Abstract
Klotho, an anti-aging protein, is believed to participate in metabolic diseases and play a potential protective role by regulating insulin sensitivity. This study aimed to explore the relationship between the triglyceride-glucose (TyG) index (a simple marker of insulin resistance) and serum soluble Klotho (S-Klotho) levels. The cross-sectional study comprised 5237 adults aged 40–79 years who participated in the National Health and Nutrition Examination Surveys (NHANES) 2007–2016. The TyG index was calculated as ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2]. The serum levels of S-Klotho were measured by enzyme-linked immunosorbent assay. The association between the TyG index and S-Klotho levels was investigated by multiple linear regression models, smoothed curve fitting, segmented linear regression models, subgroup analyses, and interaction tests. The TyG index was inversely associated with serum S-Klotho level after full adjustment (β = − 45.11, 95% CI (− 79.53, − 10.69), P = 0.011). Furthermore, we also found a non-linear correlation and saturation phenomenon between the TyG index and serum S-Klotho levels, with a turning point of 9.56. In addition, a significant interaction effect of sex was found between the two (P for interaction < 0.001), with a more pronounced association observed in females. Further studies are required to explore the mechanisms and verify the correlation.
Similar content being viewed by others
Introduction
As an aging suppressor gene, the Klotho gene was first identified in mouse models in 19971. The human Klotho gene encodes the α-Klotho protein in three forms: transmembrane Klotho, secreted Klotho, and soluble Klotho (S-Klotho)2. S-Klotho is generated through the shedding of the transmembrane Klotho extracellular domain from the cell surface into body fluids and acts as a hormone to exert endocrine effects in the bloodstream. Serum Klotho deficiency is closely connected with diseases related to aging, such as neurocognitive diseases, chronic kidney disease, and tumors. It is also associated with disability, frailty, and an elevated risk of all-cause mortality3,4. Moreover, recent research has emphasized the significance of Klotho in physiological processes like insulin sensitivity, glucose homeostasis, and lipid metabolism, with implications for metabolic diseases5,6. Serum S-Klotho concentrations are notably lower among those with type 2 diabetes mellitus and its complications than in healthy individuals7,8,9. Furthermore, studies have indicated inversely correlated serum S-Klotho concentration with the incidence and mortality of metabolic syndrome, underscoring its protective role and prognostic value in metabolic syndrome10. Recent studies have also shown the promising therapeutic potential of recombinant Klotho proteins in various metabolic diseases11,12,13. Therefore, given the current epidemiological landscape of increasing metabolic diseases, it is crucial to investigate the potential protective mechanisms of Klotho protein in metabolic regulation.
Insulin resistance (IR) is the fundamental root cause of numerous metabolic diseases, including type 2 diabetes mellitus, metabolic syndrome, and obesity. Insulin resistance, also known as decreased insulin efficacy, manifests as inadequate insulin sensitivity or decreased insulin responsiveness, posing a substantial public health challenge14. While the gold standards for assessing insulin resistance include the hyperinsulinemic–euglycemic clamp (HEC) and intravenous glucose tolerance tests, other indices, such as the homeostasis model assessment of insulin resistance (HOMA-IR) and quantitative insulin sensitivity check index (QUICKI), are also utilized15,16,17. However, these methods are often underutilized in clinical settings due to their high costs, complexity, or inability to obtain fasting insulin data. The triglyceride–glucose (TyG) index, calculated using a specific formula, has shown greater sensitivity and specificity in reflecting insulin resistance than HEC or HOMA-IR, effectively identifying individuals with reduced insulin sensitivity18,19. Additionally, it has been acknowledged as a reliable indicator for diseases like type 2 diabetes mellitus, metabolic syndrome, and metabolic dysfunction associated steatotic liver disease, and has been linked to cardiovascular events and poor prognosis20,21.
Previous genetic research on the connections between the Klotho gene and insulin resistance has produced inconsistent results. In Asian Indians, the functional KL-VS variant of the Klotho gene showed a significant association with metabolic syndrome, which was associated with glucose metabolism and insulin resistance22. In contrast to the study mentioned above, the KL-VS genotype in white British individuals did not exhibit an association with insulin resistance or type 2 diabetes mellitus23. In addition, several studies have clarified the connection between serum S-Klotho and insulin resistance-related metabolic diseases. However, the associations between them have yet to be studied. This study aimed to explore the association between the TyG index (a simple marker of insulin resistance) and serum levels of S-Klotho in the middle-aged and elderly population using data from the NHANES 2007–2016.
Materials and methods
Study population and design
This research employed a cross-sectional study design, utilizing data from the National Health and Nutrition Examination Survey (NHANES). NHANES utilizes a complex, stratified, multistage, probability sampling design to collect health-related information from a nationally representative US population on a two-year cycle24. Specifically, the survey collected demographic and health information from the subjects through questionnaires, physical examination data using standard methods, and collection of blood and urine samples for professional laboratory monitoring at a mobile screening center. The NHANES study received approval from the Research Ethics Review Board of the National Center for Health Statistics, and all participants provided written informed consent to participate in the NHANES.
