Abstract
Diabetic nephropathy, characterized by inflammation and oxidative stress, poses a management challenge. This study investigates the effect of Polygonum hyrcanicum extract on diabetic nephropathy in alloxan-induced diabetic mice. In this experimental animal study, the P. hyrcanicum extract was prepared using continuous macerations. Thirty male Albino mice, divided into five groups, were induced with alloxan-induced diabetes. They received intraperitoneal injections of the plant extract (100 and 200 mg/kg) and metformin (300 mg/kg) for four weeks. Kidney and blood samples were collected to assess protein carbonyl, glutathione, lipid peroxidation, TNF-α and IL-6 levels. The amount of total flavonoid and phenolic content in the hydroalcoholic extract of P. hyrcanicum were 7.5 ± 0.3 mg of quercetin and 88.2 ± 1.3 mg gallic acid per gram of extract respectively. The antioxidant activity level of the hydroalcoholic extract was determined to be 1.78 ± 0.51 mM equivalent per gram of extract. Alloxan administration resulted in a significant reduction in glutathione levels and a significant increase in protein carbonyl, lipid peroxidation, TNF-α, and IL-6 levels. Hydroalcoholic extract of P. hyrcanicum effectively reduced oxidative stress markers and inflammatory cytokines (TNF-α, IL-6), indicating its potential in mitigating diabetic nephropathy. However, no significant difference in efficacy was observed between the 100 mg/kg and 200 mg/kg doses in terms of reducing these toxicities.
Similar content being viewed by others
Introduction
Diabetes mellitus is a metabolic disorder characterized by hyperglycemia and metabolic changes in fat metabolism, primarily due to insufficient production of insulin by the beta cells of the pancreatic islets of Langerhans (Type 1 diabetes mellitus) or resistance of cells to insulin (Type 2 diabetes mellitus). This prevalent disease has created significant detrimental effects on society, incurring both direct and indirect costs. Complications of diabetes include both early-stage complications (diabetic ketoacidosis, hyperosmolar coma, hypoglycemia, and hyperglycemia) and long-term complications (retinopathy, nephropathy, neuropathy, cardiovascular diseases). While emergency interventions can effectively manage early-stage complications, long-term complications may persist despite appropriate medication usage1,2.
Nephropathy is one of the most significant complications of diabetes, occurring as a result of increased gene expression and activation of inflammatory factors, including various cytokines such as TNF-α, interleukins, especially interleukin-6, NFκB, and others, and their impact on the sensory, motor, and autonomic nervous system. The factor responsible for inflammation and subsequent neuropathy and nephropathy in diabetic individuals is the alteration of cytokine factors' patterns in diabetes that occur long after the onset of the disease. Prolonged elevation of blood glucose concentration activates inflammatory cells in the kidney and leads to nephropathy3,4.
Some evidence suggests that oxidative stress is one of the notable mechanisms involved in diabetic nephropathy. The elevated production of oxidant species within the glomerular microcirculation leads to a decrease in the availability of nitric oxide (NO), primarily due to the uncoupling of endothelial nitric oxide synthase (eNOS) resulting in the rise of oxidative stress-induced inflammation, endothelial dysfunction, and the detachment of podocytes from the glomerular capillaries, accompanied by an increase in glomerular permeability5. Nowadays, despite the abundance and often high impact of chemical drugs, the use of herbal medicines is increasing. However, this is mainly due to the side effects of long-term use of chemical drugs and occasional short-term use.
Phytochemicals, such as polyphenols and flavonoids found abundantly in plant-based foods, exhibit antioxidant properties that can mitigate oxidative stress, a key contributor to diabetic nephropathy. Studies have demonstrated that these bioactive compounds possess the ability to scavenge free radicals, inhibit inflammatory pathways, and modulate oxidative stress markers. By targeting oxidative stress-induced damage to renal cells and inflammation, phytoconstituents offer a promising avenue for the prevention and treatment of diabetic nephropathy6,7.
Various plants have been identified and studied for their effects on diabetes, and some of these herbal plants have entered the market as medicinal products. In addition to reducing blood glucose, some of these herbal plants contribute to controlling and improving diabetes-related complications such as various inflammations8,9.
