Abstract
Evidence suggests that maternal metabolome may be associated with child health outcomes. We analyzed the association between the maternal metabolome between 28–35 gestational weeks and child growth and development during the first year. A prospective cohort of 98 mother–child dyads was followed at birth, 1, 6, and 12 months. Maternal serum samples were collected for targeted LC–MS/MS analysis, which measured 132 metabolites. The child's growth and development were assessed at each time-point. Z-scores were calculated based on WHO growth standards, and the domains of development were assessed using the Ages and Stages Questionnaires (ASQ-3). Multiple linear mixed-effects models were performed and confounders were identified using a Diagram Acyclic Graph. The Benjamini–Hochberg correction was used for multiple comparison adjustments. We found a positive association between lysophosphatidylcholines (14:0; 16:0; 16:1; 17:0; 18:0; 18:1; 18:2; 20:4) with the z-score of weight-for-age, and lysophosphatidylcholines (14:0; 16:0; 16:1; 18:0) and taurine with the z-score of weight-for-length, and lysophosphatidylcholines (14:0; 16:0; 16:1; 17:0; 18:0; 18:1; 18:2; 20:4) and glycine with the z-score of BMI-for-age. The leucine, methionine, tryptophan, and valine were negatively associated with the fine motor skills domain. We observed an association between maternal metabolome and the growth and child's development throughout the first year.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
The period between conception and the first 2 years of a child’s life is considered a crucial phase for children's health. Because of this, maternal health could consequently impact better outcomes for the offspring throughout their lifetime1. This is aligned with the concept of Developmental Origins of Health and Disease (DOHaD), that has identified the important role of the early stages of life conditions on several outcomes throughout their life, such as inadequate nutrition, past morbidity history, maternal mental health, and social inequities2,3,4,5.
Several studies have observed an important role of maternal health on offspring outcomes6,7. During pregnancy, the woman's body undergoes physiological adaptations to provide the best conditions for fetal development and it is well documented that throughout the third trimester of pregnancy the fetus’s growth is more accelerated8,9. Therefore it is important to highlight that changes to the maternal system consequently impacts the health of the growing fetus8,9.
Child growth and development are key health indicators that reflect the child's environment10,11. Alterations in childhood growth, such as growth deficits and a heightened risk of obesity within the first 2 years, may significantly influence long-term health and nutritional status. Furthermore, inadequate development during early childhood can have lasting effects on learning, academic skills, and socioemotional abilities throughout the life course12.
Due to the importance of these indicators, using tools with predictive characteristics is a good strategy, and metabolome analysis is a valuable tool currently being used to predict maternal and child health outcomes13,14. It is a helpful tool that may be used to investigate adverse health outcomes15. In maternal and child health, the metabolome helps to identify biomarkers related to such complications as preeclampsia, gestational diabetes mellitus, preterm birth, and fetal growth restriction and understand the physiopathology of those potential diseases6,16. Besides, it is considered a vital tool for comprehending metabolic adaptations, and how the lifestyle may be related to different metabolic signature17,18.
Previous studies observed the relation of metabolome with child growth and development in different moments of life. Isganaitis et al. found a negative association between arginine, lysine, methionine, and proline with weight for age in the first month19. In another study, Moreau et al. found a positive association of the phosphatidylcholine class with neurodevelopment at 36 months and of sphingomyelins and threonine with the neurocognitive development score at 9 and 36 months20. Considering this literature is scarce, the present study aimed to explore the association between the maternal metabolome in the third trimester of pregnancy and child growth and development during the first year of life.
Results
The socio-demographic and nutrition profile of the 98 subjects that completed the follow-up did not differ for all variables, except for pre-pregnancy BMI, when comparisons were made with the 150 subjects that have initiated the baseline.
The mothers from the study had a median age of 27 years, 12 years of education, and 57% self-reported being brown/mixed. The majority of mothers were classified with normal pré-pregnancy body-mass-index (PP-BMI) (50.5%) and had a median of 40 weeks of gestational age at childbirth (Table 1). More than half of the children were exclusively breastfed in the first month (57%), were born by vaginal delivery (65%), and 78% were categorized as adequate to gestational weight (Tables 1 and 2).