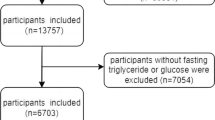
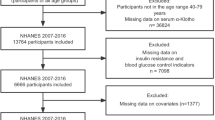
50,588 participants were initially sampled and took part in the NHANES from 2007 to 2016. Considering the availability of serum S-Klotho data, those younger than 40 years (n = 31,244) and older than 79 years (n = 1955) were excluded. Participants lacking serum S-Klotho (n = 3625), TyG index (n = 7130), weight (n = 339), other covariates (n = 1052) data, and pregnancy status (n = 6) were subsequently excluded. Ultimately, this study incorporated 5237 participants. The flowchart illustrating the screening process of study participants can be found in Fig. 1.
Measurement of the TyG index
Blood samples were collected in the morning following a minimum 8-h fasting period by the participants. Fasting glucose was determined by a hexokinase-mediated reaction and fasting triglyceride was determined through an enzymatic method. According to the literature, the calculation formula is TyG index = Ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2]18. All participants were divided into 4 categories (Q1, Q2, Q3, and Q4) based on their TyG index quartiles.
Measurement of serum S-Klotho levels
Serum S-Klotho levels (pg/ml) were measured in adults aged 40–79 years from NHANES 2007–2016 with an enzyme-linked immunosorbent assay (ELISA) kit made by IBL International, Japan. The samples were stored at − 80 °C and shipped to the Northwestern Lipid Metabolism and Diabetes Research Laboratory in the Division of Metabolism, Endocrinology, and Nutrition at the University of Washington for analysis. A duplicate analysis of samples was conducted, and the average of the two readings was used for the ultimate result. In addition, strict quality control is performed, and if a quality control sample’s value falls outside 2 standard deviations of the specified value, the sample will be repeated. For more detailed information on the S-Klotho level measurements, please visit https://wwwn.cdc.gov/Nchs/Nhanes/2007-2008/SSKL_I.htm.
Assessment of covariates
Considering insights from previous studies and clinical practice, general information, lifestyle, physical examination, laboratory data, and personal medical history were collected from the NHANES database. General information included sex, age, race, poverty-to-income ratio (PIR), education level, and marital status. The PIR was defined as the ratio of total household income to the poverty line for households with specific characteristics. Lifestyle included smoking status and alcohol consumption. Smoking status was categorized as never, former, or now based on two questions in the interview questionnaire: “Have you ever smoked less than 100 cigarettes in your lifetime?” and “Are you a current smoker?”. Alcohol consumption was categorized based on whether or not they had less than 12 drinks per year. Physical examination data included waist circumference and body mass index (BMI) calculated by dividing weight (kilogram) by the square of height(meter), according to which < 25 kg/m2 was considered low/normal weight, 25–30 kg/m2 was considered overweight, and ≥ 30 kg/m2 was considered obese. Laboratory data included fasting glucose and fasting triglyceride levels. Disease history included cardiovascular disease, chronic obstructive pulmonary disease, cancer, hypertension, diabetes, and chronic kidney disease. The first three were self-reported. Diabetes was defined as fasting glucose levels ≥ 7.0 mmol/L, random blood glucose or 2-h oral glucose tolerance test (OGTT) value ≥ 11.1 mmol/L, glycosylated Hemoglobin A1c (HbAlc) ≥ 6.5%, receiving hypoglycemic therapy, or self-reported physician diagnosis. Subjects with three consecutive measurements of systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg, self-reported physician-diagnosed hypertension, or using antihypertensive medication were classified as having hypertension. Estimated glomerular filtration rate (eGFR) ≤ 60 ml/min/1.73 m2 or a urinary albumin–creatinine ratio (ACR) ≥ 30 mg/g were used to diagnose chronic kidney disease.