Polygonum hyrcanicum Rech.f. commonly known as “Haftband” in Persian, is a plant that has shown anti-diabetic effects. P. hyrcanicum is an endemic species that grows widely in northern areas of Iran. It belongs to the Polygonaceae family. This plant exhibits unique astringent effects and is often rich in flavonoids, quinones, anthraquinones, and tannins10,11,12,13. According to traditional Iranian medicine texts, this plant has a cool and dry nature. It has been reported to be used for preventing bleeding due to excessive coagulability, treating inflammation and wounds, and alleviating gastric inflammation. Moreover, this plant has blood sugar-lowering and anti-diabetic effects14.
The efficacy of the Polygonum multiflorum plant in glycation processes related to diabetes demonstrated its effectiveness in reducing diabetic complications15. Similarly, the aqueous extract of Rheum ribes L. from the Polygonaceae family for treating diabetic mice induced by alloxan decreased blood sugar levels16.
Given these findings, this study aims to investigate the effects of P. hyrcanicum extract on diabetic nephropathy in male Albino mice induced by alloxan and to explore the therapeutic potential of P. hyrcanicum in mitigating oxidative stress and inflammation, which are key factors in the progression of diabetic nephropathy, thereby providing a possible natural treatment option for managing this condition.
Materials and methods
Chemicals and materials
Mannitol, Sucrose, Monopotassium phosphate(KH2PO4), Ethylenediaminetetraacetic acid (EDTA), Phosphoric acid, Disodium hydrogen phosphate, Methanol, aluminum chloride, Thiobarbituric acid (TBA), HCl, Trichloro acetic acid (TCA), Folin–Ciocâlteu, 2, 4, 6-tripyridyl-s-triazine (TPTZ), Acetic acid (glacial), quercetin, Gallic acid, Sodium carbonate, Potassium acetate, Sodium acetate, Iron trichloride, Ferrous sulfate, Dimethyl sulfoxide (DMSO), and n-butanol were purchased from Merck(Germany). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), Alloxan monohydrate, Metformin, and Tris–HCl were purchased from Sigma Aldrich (USA). IL-6 and TNF-α detection kits were purchased from Thermo Fisher Scientific (USA). Potassium Chloride, normal saline, methanol, and all other chemicals and solvents were of the highest grade commercially available.
Collection and extraction
Polygonum hyrcanicum were collected from the Caspian Hyrcanian forests (Hezar Jarib-Neka), Mazandaran, Iran. The voucher specimen (MAZ‑E1-17-141) was deposited in the herbarium of Mazandaran University of medical sciences. Subsequently, the whole aerial part of the plant material was air-dried under shade at room temperature before manual grinding using a grinder. The preparation of the extract involved continuous macerations using n-Hexane and 80% methanol solvents. Initially weighing approximately 500 g, the ground plant material underwent a decanting procedure where a cotton pad was placed at the bottom of the flask to facilitate successful extraction. n-Hexane, the preferred solvent, was employed to remove lipid compounds and pigments. The extraction process involved pouring n-Hexane over the plant in a decanter flask, allowing it to exceed the plant level by 2 cm. After 48 h extract was collected, and the n-Hexane solvent was replaced with fresh solvent for three repeated cycles. Subsequently, the residual plant material was soaked in an 80% methanol solution using a maceration technique similar to the previous extractions, resulting in the formation of alcoholic solutions for further analysis of residue content. The hydroalcoholic extracts were concentrated using a rotary evaporator. The concentrated extracts were then subjected to freeze-drying at − 45 °C to obtain a powdered extract. Finally, the powdered extracts were stored in glass containers in a refrigerator, away from heat and light sources. Finally, the powdered extracts were stored in glass containers in a refrigerator, away from heat and light sources.
Experimental design
A total of 30 albino mice, each weighing between 20 and 25 g and aged between 8 and 10 weeks, were obtained from the Animal Research Institute at Mazandaran University of Medical Sciences in Sari, Iran. The mice were housed for two weeks in standard conventional cages, maintaining a controlled temperature of 25 ± 1°C and a 12-h light/dark cycle. Food and water were available ad libitum. All experimental procedures adhered to guidelines approved by the Research Ethics Committee of Mazandaran University of Medical Sciences, Iran (IR.Mazums.Rec.1396.2922).
Diabetes was induced in the albino mice by injecting alloxan. Alloxan was dissolved in normal saline and administered intraperitoneally at a dose of 150 mg/kg. Blood glucose levels were measured before the experiment and one week after the alloxan injection. Mice with blood glucose levels higher than 200 mg/dl were considered diabetic17.
After confirming diabetes and determining the effective dose of the extract, the animals were randomly divided into five groups (Groups I–V, n = 6 for each group):
-
1.