The z-score trajectory of weight-for-age, weight-for-length, and body mass index (BMI)-for-age varied significantly with time, whereas the length-for-age and head-circumference-for-age did not (Fig. 1). The developmental scores of communications, gross motor, fine motor, and problem-solving domains varied significantly with time, whereas personal-social skills did not (Fig. 2).
Child growth trajectory throughout the first year after delivery. Note: The child anthropometric z-score was calculated according to the child growth standards of the World Health Organization (2006). Linear mixed regression models were performed to evaluate the association of child growth trajectory with time. The anthropometric measures were taken at birth (n = 94), 28–50 days (~ 1 month; n = 85), 180–216 days (~ 6 months; n = 70), and 305–404 days (~ 12 months; n = 58) after birth. β coefficients represent the effect estimates by linear mixed models. CI confidence interval.
Child development trajectory throughout the first year after delivery, according to the developmental domains. Note: The child's development was estimated by the scores of each domain, according to the Ages and Stages questionnaire version 3. Linear mixed regression models were performed to evaluate the association of child developmental trajectory with time. The questionnaire was applied in the following periods: 28–50 days (~ 1 month; n = 85), 180–216 days (~ 6 months; n = 70), and 305–404 days (~ 12 months; n = 57) after birth. β coefficients represent the effect estimates by linear mixed models. CI confidence interval.
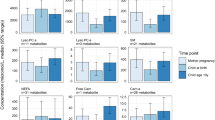
We did not find a statistically significant association between maternal third-trimester metabolome and the z-score of length-for-age and head-circumference-for-age. Nonetheless we observed that the lysophosphatidylcholines (LPCs) (14:0, 16:0, 16:1, 17:0, 18:0, 18:1, 18:2, 20:4) were positively associated with weight-for-age z-score, the LPCs (14:0, 16:0, 16:1, 18:0) and taurine were positively associated with the z-score of weight-for-length, and the LPCs (14:0, 16:0, 16:1, 17:0, 18:0, 18:1, 18:2, 20:4) and glycine were positively associated with the z-score of BMI-for-age (Fig. 3 and Supplementary Table 2).
Associations between the maternal serum metabolites and child growth throughout the first year of life. Note: Adjusted linear mixed models were built having the following outcomes: weight-for-age z-score (A), weight-for-length z-score (B), and body mass index for-age z-score (C) at birth (n = 94), 28–50 days (~ 1 month; n = 85), 180–216 days (~ 6 months; n = 70), and 305–404 days (~ 12 months; n = 58) after birth. The concentration of metabolites was centered in the mean to be represented graphically. Only the metabolites with p-value < 0.1 after Benjamini–Hochberg correction for multiple comparisons (q-value) are shown. All models were adjusted for pre-pregnancy body mass index (kg/m2), and gestational age at birth, as the minimal adjustments suggested by the Directed Acyclic Graphics. The vertical dashed red line represents the absence of associations. The horizontal bars indicate the confidence interval of the regression with 95% confidence, and the bullet in the middle of the bar represents the coefficient. The z-score was calculated according to the child growth standards of the World Health Organization. CI confidence interval.
We did not find a statistically significant association between maternal third-trimester metabolome and the children’s domains of communication, gross motor skill, personal-social, and problem-solving during the first year. Nonetheless, the maternal third-trimester metabolites leucine, methionine, tryptophan, and valine showed a negative association with the fine motor skill domain (Fig. 4 and Supplementary Table 3).
Association between the maternal serum metabolites and fine motor skills domain throughout the first year of life. Note: Adjusted linear mixed regression models were built having the child development scores as the outcomes evaluated in the period of 28–50 days (~ 1 month; n = 85), 180–216 days (~ 6 months; n = 70), and 305–404 days (~ 12 months; n = 57) after birth. The concentration of metabolites was centered in the mean to be represented graphically. Only the metabolites with p-value < 0.1 after Benjamini–Hochberg correction for multiple comparisons (q-value) are shown. All models were adjusted for pre-pregnancy body mass index (kg/m2), and gestational age at birth, as the minimal adjustments suggested by the Directed Acyclic Graphics. The vertical dashed red line represents the absence of effects. The horizontal bars indicate the confidence interval of regression with 95% confidence, and the bullet in the middle of the bar represents the coefficient. The domains and scores were accessed and classified according to Ages and Stages questionnaires 3. CI confidence interval.