Statistical analysis
The complex survey design was considered, and the fasting subsample weight was employed in this study24. Continuous variables were given as the mean ± standard error (mean ± SE) (normal distribution) or as median with interquartile range (IQR) (skewed distribution), whereas categorical variables were expressed as percentages (%). Weighted one-way analysis of variance (ANOVA) or Kruskal–Wallis tests, and chi-square tests were used to assess the differences among groups categorized by TyG index quartiles for continuous and categorical variables, respectively. Weighted multiple linear regression analyses were utilized to explore the association between the TyG index and serum S-Klotho levels, and beta (β) coefficients and 95% confidence interval (CI) were calculated. Following the STROBE statement25, we constructed three models: Model 1 adjusted for none; Model 2 adjusted for age and sex; Model 3 adjusted for age, sex, race, marital status, education level, PIR, smoking status, alcohol consumption, BMI, waist circumference, fasting glucose, fasting triglyceride, diabetes, hypertension, chronic kidney disease, cardiovascular disease, chronic obstructive pulmonary disease, and cancer. The medians of each TyG index category were also included in the models as continuous variables for trend testing. To investigate potential non-linear relationships, the study utilized smoothed curve fitting and a generalized additive model. Additionally, segmented linear regression models were utilized to test for threshold effects and calculate inflection points. To assess the impact of age, sex, and disease status (including diabetes, hypertension, cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, and cancer) on the correlations, we conducted subgroup analyses and interaction tests. All statistical analyses were conducted using R version 4.3.3 (http://www.R-project.org, The R Foundation) and EmpowerStats version 4.2.0 (www.empowerstats.com; X&Y Solutions, Inc., Boston, MA), and the significance level was set at 0.05 (two-tailed).
Ethical statement
The studies involving human participants received approval from the Research Ethics Review Board of the National Center for Health Statistics, and all participants provided written informed consent to participate in the NHANES. All methods involved in this study were performed in accordance with the relevant guidelines and regulations (Helsinki Declaration).
Results
Baseline characteristics of the study participants
A total of 5237 adults were eligible for the study, and the average age of the participants was 56.29 ± 0.21 years. Overall, 51.94% of the participants were females, and 48.06% were males. The mean ± SE of the TyG index was 8.71 ± 0.02, and the mean ± SE of serum S-Klotho levels was 845.35 ± 7.42 pg/ml. Participants were categorized into 4 groups according to TyG index quartiles: 5.65 ≤ Q1 ≤ 8.29, 8.29 < Q2 ≤ 8.68, 8.68 < Q3 ≤ 9.11, and 9.11 < Q4 ≤ 12.84, as shown in Table 1. Individuals in the highest quartile (Q4) had the lowest serum S-Klotho levels (821.62 ± 12.72 pg/ml) and PIR (3.06 ± 0.07), as well as the highest waist circumference (109.65 ± 0.72 cm), fasting glucose levels (134.38 ± 2.19 mg/dL), and fasting triglyceride levels (219.00(184.00, 282.00) mg/dL). Additionally, they were more likely to be male (55.22%), obese (57.58%), with a higher incidence of chronic diseases including diabetes (44.80%), hypertension (64.78%), cardiovascular disease (15.57%), and chronic kidney disease (23.51%). All variables, excluding marital status, alcohol consumption, chronic obstructive pulmonary disease, and cancer, displayed statistically significant variations across the four groups (all P < 0.05).
Variables associated with serum S-Klotho levels
The associations between different baseline characteristics and serum S-Klotho levels were analyzed through univariate analysis, as detailed in Supplementary Table S1. Serum levels of S-Klotho were found to be correlated with the TyG index, sex, age, race, smoking status, alcohol consumption, BMI, waist circumference, fasting glucose levels, fasting triglyceride levels, as well as the prevalence of cardiovascular disease and chronic kidney disease (all P < 0.05).
Relationship between the TyG index and the serum S-Klotho levels
The association between the serum S-Klotho levels and the TyG index was estimated using three multivariate linear regression models, as presented in Table 2. An inverse association between the TyG index and S-Klotho levels persisted in the fully adjusted model. Specifically, serum S-Klotho levels were reduced by 45.11 pg/ml for every additional unit of the TyG index (95% CI (− 79.53, − 10.69), P = 0.011). When the TyG index was divided into quartiles and the lowest quartile was chosen as the reference, a significant negative correlation between TyG and serum S-Klotho levels could also be observed in all three models in the Q3 group and Q4 group. In comparison to Q1 group participants, those in Q4 group exhibited significantly reduced serum S-Klotho levels in Model 1 [β = − 55.22, 95% CI (− 93.01, − 17.44), P = 0.005], Model 2 [β = − 47.86, 95% CI (− 85.06, − 10.66), P = 0.012] and Model 3 [β = − 50.01, 95% CI (− 91.17, − 8.86), P = 0.020]. All three models across quartiles were tested for trend, and the P values were less than 0.05.
Non-linear correlation between the TyG index and serum S-Klotho levels
The generalized additive model was conducted to explore the potential non-linear relationship between the TyG index and serum S-Klotho levels. (Fig. 2; Table 3). The adjusted smoothed plot displayed a non-linear correlation between them (Fig. 2). The segmented regression analysis revealed a turning point of 9.56 for the TyG index, with a log-likelihood ratio P value of below 0.05, confirming the statistical significance of the turning point (Table 3). Notably, for every additional unit of the TyG index below 9.56, there was a corresponding decrease of 39.20 pg/ml in the serum S-Klotho level (95% CI (− 64.30, − 14.09), P = 0.0022). Nevertheless, a notable correlation was not detected within the TyG index range of 9.56–12.84 (P > 0.05), indicating a potential saturation effect.