Healthy mice receiving normal saline (Group I).
-
2.
Diabetic mice receiving normal saline as a negative control (Group II).
-
3.
Diabetic mice receiving metformin (300 mg/kg) as a positive control (Group III).
-
4.
Diabetic mice receiving a hydroalcoholic extract (100 mg/kg) of the plant (Group IV).
-
5.
Diabetic mice receiving a hydroalcoholic extract (200 mg/kg) of the plant (Group V)18.
The extract and metformin were administered via intraperitoneal injection for four weeks after confirming the induction of diabetes. Metformin may exert protective effects on the kidneys, making it a relevant comparator for evaluating the extract's efficacy19. After the treatment period, the mice were euthanized, and their kidneys and blood samples were collected for evaluating oxidative stress parameters, determining urea and creatinine levels, and measuring the inflammatory factors interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α).
Measurement of total phenolic content
Total phenol content was determined by the Folin-Ciocalteu reagent, standard of gallic acid, using spectrophotometry method and standard curve drawing with some modifications20. A mixture containing 25 mL of distilled water, 1 mL of the sample, 5 mL of Folin–Ciocalteu's reagent, and 4 mL of 6% Na2CO3 was prepared. The mixture was incubated at room temperature for 60 min, and then the absorbance was measured at 765 nm against a blank sample. The quantification was performed based on the standard curve of gallic acid. The results were reported as milligrams of gallic acid equivalents (GAE) per gram of dried extracts.
Measurement of total tannin content
Tannins were extracted from the sample, and the remaining phenolic compounds measured using the Folin–Ciocalteu method. By having the levels of total phenols and phenols in tannin-free samples, the amount of total tannins can be calculated21. Polyvinylpolypyrrolidone (PVPP) is one of the binding agents for tannins.
A 100 mg quantity of PVPP was weighed and placed in a 100 × 12 mm test tube. Following this, 1.0 mL of distilled water and 1.0 mL of the tannin-containing extract were added to the tube. The mixture was vortexed and then kept at 4 °C for 15 min. After vortexing again, the tube was centrifuged at 3000 × g for 10 min, and the supernatant was collected. The collected supernatant contains only simple phenolics other than tannins, as the tannins would have precipitated along with the PVPP. The phenolic content of the supernatant was measured as previously described, ensuring to take at least double the volume (preferably three times) used for total phenol estimation due to the two-fold dilution of the extract and expected loss of tannin-phenols through binding with PVPP. Finally, the content of non-tannin phenols was expressed on a dried matter basis.
Measurement of total flavonoid content
The total flavonoid content was assessed using the aluminum chloride colorimetric method with slight modifications22. A calibration curve was created using quercetin, where 10 mg of the substance were dissolved in 80% ethanol and then diluted to concentrations of 25, 50, and 100 μg/mL. Each standard solution was mixed with a solution consisting of 0.5 mL of the diluted solution, 1.5 mL of 95% ethanol, 0.1 mL of 10% aluminum chloride, 0.1 mL of 1M potassium acetate, and 2.8 mL of distilled water individually.
After a 30 min incubation period at room temperature, the absorbance of the reaction mixture was measured using a UV–Vis spectrophotometer at 415 nm. Distilled water was used as a blank solution, replacing the equivalent amount of 10% aluminum chloride. The quantification of flavonoid content was carried out by reacting ethanol extracts with fifteen standard flavonoid solutions, each at a concentration of 100 ppm, using aluminum chloride according to the previously described protocol.
Measurement of FRAP
The determination of total antioxidant activity (TAC) was performed using the FRAP (ferric-reducing ability of plasma) method which is based on the reduction of \({Fe}^{III}\) (ferric) ions to \({Fe}^{II}\) (ferrous) ions by biological fluids in the presence of a substance called TPTZ. In this method, serum samples (50 µL) were mixed with 1.5 mL of the FRAP reagent (consisting of 25 mL of 300 mM acetate buffer (pH 3.6), 2.5 mL of 10 mM TPTZ solution in 40 mM HCl, and 2.5 mL of 20 mM FeCl3) using vortexing. The samples, along with blank tubes, were then incubated in a water bath at 37 °C for 5 min. The absorbance of the samples was measured at a wavelength of 593 nm. A stock solution was prepared to create a standard curve, with stock solutions of FeSO4⋅7H2O at concentrations of 125, 250, 500, 750, and 1000 µM in an aqueous solution. The values obtained from the curve were expressed as mM Fe (II)/g dried mass23.