Discussion
This study examined the role of the maternal third-pregnancy trimester metabolome and the association of these metabolites with growth and child development in a cohort of 98 Brazilian mother–child dyads. We found a positive association of several LPCs, glycine, and taurine with z-score growth trajectories, while leucine, valine, methionine, and tryptophan were negatively associated with fine motor skills. The number of studies that assessed the association between metabolome and child growth and development is still scarce. Therefore, developing a discussion incorporating findings from various study designs is challenging. To the best of our knowledge, this is the first study to analyze the association between metabolome in the third trimester and child trajectories of growth and development through the first year of age.
Previous studies observed a positive association of cord LPC with birth weight but not from maternal blood in pregnancy21,22. Although these studies found no significant associations with maternal blood samples, they presented a different metabolome time collection. Voerme et al. measured the metabolome during early pregnancy, and Labarre et al. in the first trimester (8–14 weeks of pregnancy) and at birth. In contrast, our study measured the metabolome during the third pregnancy trimester, a period characterized as the most accelerated fetal growth22.
We found that glycine was positively associated with child growth, a similar result found by a retrospective cohort with blood spot samples collection taken between 12 h and 8 days after birth. These authors found higher concentrations of child blood glycine in small for gestational age newborns compared to adequate to gestational age23. Nonetheless, a Chinese study also has found an inverse association between child blood glycine over the first 2 years: 42 days, 6 months, 1 year (10–14 months), and 2 years (22–26 months) with the abdominal skinfold thickness24. However, those findings differ from ours in presenting child metabolome analysis, while we observed the maternal blood metabolite.
Taurine was positively associated with child weight for length in our study, and the literature shows that this metabolite is related to the nervous system and is abundant in the heart, breast, gallbladder, and kidney25. A case–control study with mothers with and without maternal gestational diabetes enrolled in an antenatal care system in Tianjin, China, observed that the pregnancy serum taurine has been positively associated with persistent obesity growth patterns in their offspring26.
Interestingly, our findings revealed a negative association of some branched-chain amino acids (BCAA), which include leucine and valine, with fine motor skills. BCAA are important in brain function and are involved in stress, energy, and muscle human metabolism27,28. The breakdown of leucine and valine can generate acetyl-CoA, an essential component of the citric acid cycle, indirectly contributing to energy metabolism29.
Similar results between BCAA and child development were observed in an ongoing cohort of 626 mother–child dyads from Boston, United States, that started at birth. The children’s development was assessed using a scale provided by a physician diagnoses per International Classification of Diseases (ICD) 9 or 10 in electronic medical records. The metabolome samples were analyzed from maternal and umbilical cord blood collected at birth. The results showed that the levels of cord blood BCAA were higher in those diagnosed with attention deficit hyperactivity disorder than in children with neurotypical development. The third tertile of cord blood leucine, isoleucine, and valine was associated with higher odds of presenting attention deficit hyperactivity disorder27. This study found no statistically significant associations between maternal blood BCAA and child development, a result that contrasts with our findings, which may be attributed to differences in the period of the blood samples collection27.
Our findings showed a negative association between methionine and fine motor skills. Methionine is an essential amino acid, described as a substance required for adequate growth and development, and is presented in the single-carbon metabolism and sulfur-containing amino acids25,29. The methionine was also associated with child development in a prospective cohort that observed the association of child urinary methionine collected at 3, 6, and 12 months with child development. The authors also found an association of lower methionine with non-typical development when compared to children with typical development30.
The tryptophan showed a negative association with fine motor skills in our study. This metabolite is presented in the aromatic amino acid metabolism and was observed as a metabolite that, when increased in cord blood, was related to an elevated risk of attention-deficit hyperactivity disorder diagnosis in their offspring29,31.