Smooth curve fitting for the TyG index and serum S-Klotho. The middle solid line represents the smooth curve adjustment between the variables. The dotted lines on both sides represent the matching result’s 95% Confidence interval (CI). The black and white strip at the bottom represents the population density calculated on the TyG index. The model adjusted for age, sex, race, marital status, education level, PIR, smoking status, alcohol consumption, BMI, waist circumference, fasting glucose, fasting triglyceride, diabetes, hypertension, cardiovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, and cancer.
Subgroup analysis
Subgroup analyses and interaction tests were performed to further examine the association between the TyG index and S-Klotho levels and evaluate potential factors that may influence this correlation. Subgroups were categorized based on sex (Male or Female), age (< 60 or ≥ 60 years), diabetes (Yes or No), hypertension (Yes or No), cardiovascular disease (Yes or No), chronic kidney disease (Yes or No), chronic obstructive pulmonary disease (Yes or No), and cancer (Yes or No). A significant sex-specific interaction effect was found on the association between the TyG index and serum S-Klotho levels (P for interaction < 0.001), with a more pronounced association observed in females (Fig. 3). No significant interactions were detected in other subgroups.
Subgroup analysis of the association between TyG index and serum S-Klotho. β was the effect size (pg/ml) of the change in serum S-Klotho level, and the 95% CI indicated the 95% Confidence interval. Adjusted for age, sex, race, marital status, education level, PIR, smoking status, alcohol consumption, BMI, waist circumference, fasting glucose, fasting triglyceride, diabetes, hypertension, cardiovascular disease (CVD), chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), and cancer. The strata variable was not included in the model when stratifying by itself.
Discussion
The large cross-sectional study investigated the correlation between the TyG index and serum S-Klotho levels in a nationally representative US population for the first time. It indicated a negative correlation between the TyG index and serum levels of S-Klotho in middle-aged and elderly individuals. Notably, a non-linear relationship was observed, with the TyG index turning point at 9.56. An inverse correlation existed before the turning point but not after, indicating a saturation effect on the association between the TyG index and serum S-Klotho levels. Furthermore, a significant interaction effect of gender was discovered in this relationship.
Primarily expressed in the kidney, α-Klotho protein can function as a humoral factor that mitigates oxidative stress, reduces chronic inflammation, enhances endothelial function, and safeguards blood vessels. Research has shown a negative relationship between S-Klotho levels and cardiometabolic risk, and various cardiovascular risk factors including high BMI, elevated blood lipids, drinking, and smoking26,27. Furthermore, low levels of serum S-Klotho have been closely linked to various cardiometabolic diseases (CMDs), such as cardiovascular disease, type 2 diabetes mellitus, hyperlipidemia, obesity, nonalcoholic fatty liver disease, and metabolic syndrome6. Recent studies suggested that treatment with recombinant S-Klotho can improve heart failure, decrease myocardial fibrosis, combat atherosclerosis, reduce obesity and hepatic steatosis, and alleviate type 2 diabetes mellitus and its complications, such as diabetic nephropathy, diabetic retinopathy, and diabetic cardiomyopathy6. These findings imply that Klotho may not only be a marker of aging but also a valuable indicator of cardiometabolic risk, offering protective benefits in CMDs.
As a core pathophysiological feature of cardiometabolic diseases (CMDs), insulin resistance is not only closely associated with hyperglycemia, lipid metabolism disorders, and hypertension but also promotes the development of cardiovascular disease28. Several studies have highlighted the predictive capability of the TyG index to CMDs and its strong correlation with adverse cardiovascular outcomes. Nevertheless, there is currently a gap in research on the potential association between the TyG index and the serum anti-aging protein Klotho.
Previous research has explored the correlation between S-Klotho and insulin resistance based on the homeostasis model assessment of insulin resistance (HOMA-IR). However, the findings from these studies have shown inconsistency. Ismail and colleagues conducted a study that revealed lower serum S-Klotho levels in individuals with polycystic ovary syndrome in comparison to healthy controls and found a negative correlation between serum S-Klotho levels and HOMA-IR, BMI, and waist circumference29. Similarly, serum S-Klotho levels were inversely related to albumin–creatinine ratio and HOMA-IR among individuals with type 2 diabetes mellitus and chronic kidney disease30. In addition, Amaro-Gahete et al.26 observed a positive correlation between serum S-Klotho levels and quantitative insulin sensitivity check index (QUICKI) while noting a negative correlation between serum S-Klotho levels and HOMA-IR and cardiometabolic risk in a healthy population aged 40–65 years. In contrast, Socha-Banasiak et al.31 reported that serum S-Klotho levels were higher in children with insulin resistance than insulin-sensitive children. Recent studies revealed a positive association between serum S-Klotho levels and HOMA-IR, as well as the incidence of insulin resistanc32,33. Furthermore, Amaro-Gahete et al.26 and Agnieszka et al.34 reported no correlation between serum S-Klotho and insulin resistance in healthy young adults aged 18–25 and healthy men aged 30–45, respectively. Discrepancies in these results may be attributed to factors such as age, health status, sample size, and study design, which further emphasizes the importance of conducting large-scale longitudinal studies to verify this association. The current study included 5237 adults aged 40–79 years and revealed that serum levels of S-Klotho were negatively related to the TyG index, a reliable marker of insulin resistance. However, Given the absence of serum S-Klotho data in the NHANES database for individuals under 40 years old, additional research is necessary to test the correlation between the TyG index and serum S-Klotho within this specific age group.