Measurement of oxidative stress parameters
Measurement of protein carbonyl content
Protein carbonyl contents were determined using a spectrophotometric method. Initially, 250 μg of kidney tissues were homogenized, and the protein contents of all groups underwent precipitation with 500 μL of cold TCA 20% and were then separated through centrifugation at 6500 × g for 15 min. Subsequently, 500 μL of 10 mM DNPH in 500 μL of 2M HCl was added to the pellet of each group, and the samples were kept in a dark chamber for 30 min at room temperature. During this time, vortexing was performed at 10-min intervals. Once again, protein precipitation was carried out using TCA 20%, followed by centrifugation at 6500 × g for 10 min. The resulting protein-containing pellets were collected and subjected to three washes with ethanol/ethyl acetate (1:1) to remove free DNPH. The protein contents were then solubilized by resuspending the samples in 6M Guanidine hydrochloride. Finally, the optical absorption was measured at 365 nm absorbance24.
Measurement of lipid peroxidation
The assessment of lipid peroxidation involved measuring the formation of thiobarbituric acid reactive substances (TBARs). The concentration of malondialdehyde (MDA) in μM served as the indicator for lipid peroxidation. A 0.2 mL of tissue homogenates were treated with 0.1 mL Thiobarbituric acid (TBA) reagent (consisting of 15%w/v TCA and 0.3%w/v TBA in 0.5 N HCl) and appropriately vortexed. The samples were then incubated in boiling water for 30 min. Following this, the samples were transferred to an ice bath, and, 0.2 mL n-butanol was added. Finally, the n-butanol layer was separated through centrifugation at 1500 × g for 10 min, and the absorbance of the n-butanol layer was measured at a wavelength of 532 nm, and the amount of TBARs was calculated from the standard curve25.
Measurement of glutathione (GSH) concentration
The reduced GSH content was assessed by employing 5,5ʹ-dithiobis-(2-nitrobenzoic acid) (DTNB) as the indicator. For each group, samples were individually mixed with TCA 20% to precipitate protein contents. Subsequently, the mixtures underwent centrifugation at 1000 × g for 20 min. The resulting supernatants were then combined with 0.3M Disodium hydrogen phosphate and DTNB 40%. The interaction between DTNB and the sulfhydryl groups present in the glutathione structure led to the formation of a detectable yellow thiolate anion group. Finally, the absorbance for each group was measured at 412 nm using a spectrophotometer, and GSH concentration was quantified in μM26.
HPLC analysis of the extract
High-Performance Liquid Chromatography (HPLC) analysis was conducted to determine the quercetin component of the extract. The content quantification was carried out using a calibration curve equation, and the results were reported as milligrams per gram of dried weight (mg/g DW) of the extract27.
The chromatographic assay used a 12.5 cm × 4 mm with a pre-column, KNAUER LPG system. Two solvents, solvent A (methanol, v/v) and solvent B (0.3 g/L phosphoric acid solution adjusted to PH 2.0, v/v), were used. The method utilized a linear gradient elution with the following parameters: 0–1 min (60% → 40% B), 1–2 min (45 → 55% B), 20–21 min (0% → 100% B), and 21–25 min (100% B). The flow rate and column temperature were maintained at 1.0 mL/min and 25 °C, respectively. Detection occurred at a fixed wavelength of 370 nm, monitored for 25 min per sample. Using a Hamilton syringe, 20 µL of the prepared solution were injected into the HPLC apparatus, and the quercetin content of the extract was calculated.
Inflammation evaluation
Measurement of TNF-α and IL-6 levels
The quantitative assessment of TNF-α and IL-6 levels in kidney tissue was conducted through enzyme-linked immunosorbent assay (ELISA) using mice-specific ELISA kits. The absorbance of the final colored product was measured at 450 nm as the primary wavelength, with a reference wavelength of 620 nm. The levels of these cytokines were expressed as picograms per milligram (pg/mg)28.
Statistical analysis
The results were presented as mean ± standard deviation (SD). Statistical analyses were conducted using SPSS software, version 17. All assays were carried out in triplicate, and the mean values were utilized for statistical analysis. The one-way ANOVA test was employed, followed by the post hoc Tukey test to determine statistical significance. Significance was set at P < 0.05.