The strengths of this study comprise the use of a metabolome preprocessing technique considered adequate for epidemiologic studies with good reproducibility and reflecting properly the concentration and the measuring of risk32, robust statistical analysis using confounders' definitions based on literature through Directed Acyclic Graphics (DAG), which minimizes the risk of bias. Another positive aspect of this study was the use of longitudinal data, using four points to evaluate the growth and three points of development. This study has some limitations that are worth describing: the small sample size, the use of q-values < 0.10 in some instances as statistical significant results, the fact that it had just one point of metabolome analysis during pregnancy, the potential for low external validity as the study was conducted in a single public health unit located in a low-income region of Rio de Janeiro, and the analyses were limited to individual metabolites, without consider a mixture or combination of them. Altogether, these factors could alter the strength of the associations.
In summary, we observed an association between the third trimester maternal blood metabolites classes of amino acids, biogenic amines, and LPCs and the growth and development trajectory through the first year. Our findings revealed that the maternal metabolic signature could influence their offspring's outcomes, illustrating the importance of early health care. Thus, more studies with other populations and exploring other moments of pregnancy could be interesting to understand maternal and child health implications comprehensively.
Methods
Study design
A non-probabilistic sample of 147 pregnant women was recruited in the third gestational trimester (28–35 weeks) during prenatal care appointments in a public health center from the Brazilian Unified Health System located in a low-income region of Rio de Janeiro, Brazil. Data collection occurred between February 2017 and April 2019. The study was conceptualized as exploratory research, and the protocol has already been described elsewhere33,34.
The study design comprised a prospective cohort with a baseline at birth and five follow-up visits during postpartum at the periods of 2–8 days (visit 1), 28–50 days (visit 2; ~ 1 month), 88–119 days (visit 3; ~ 3 months), 180–216 days (visit 4; ~ 6 months), and 365–404 days (visit 5; ~ 12 months).
The eligibility criteria of the principal study included living in the catchment area of the public health center, being between 18 and 40 years of age, not presenting infectious or chronic non-communicable diseases (except obesity), and singleton pregnancy. The exclusion criteria comprised the development of any gestational morbidity throughout the study, having a preterm delivery or a stillbirth, and having a newborn with any disease.
This manuscript uses data from a subset of 98 mother-dyads that fulfilled the eligibility criteria, which comprised having data on weight and length at birth, blood sample collection at pregnancy, and data of child growth and/or development during at least one visit at one (n = 86), six (n = 70), or 12 (n = 58) months postpartum (Fig. 5).
Maternal blood sample collection
The women's blood sample collection was carried out between the 28th and 35th gestational weeks, in the morning, which required fasting for 8–12 h before the blood was drawn. The collection was conducted by a trained nurse affiliated with the research team.
Samples were homogenized after the collection and maintained in the rack until the total coagulation. After 30 min, the samples were centrifuged (1100–1300g) for 20 min at 4 °C to obtain aliquots of serum. Then, the serum aliquots (1 mL) were stored in cryogenic tubes at − 80 °C until further analysis.
Metabolome analysis
The serum samples were analyzed using a targeted metabolomic platform, where AbsoluteID p180 assay (Biocrates Life Sciences, Innsbruck, Austria) was used to identify and quantify a total of 132 metabolites, including amino acids, biogenic amines, acylcarnitines, LPCs, diacyl phosphatidylcholines (PC), alkyl:acyl phosphatidylcholines (PC-O), sphingomyelins with [SM(OH)] and without hydroxylation (SM) and the sum of hexoses (Supplementary Table 1).
The sample analysis and preparation were performed according to the manufacturer’s instructions of the Biocrates p180 assay previously described elsewhere32. Briefly, the serum samples were centrifuged (2750g for 5 min, at 4 °C), then 10 µL of supernatant was transferred to a 96-well plate and dried under nitrogen. The amino acids and biogenic amines were derivatized by adding 50 µL of 5% phenyl isothiocyanate (PITC) and kept at room temperature for 25 min. Following this, the plate was dried for 1 h, and 300 µL of 5 mM ammonium acetate/methanol was added. The amino acids and biogenic amines were measured using liquid chromatography-mass spectrometry (LC–MS) (AB Sciex, Darmstadt, Germany). The plate was then centrifugated at 500×g for 2 min, and the 50 µL of eluate was diluted with 450 µL methanol for FIA-MS. Acylcarnitines and other lipids were measured semi-quantitatively using flow injection analysis-mass spectrometry (FIA-MS) (AB Sciex, Darmstadt, Germany). A total of 150 µL of eluate was diluted with 150 µL of HPLC grade water for a liquid chromatography-tandem mass spectrometry (LC–MS/MS) run.