Klotho has diverse physiological functions and a complex role in metabolic regulation. Previous experimental studies have presented conflicting results regarding the impact of Klotho on insulin resistance. For instance, Kurosu et al.35 reported Klotho inhibited insulin/ insulin-like growth factor-I (IGF-1) signaling and Klotho overexpression induced insulin resistance. In addition, Hasannejad et al.36 proposed a mechanism where Klotho may induce insulin resistance in adipocytes by interfering with insulin-mediated signaling pathways. Conversely, a recent study in type 2 diabetes mellitus mice showed that S-Klotho overexpression improved insulin resistance status and myocardial fibrosis in diabetic cardiomyopathy37. This finding aligns with our research indicating that S-Klotho may act as a hormone that inhibits the onset of insulin resistance, emphasizing the therapeutic promise of S-Klotho as a target for metabolic disorders. However, Olivier et al. found that S-Klotho could not directly inhibit insulin/IGF-1 signaling, and whether Klotho indirectly regulates insulin sensitivity through the activation of fibroblast growth factor 23 (FGF23) remains to be investigated38.To sum up, how Klotho regulates the balance of insulin resistance or insulin sensitivity is unclear, and more evidence is needed to clarify relevant mechanisms. Notably, the above Klotho-related studies were conducted in animal models, and caution is required when extending them to humans.
The study found a significant association between high TyG index and lower serum S-Klotho levels, which was analyzed for several reasons. First, Klotho can reduce inflammation and oxidative stress by regulating pro-inflammatory cytokines and participating in the oxidative stress buffer system39,40. These effects contribute to insulin sensitivity and influence the TyG index. Moreover, increased endothelial Na channel (EnNaC) activity leads to endothelial dysfunction through impaired endothelial nitric oxide (NO) release and increased oxidative stress-mediated NO destruction in the state of insulin resistance41. On the other hand, S-Klotho reduces and prevents endothelial dysfunction by attenuating vascular calcification, increasing endothelial NO production, and downregulating endothelial cell inflammatory mediators42. Finally, the TyG index combines fasting glucose and triglyceride levels, with high values reflecting disturbed glucose and lipid metabolism. Research has demonstrated that Klotho is involved in regulating glucose and lipid metabolism through a variety of mechanisms, such as modulating the expression of TRPV2 to enhance insulin secretion and regulating the activity of peroxisome proliferator-activated receptor (PPAR)-γ43. In addition, S-Klotho interacts with the insulin-like growth factor-I (IGF-1) receptor to regulate signaling and increase PPAR-α activity, thereby improving hepatic glucose-lipid homeostasis and insulin sensitivity44. The present finding indicated that S-Klotho appears to be a hormone that slows insulin resistance in middle-aged and older adults. However, as the metabolic functions and molecular mechanisms of Klotho are complex, further research is required to understand its effects fully.
The current study also discovered a non-linear correlation and saturation phenomenon between the TyG index and serum S-Klotho levels, with a turning point of 9.56. It seems consistent with the results of another NHANES database-based study in which the visceral obesity index (VAI) was non-linearly and negatively correlated with serum S-Klotho, showing a saturation effect45. The VAI has been reported to not only assess visceral obesity dysfunction but also identify insulin-resistant individuals46. The above studies illustrated the correlation between serum S-Klotho and insulin resistance from varying perspectives and provided new insights for the study of insulin resistance and aging. Nevertheless, further mechanistic exploration is warranted to verify the reasons behind the saturation effect.
The study demonstrated a gender interaction between the TyG index and serum S-Klotho levels, with a more pronounced association observed in females. This finding aligns with previous research, such as a large study on the US population, which showed that metabolic syndrome was significantly negatively correlated with serum S-Klotho, particularly in females47. However, the mechanism of sex differences in the relationship between the TyG index and serum S-Klotho levels remains unclear, with one possible explanation being differences in sex hormones. It has been revealed that testosterone increases the expression of the Klotho gene through an androgen receptor-mediated pathway48. Furthermore, research has linked low testosterone levels in men not only to insulin resistance but also to conditions such as Alzheimer’s disease, cardiovascular disease, and accelerated organ aging49. Therefore, it can be hypothesized that the correlation between the TyG index and S-Klotho levels in men may be influenced by testosterone. Another possible reason is gender variations in the regulation of free radical balance, as shorter-lived female mice have been shown to exhibit increased oxidative stress50. It is worth noting that both Klotho and insulin resistance are linked to oxidative stress.
