Dr. Dandan Sun and Dr. Tim Greenamyre for the use of microscopy equipment, as well as Dr. Kyle Farmer for assistance with imaging.
Author information
Authors and Affiliations
Contributions
T.D.M contributed to the conception, design, data acquisition, data analysis, interpretation of data, and drafting of the manuscript. Y.L., S.T., and P.C.A. contributed to the design, data acquisition, data analysis, interpretation of data, and review of the manuscript. E.K., K.M., and C.B. contributed to data analysis and review of the manuscript. M.W. contributed to data acquisition and review of the manuscript. G.E.H. contributed to data acquisition and review of the manuscript. A.C.S. contributed to design, interpretation of data, and review of the manuscript. S.D.M. contributed to design, interpretation of data, and review of the manuscript. M.J.P. contributed to the conception, design, interpretation of data, and drafting of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Myers, T.D., Li, Y., Taiclet, S. et al. Neuromuscular dysfunction and pathogenesis in triosephosphate isomerase deficiency. Sci Rep 14, 18575 (2024). https://doi.org/10.1038/s41598-024-69618-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69618-7
- Springer Nature Limited