The LC–MS system included the Sciex ExionLseries of the UHPLC system coupled to a QTRAP 6500+ on ionization mode by electrospray (ESI) (AB Sciex, Darmstadt, Germany). 5 µL of the sample were injected onto a UHLPC column provided with the p180 assay with 0.2% formic acid in water as solvent A and 0.2% formic acid in acetonitrile as solvent B with a gradient of 5.8 min as per p180 instructions. During the FIA analysis, 15 µL of the sample was injected with methanol. All metabolites were identified in positive mode and quantified according to the multiple reactions monitoring (MRM) method.
The amino acids and biogenic amines were quantified based on their internal standards, which were isotopically marked, and 7-point calibration curves and metabolome data were processed. Other metabolites, such as acylcarnitines, LPCs, PCs, SMs and hexose were semi-quantified by using 14 internal standards in the MetIDQ™ software (Biocrates Life Sciences, Innsbruck, Áustria). The metabolite concentration was exported in µM, and only the metabolites with a concentration above the detection limit in > 75% serum samples were included in statistical analysis. All the data acquisition and processing were applied by AB Sciex Analyst version 1.7.2 software (SCIEX, Toronto, Canada).
Child growth
The childbirth weight and length were collected from the child's health handbook on visit 1, and the subsequent measurements were assessed on visit 2, 4, and 5, including weight (cm), length (cm), and head circumference (cm). These measurements were performed by trained interviewers.
Children were weighed without clothes and accessories using the Seca model 336 scale (Seca, Hamburg, Germany). The length was measured using the Seca model 417 infantometer (Seca, Hamburg, Germany). The head-circumference measurements were performed using a disposable measuring tape of Seca model 211 (Seca, Hamburg, Germany). Length and head-circumference measurements were taken in duplicate. If the differences were within a range of 0.5 cm for length and 0.2 cm for head circumference, an arithmetic mean was calculated. If the difference was greater than these values, it was necessary to take a third measure.
The indices weight-for-age, height/length-for-age, weight-for-length, BMI-for-age, and head circumference-for-age were used to evaluate child growth according to the World Health Organization (WHO) growth standards35. The package anthro in RStudio was used to classify the z-scores of children35,36.
Child development
The Age and Stages Questionnaire version 3 (ASQ-3), translated and cross-culturally adapted for the Brazilian population, was used to evaluate early childhood development37. ASQ-3 is a screening instrument used for follow-up and monitoring the child's development according to age since the first infancy38. It considers the observation of their caregivers.
This questionnaire comprises six questions for five developmental domains: communication, gross motor skills, fine motor skills, problem-solving, and personal-social. The response options for each question regarding whether the child has already performed activities of each domain could be scored as follows: 'yes' receiving 10 points, 'sometimes', receiving 5 points, and 'not yet' receiving 0 points. The final score was calculated by the sum of points for each domain and ranges from 0 to 60. The higher the score, the closer to the development expected for the child's age. No cut-off has been published for the Brazilian population yet. However, the United States of America's population cut-off points are usually used to classify and describe the child development status described in previous Brazilian studies37,38,39.
Covariates
A structured questionnaire was administered at the baseline to obtain data on maternal age (years), self-declared skin color (white, brown/mixed, black, yellow), education (schooling years), marital status (lives with or without a partner), parity (primiparous or multiparous), smoking status (smoking during pregnancy), alcohol intake in any gestational trimester, and self-reported pre-pregnancy weight.
The maternal PP-BMI (kg/m2) was calculated using the self-reported pre-pregnancy weight (kg) and height measured at 28–50 days postpartum and was categorized as underweight (< 18.5 kg/m2), normal weight (≥ 18.5 to < 25.0 kg/m2), overweight (≥ 25.0 to < 30.0 kg/m2), and obese (≥ 30.0 kg/m2)40.