There is no denying that the study has some limitations that need to be considered. First, the causal relationship between the TyG index and serum S-Klotho levels may not be established because this was a cross-sectional study. Second, like other epidemiologic investigations, residual confounding variables may affect the association despite efforts to adjust for multiple confounding variables. For example, medications (e.g., PPAR-γ agonists, renin-angiotensin system blockers, statins) and vitamin D levels affect serum S-Klotho levels51. However, these potentially relevant variables were not included in this study. Third, data on the TyG index and S-Klotho were gathered at a single time point, preventing dynamic monitoring. Despite these limitations, the study is meaningful as it first reported the association between the TyG index and serum S-Klotho levels, revealing a non-linear association and identifying a turning point. Moreover, the study uncovered a sex interaction between the TyG index and serum S-Klotho levels, emphasizing the importance of further research to understand the underlying mechanisms involved. Finally, the study employed a sizable sample size and implemented a weighted design to reduce potential bias.
Conclusion
This study indicated a non-linear correlation and saturation effect between the TyG index and the serum S-klotho in middle-aged and older Americans. Serum S-klotho levels decreased significantly with increasing TyG index (TyG index < 9.56). The TyG index can serve as a potential indicator of serum levels of the anti-aging protein Klotho, in addition to reflecting insulin resistance. Furthermore, the relationship between the TyG index and S-klotho levels is more pronounced in females, indicating a potential sex difference. Further studies are required to investigate the mechanisms behind this observed association.
Data availability
All data analyzed in the current study are available at https://www.cdc.gov/nchs/nhanes/index.htm.
References
Kuro-o, M. et al. Mutation of the mouse Klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51. https://doi.org/10.1038/36285 (1997).
Xu, Y. & Sun, Z. Molecular basis of Klotho: From gene to function in aging. Endocr. Rev. 36, 174–193. https://doi.org/10.1210/er.2013-1079 (2015).
Abraham, C. R. & Li, A. Aging-suppressor Klotho: Prospects in diagnostics and therapeutics. Ageing Res. Rev. 82, 101766. https://doi.org/10.1016/j.arr.2022.101766 (2022).
Veronesi, F., Borsari, V., Cherubini, A. & Fini, M. Association of Klotho with physical performance and frailty in middle-aged and older adults: A systematic review. Exp. Gerontol. 154, 111518. https://doi.org/10.1016/j.exger.2021.111518 (2021).
Prud’homme, G. J., Kurt, M. & Wang, Q. Pathobiology of the Klotho antiaging protein and therapeutic considerations. Front. Aging 3, 931331. https://doi.org/10.3389/fragi.2022.931331 (2022).
Liu, Y. & Chen, M. Emerging role of α-Klotho in energy metabolism and cardiometabolic diseases. Diabetes Metab. Syndr. 17, 102854. https://doi.org/10.1016/j.dsx.2023.102854 (2023).
Zhang, L. & Liu, T. Clinical implication of alterations in serum Klotho levels in patients with type 2 diabetes mellitus and its associated complications. J. Diabetes Complicat. 32, 922–930. https://doi.org/10.1016/j.jdiacomp.2018.06.002 (2018).
Corcillo, A. et al. Low levels of circulating anti-ageing hormone Klotho predict the onset and progression of diabetic retinopathy. Diabetes. Vasc. Dis. Res. 17, 1479164120970901. https://doi.org/10.1177/1479164120970901 (2020).
Pan, H. C., Chou, K. M., Lee, C. C., Yang, N. I. & Sun, C. Y. Circulating Klotho levels can predict long-term macrovascular outcomes in type 2 diabetic patients. Atherosclerosis 276, 83–90. https://doi.org/10.1016/j.atherosclerosis.2018.07.006 (2018).
Yuguang, L. et al. Serum klotho as a novel biomarker for metabolic syndrome: Findings from a large national cohort. Front. Endocrinol. (Lausanne) 15, 1295927. https://doi.org/10.3389/fendo.2024.1295927 (2024).
Rao, Z. et al. Administration of alpha klotho reduces liver and adipose lipid accumulation in obese mice. Heliyon 5, e01494. https://doi.org/10.1016/j.heliyon.2019.e01494 (2019).
Li, X., Li, Z., Li, B., Zhu, X. & Lai, X. Klotho improves diabetic cardiomyopathy by suppressing the NLRP3 inflammasome pathway. Life Sci. 234, 116773. https://doi.org/10.1016/j.lfs.2019.116773 (2019).