Data on type of delivery (vaginal/cesarean), newborn’s sex (male/female), and birth date was collected at the first visit 1. The gestational age at birth was calculated according to the first ultrasound when it was performed ≤ 24 gestational weeks (n = 96, 98.0%) or based on the date of the last menstrual period (n = 2, 2.0%) in case the first ultrasound was considered unavailable41. The breastfeeding status was collected during the follow-up visits and classified as exclusive breastfeeding, complementary, or no breastfeeding42.
Statistical analysis
The normality of the variables was investigated using the Shapiro–Wilk test. We used absolute (n) and relative frequencies (%) for categorical variables and median and interquartile range (IQR) for quantitative variables. Metabolite values with a median ± 5 standard deviations of the log-transformed data were considered outliers43. Outlier analysis removed 0.16% (n = 25) of measurements from downstream analysis.
We compared the distribution or mean values for selected variables between the initial group at the baseline (n = 151) with those that reached the final follow-up (n = 98) using the chi-square test for categorical and the Wilcoxon–Mann–Whitney test for continuous variables.
A trajectory graph for growth and development parameters during the first year was built using a longitudinal linear mixed-effects (LME) model to evaluate the difference in the scores' trajectories during the first year (in days).
LME models evaluated the association between maternal third-pregnancy trimester metabolites and child growth and development throughout the first year after delivery. A random intercept and slope model was performed using the “lme4” and “lmerTest” packages. A loop repetition model was used to assess the association of the response variable with each of the 132 metabolites. The performance of the random slope for the temporal models was evaluated using a spaghetti plot and the Akaike Information Criteria (AIC) using ANOVA to observe statistical significance. A likelihood ratio test was implemented using the linear mixed-effects model to observe the necessity of using the interaction term between the response variable and days. All the LME models were implemented using the 'lmerControl("bobyqa")' configuration to optimize model fitting.
Confounders were defined based on DAG using the DAGitty software freely available at https://dagitty.net/ (Institute for Computing and Information Sciences, Radboud University, The Netherlands, Johannes Textor)44. DAGs can help identify sufficient minimum adjustment sets of confounding variables based on the current literature review, where the minimum adjustments are obtained from the causal relationship between the variables. The DAG has suggested that gestational age and PP-BMI were applied as minimum adjustments for the LME models (Supplementary Fig. 1).
The model’s quality was evaluated using a quantile–quantile (QQ) plot that was performed based on the residuals model. The residual showed reasonably normal distribution, except for child development. Scores of child development were box-cox-transformed to get better residual distributions.
The results were considered statistically significant when the adjusted p-values (q-values) were < 0.1 after the Benjamini–Hochberg correction for multiple comparisons for models with interaction. A less rigorous level of significance has been deemed acceptable for models with interaction, according to Altman et al.45. For the other analyses, p-value < 0.05 was considered. Effect estimates were presented as beta coefficients (β) and 95% confidence intervals (CI 95%). All statistical analyses were performed in R 4.3.1 (R Core Team, Vienna, Austria) and STATA 15 (College Station, Texas, USA).
Ethical aspects
The study protocol followed the guidelines laid down in the Declaration of Helsinki and has been approved by the Ethical Committee from the Municipal Health Secretary from Rio de Janeiro (No. 49218115.0.3001.5279) and the Ethical Committee from the Maternity School of Federal University of Rio de Janeiro (No. 49218115.0.0000.5275). The participants have freely and spontaneously signed the written informed consent.
Data availability
The data will be made available upon request to the corresponding author Dr Gilberto Kac (gilberto.kac@gmail.com).
References
Darling, J. C., Bamidis, P. D., Burberry, J. & Rudolf, M. C. J. The First Thousand Days: Early, integrated and evidence-based approaches to improving child health: Coming to a population near you?. Arch. Dis. Child. 105, 837–841 (2020).
Grandi, C. et al. Developmental Origins of Health and Disease Concept: The environment in the first 1000 days of life and its association with noncommunicable diseases. Archivos Argentinos de Pediatria 118, S118–S129 (2020).
Neumann, D. et al. A longitudinal study of antenatal and perinatal risk factors in early childhood cognition: Evidence from Growing Up in New Zealand. Early Hum. Dev. 132, 45–51 (2019).