Lee, J. et al. Klotho ameliorates diabetic nephropathy via LKB1-AMPK-PGC1α-mediated renal mitochondrial protection. Biochem. Biophys. Res. Commun. 534, 1040–1046. https://doi.org/10.1016/j.bbrc.2020.10.040 (2021).
Yaribeygi, H., Farrokhi, F. R., Butler, A. E. & Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell Physiol. 234, 8152–8161. https://doi.org/10.1002/jcp.27603 (2019).
Borai, A., Livingstone, C. & Ferns, G. A. The biochemical assessment of insulin resistance. Ann. Clin. Biochem. 44, 324–342. https://doi.org/10.1258/000456307780945778 (2007).
Katz, A. et al. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 85, 2402–2410. https://doi.org/10.1210/jcem.85.7.6661 (2000).
Rabasa-Lhoret, R. & Laville, M. How to measure insulin sensitivity in clinical practice?. Diabetes Metab. 27, 201–208 (2001).
Guerrero-Romero, F. et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 95, 3347–3351. https://doi.org/10.1210/jc.2010-0288 (2010).
Vasques, A. C. et al. TyG index performs better than HOMA in a Brazilian population: A hyperglycemic clamp validated study. Diabetes Res. Clin. Pract. 93, e98–e100. https://doi.org/10.1016/j.diabres.2011.05.030 (2011).
Kurniawan, L. B. Triglyceride–glucose index as a biomarker of insulin resistance, diabetes mellitus, metabolic syndrome and cardiovascular disease: A review. EJIFCC 35, 44–51 (2024).
Zheng, R., Du, Z., Wang, M., Mao, Y. & Mao, W. A longitudinal epidemiological study on the triglyceride and glucose index and the incident nonalcoholic fatty liver disease. Lipids Health Dis. 17, 262. https://doi.org/10.1186/s12944-018-0913-3 (2018).
Majumdar, V. & Christopher, R. Association of exonic variants of Klotho with metabolic syndrome in Asian Indians. Clin. Chim. Acta 412, 1116–1121. https://doi.org/10.1016/j.cca.2011.02.034 (2011).
Freathy, R. M. et al. The functional “KL-VS” variant of KLOTHO is not associated with type 2 diabetes in 5028 UK Caucasians. BMC Med. Genet. 7, 51. https://doi.org/10.1186/1471-2350-7-51 (2006).
Johnson, C. L. et al. National health and nutrition examination survey: Analytic guidelines, 1999–2010. Vital Health Stat. 2, 1–24 (2013).
Vandenbroucke, J. P. et al. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 12, 1500–1524. https://doi.org/10.1016/j.ijsu.2014.07.014 (2014).
Amaro-Gahete, F. J. et al. Relationship between plasma S-Klotho and cardiometabolic risk in sedentary adults. Aging (Albany NY) 12, 2698–2710. https://doi.org/10.18632/aging.102771 (2020).
Lee, J. et al. Association between serum klotho levels and cardiovascular disease risk factors in older adults. BMC Cardiovasc. Disord. 22, 442. https://doi.org/10.1186/s12872-022-02885-2 (2022).
DeFronzo, R. A. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: The missing links. The Claude Bernard Lecture 2009. Diabetologia 53, 1270–1287. https://doi.org/10.1007/s00125-010-1684-1 (2010).
Biyik, I. et al. Comparison of serum human Klotho levels and thiol/disulfide homeostasis in women with polycystic ovary syndrome and in healthy women. Taiwan J. Obstet. Gynecol. 60, 487–491. https://doi.org/10.1016/j.tjog.2021.03.017 (2021).
Silva, A. P. et al. Klotho levels: Association with insulin resistance and albumin-to-creatinine ratio in type 2 diabetic patients. Int. Urol. Nephrol. 49, 1809–1814. https://doi.org/10.1007/s11255-017-1646-3 (2017).
Socha-Banasiak, A. et al. Klotho and fibroblast growth factors 19 and 21 serum concentrations in children and adolescents with normal body weight and obesity and their associations with metabolic parameters. BMC Pediatr. 20, 294. https://doi.org/10.1186/s12887-020-02199-2 (2020).
Yan, L., Hu, X., Wu, S. & Zhao, S. Serum Klotho and insulin resistance: Insights from a cross-sectional analysis. Medicine (Baltimore) 103, e37971. https://doi.org/10.1097/md.0000000000037971 (2024).
Xiao, Y., Hou, Y., Zeng, J., Gong, Y. & Ma, L. Association between the serum α-Klotho level and insulin resistance in adults: NHANES 2007–2016. Endocr. Res. https://doi.org/10.1080/07435800.2024.2350428 (2024).
Żelaźniewicz, A., Nowak-Kornicka, J. & Pawłowski, B. S-Klotho level and physiological markers of cardiometabolic risk in healthy adult men. Aging (Albany NY) 14, 708–727. https://doi.org/10.18632/aging.203861 (2022).
Kurosu, H. et al. Suppression of aging in mice by the hormone Klotho. Science 309, 1829–1833. https://doi.org/10.1126/science.1112766 (2005).
Hasannejad, M., Samsamshariat, S. Z., Esmaili, A. & Jahanian-Najafabadi, A. Klotho induces insulin resistance possibly through interference with GLUT4 translocation and activation of Akt, GSK3β, and PFKfβ3 in 3T3-L1 adipocyte cells. Res. Pharm. Sci. 14, 369–377. https://doi.org/10.4103/1735-5362.263627 (2019).
Li, J. M. et al. Soluble Klotho-integrin β1/ERK1/2 pathway ameliorates myocardial fibrosis in diabetic cardiomyopathy. FASEB J. 35, e21960. https://doi.org/10.1096/fj.202100952R (2021).
Lorenzi, O. et al. Evidence against a direct role of klotho in insulin resistance. Pflugers Arch. 459, 465–473. https://doi.org/10.1007/s00424-009-0735-2 (2010).
Dalton, G. D., Xie, J., An, S. W. & Huang, C. L. New insights into the mechanism of action of soluble Klotho. Front. Endocrinol. (Lausanne) 8, 323. https://doi.org/10.3389/fendo.2017.00323 (2017).
Landry, T., Shookster, D. & Huang, H. Circulating α-klotho regulates metabolism via distinct central and peripheral mechanisms. Metabolism 121, 154819. https://doi.org/10.1016/j.metabol.2021.154819 (2021).
Hill, M. A., Jaisser, F. & Sowers, J. R. Role of the vascular endothelial sodium channel activation in the genesis of pathologically increased cardiovascular stiffness. Cardiovasc. Res. 118, 130–140. https://doi.org/10.1093/cvr/cvaa326 (2022).
Wang, Y. & Sun, Z. Current understanding of klotho. Ageing Res. Rev. 8, 43–51. https://doi.org/10.1016/j.arr.2008.10.002 (2009).
Lin, Y. & Sun, Z. Antiaging gene Klotho enhances glucose-induced insulin secretion by up-regulating plasma membrane levels of TRPV2 in MIN6 β-cells. Endocrinology 153, 3029–3039. https://doi.org/10.1210/en.2012-1091 (2012).
Gu, H. et al. Soluble klotho improves hepatic glucose and lipid homeostasis in type 2 diabetes. Mol. Ther. Methods Clin. Dev. 18, 811–823. https://doi.org/10.1016/j.omtm.2020.08.002 (2020).
Cui, J. et al. A cross-sectional analysis of association between visceral adiposity index and serum anti-aging protein Klotho in adults. Front. Endocrinol. (Lausanne) 14, 1082504. https://doi.org/10.3389/fendo.2023.1082504 (2023).
Amato, M. C. et al. Visceral adiposity index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 33, 920–922. https://doi.org/10.2337/dc09-1825 (2010).
Orces, C. H. The association between metabolic syndrome and the anti-aging humoral factor klotho in middle-aged and older adults. Diabetes Metab. Syndr. 16, 102522. https://doi.org/10.1016/j.dsx.2022.102522 (2022).
Hsu, S. C. et al. Testosterone increases renal anti-aging klotho gene expression via the androgen receptor-mediated pathway. Biochem. J. 464, 221–229. https://doi.org/10.1042/bj20140739 (2014).
Elenkov, A., Al-Jebari, Y., Giwercman, Y. L. & Giwercman, A. Testosterone replacement therapy in men who conceived with intracytoplasmic sperm injection: Nationwide register study. Eur. J. Endocrinol. 182, 423–428. https://doi.org/10.1530/eje-19-0734 (2020).
Ali, S. S. et al. Gender differences in free radical homeostasis during aging: Shorter-lived female C57BL6 mice have increased oxidative stress. Aging Cell 5, 565–574. https://doi.org/10.1111/j.1474-9726.2006.00252.x (2006).
Poursistany, H., Azar, S. T., Azar, M. T. & Raeisi, S. The current and emerging Klotho-enhancement strategies. Biochem. Biophys. Res. Commun. 693, 149357. https://doi.org/10.1016/j.bbrc.2023.149357 (2024).
Acknowledgements
We are grateful to all NHANES participants and staff for their contributions and to the NHANES databases for providing accessible data.
Author information
Authors and Affiliations
Contributions
L.X.L. designed the study and wrote the manuscript. L.X.L. collected, analyzed, and interpreted the data. T.C. reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lai, X., Chen, T. Association between the triglyceride–glucose index and serum soluble Klotho in middle-aged and older adults from NHANES 2007–2016. Sci Rep 14, 18408 (2024). https://doi.org/10.1038/s41598-024-69226-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69226-5
- Springer Nature Limited