Reynolds, L. P. et al. Developmental programming of fetal growth and development. Vet. Clin. North Am. Food Anim. Pract. 35, 229–247 (2019).
Barker, D. J. The origins of the developmental origins theory. J. Intern. Med. 261, 412–417 (2007).
Colicino, E. et al. Non-linear and non-additive associations between the pregnancy metabolome and birthweight. Environ. Int. 156, 106750 (2021).
Zhai, X. et al. Nontargeted metabolomics reveals the potential mechanism underlying the association between birthweight and metabolic disturbances. BMC Pregnancy Child. 23, 14–14 (2023).
Kepley, J. M., Bates, K. & Mohiuddin, S. S. Physiology, maternal changes. In StatPearls (StatPearls Publishing, 2024).
Institute of Medicine Committee on Nutritional Status During Pregnancy & Lactation in Nutrition During Pregnancy: Part I Weight Gain: Part II Nutrient Supplements (National Academy of Sciences, 1990).
Ministério da Saúde. Atenção ao Pré-Natal de Baixo Risco (Governo Federal Ministério da Saúde, 2012).
WHO. Improving Early Childhood Development: WHO Guideline (World Health Organization, 2020).
Balasundaram, P. & Avulakunta, I. D. Bayley scales of infant and toddler development. In StatPearls (StatPearls Publishing, 2023).
Orozco, J. S., Hertz-Picciotto, I., Abbeduto, L. & Slupsky, C. M. Metabolomics analysis of children with autism, idiopathic-developmental delays, and Down syndrome. Transl. Psychiatry 9, 243 (2019).
Kadakia, R. et al. Cord blood metabolomics: Association with newborn anthropometrics and C-peptide across ancestries. J. Clin. Endocrinol. Metab. 104, 4459–4472 (2019).
Bar, N. et al. A reference map of potential determinants for the human serum metabolome. Nature 588, 135–140 (2020).
Delplancke, T. D. J. et al. Metabolomics of pregnancy complications: Emerging application of maternal hair. BioMed Res. Int. 2018, 2815439 (2018).
Fiehn, O. Combining genomics, metabolome analysis, and biochemical modelling to understand metabolic networks. Comp. Funct. Genomics 2, 155–168 (2001).
Fiehn, O. et al. Metabolite profiling for plant functional genomics. Nat. Biotechnol. 18, 1157–1161 (2000).
Isganaitis, E. et al. Maternal obesity and the human milk metabolome: Associations with infant body composition and postnatal weight gain. Am. J. Clin. Nutr. 110, 111–120 (2019).
Moreau, G. B. et al. Childhood growth and neurocognition are associated with distinct sets of metabolites. EBioMedicine 44, 597–606 (2019).
Voerman, E. et al. Associations of maternal and infant metabolite profiles with foetal growth and the odds of adverse birth outcomes. Pediatr. Obes. 17, e12844 (2022).
LaBarre, J. L. et al. Maternal lipid levels across pregnancy impact the umbilical cord blood lipidome and infant birth weight. Sci. Rep. 10, 14209 (2020).
McCarthy, M. E. et al. The independent and combined influences of small for gestational age and socioeconomic status on newborn metabolite levels. J. Maternal-Fetal Neonatal Med. 35, 6192–6198 (2022).
Chen, Y. et al. High-risk growth trajectory related to childhood overweight/obesity and its predictive model at birth. J. Clin. Endocrinol. Metab. 107, e4015–e4026 (2022).
Wishart, D. S. et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 50, D622–D631 (2022).
Li, N. et al. Serum sulfur-containing amino acids and risk of maternal gestational diabetes and adverse growth patterns in offspring. Nutrients 15, 4089 (2023).
Anand, N. S. et al. Maternal and cord plasma branched-chain amino acids and child risk of attention-deficit hyperactivity disorder: A prospective birth cohort study. J. Child Psychol. Psychiatry Allied Discipl. 62, 868–875 (2021).
Etzel, M. R. Manufacture and use of dairy protein fractions. J. Nutr. 134, 996s–1002s (2004).
Stanford. Pathways of Human Metabolism. Stanford School of Medicine. Version 10.18 (2023)
Sotelo-Orozco, J., Schmidt, R. J., Slupsky, C. M. & Hertz-Picciotto, I. Investigating the urinary metabolome in the first year of life and its association with later diagnosis of autism spectrum disorder or non-typical neurodevelopment in the MARBLES study. Int. J. Mol. Sci. 24, 9454 (2023).
Raghavan, R. et al. Association between cord blood metabolites in tryptophan pathway and childhood risk of autism spectrum disorder and attention-deficit hyperactivity disorder. Transl. Psychiatry 12, 270 (2022).
Yin, X., Prendiville, O., McNamara, A. E. & Brennan, L. Targeted metabolomic approach to assess the reproducibility of plasma metabolites over a four month period in a free-living population. J. Proteome Res. 21, 683–690 (2022).
Batalha, M. A. et al. Factors associated with longitudinal changes in B-vitamin and choline concentrations of human milk. Am. J. Clin. Nutr. 114, 1560–1573 (2021).
Ferreira, A. L. et al. Human milk oligosaccharide profile variation throughout postpartum in healthy women in a Brazilian cohort. Nutrients 12, 790 (2020).
WHO. WHO child growth standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development (World Health Organization, 2006).
WHO (World Health Organization, 2007).
Squires, J., Bricker, D. D. & Twombly, E. Ages and Stages Questionnaires (Paul H. Brookes, 2009).
Ramos, M. & Barba, P. C. S. D. Ages and Stages Questionnaires Brazil in monitoring development in early childhood education. An. Acad. Bras. Ciênc. 93, e20201838 (2021).
Rio de Janeiro. Manual de Uso do ASQ-3: guia rápido para aplicação do ASQ-3 (IETS, 2010).
WHO. Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Org. Tech. Rep. Ser. 854, 1–452 (1995).
Committee Opinion No 700: Methods for Estimating the Due Date. Obstet. Gynecol. 129, e150–e154 (2017).
Ministério da Saúde. Caderno de atenção básica—Saúde da criança: Nutrição Infantil, Aleitamento Materno e Alimentação Complementar. Ministério da saúde 23 (2009).
Beuchel, C. et al. Clinical and lifestyle related factors influencing whole blood metabolite levels—A comparative analysis of three large cohorts. Mol. Metab. 29, 76–85 (2019).
Textor, J., van der Zander, B., Gilthorpe, M. S., Liśkiewicz, M. & Ellison, G. T. Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. Int. J. Epidemiol. 45, 1887–1894 (2017).
Altman, D. G. & Bland, J. M. Interaction revisited: The difference between two estimates. BMJ 326, 219 (2003).
Villar, J. et al. Postnatal growth standards for preterm infants: The Preterm Postnatal Follow-up Study of the INTERGROWTH-21(st) Project. Lancet Glob. Health 3, e681-691 (2015).
Acknowledgements
We thank all cohort participants and their children from the public health care center, where the data collection happened. We also thank the financial support provided by the National Council for Scientific and Technological Development—CNPq by grant #309703/2019-0 (call CNPq 06/2019—Research Productivity Scholarship) and the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro—FAPERJ by the fellowships CNE-FAPERJ (E-26/202.805/2017; “Cientista do Nosso Estado”).
Author information
Authors and Affiliations
Contributions
The author’s contributions were: S.S.R.F., M.P. and G.K. study conception and design; A.L.L.F., A.C.C.F., N.C.F.C., L.B. and G.K. carried out the research data collection and transfer. M.P., L.B., and X.Y.: created and managed the original metabolome database; S.S.R.F., M.P., A.L.L.F. and R.M.S.: assistance in statistical analysis decisions; S.S.R.F.: analyzed and performed the statistical data analysis and written the first version of the manuscript; S.S.R.F., M.P., A.L.L.F., R.M.S., A.C.C.F., N.C.F.C., X.Y., L.B. and G.K.: reviewed, edited, critical evaluated, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
da Silva Rosa Freire, S., Padilha, M., Lima Ferreira, A.L. et al. Association between the third trimester maternal serum metabolome and child growth and development through the first year of life. Sci Rep 14, 18360 (2024). https://doi.org/10.1038/s41598-024-69247-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69247-0
- Springer Nature Limited