Ethics approval
All experimental procedures adhered to the guidelines approved by the Research Ethics Committee of Mazandaran University of Medical Sciences, Iran (IR.Mazums.Rec.1396.2922).
Results
This study examined the impact of different concentrations (200 mg/kg, 100 mg/kg) of the “P. hyrcanicum” plant extract on various parameters in diabetic Albino mice.
Quantification of phytoconstituents
The quantification of total phenolic content in the hydroalcoholic extract of P. hyrcanicum was carried out using a standard curve (y = 0.010x + 0.018, \({r}^{2}\) = 0.997). The average value with standard deviation was found to be 88.2 ± 1.3 mg gallic acid per gram of extract.
By determining the levels of total phenols and the phenols in tannin-free samples (using PVPP), the total tannin content was calculated to be 27.6 ± 0.8 mg per gram of dried extract.
The amount of total flavonoid content in the hydroalcoholic extract of P. hyrcanicum was carried out using a standard curve (y = 0.008x − 0.034, \({r}^{2}\) = 0.997). The average value with standard deviation is 7.5 ± 0.3 mg of quercetin per gram of dried extract.
HPLC analysis
The concentration of quercetin in the sample was determined using HPLC analysis, calibrated against a standard curve (y = 13945x + 4E + 06, \({r}^{2}\) = 0.992). The area under the curve for the quercetin peak in the sample showed the retention time in 25.367 min. Based on the standard curve, the calculated quercetin level in the sample was found to be 0.003% of dried extract. This value represents the mean of triplicate measurements, with a standard deviation of ± 0.001%. The HPLC method demonstrated high precision and sensitivity in quantifying quercetin levels in the sample (Fig. 1).
FRAP assay
The FRAP assay is a commonly used method for assessing the total antioxidant capacity of serum samples. The values were obtained from the standard ferrous (Fe2+) sulfate curve (y = 0.0007x + 0.015, \({r}^{2}\) = 0.998). The antioxidant activity level of the hydroalcoholic extract of P. hyrcanicum was determined to be 1.78 ± 0.51 mM equivalent per gram of extract.
Blood glucose level
The blood glucose levels were measured in the experimental groups, including diabetic mice, and after the administration of the hydroalcoholic extract at different concentrations of 200 mg/kg, 100 mg/kg, and 300 mg/kg of metformin. Table 1 shows the blood glucose levels in mg/dl as mean ± standard deviation for the different groups.
According to Table 1, the blood glucose of the mice significantly increased in all groups compared to the control group after induction of diabetes with alloxan (p < 0.001). Even after the administration of metformin and the 100 mg/kg and 200 mg/kg doses of the plant extract, the blood glucose levels in the diabetic group remained significantly elevated compared to the control group after 28 days of administration (p < 0.001).
In the metformin-treated group, there was a noticeable reduction in blood glucose levels, and a significant difference was observed compared to the diabetic group (p < 0.001). Additionally, the group receiving the plant extract at doses of 100 mg/kg and 200 mg/kg showed a significant reduction in blood glucose levels (respectively p < 0.05 and p < 0.001) compared to the diabetic group.
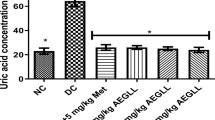
Blood urea nitrogen and creatinine levels
The blood urea nitrogen (BUN) and creatinine (CR) levels were measured in the experimental groups, including diabetic mice, and after the administration of the hydroalcoholic extract at different concentrations of 200 mg/kg, 100 mg/kg, and 300 mg/kg of metformin. Table 2 shows the BUN & CR levels in mg/dl as mean ± standard deviation for the different groups.
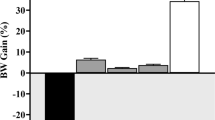
According to Table 2 and Figs. 2 and 3, the highest levels of BUN and CR were observed in the diabetic group. There was a significant difference in BUN and CR levels between the diabetic group and the control group (p < 0.05). Additionally, a significant decrease in BUN and CR levels compared to the diabetic group was observed in the group receiving the plant extract and the group receiving metformin (p < 0.05). However, there was no significant difference in BUN and CR levels between the metformin-treated group and the group receiving the 200 mg/kg extract of the plant.
Protein carbonyl content
According to Fig. 4, the highest carbonyl protein levels were observed in the diabetic group. Metformin and plant extract significantly (P < 0.05) decreased carbonyl protein in the kidney tissue compared to the diabetic group. Also, there wasn’t a significant difference (P < 0.05) in carbonyl protein levels between the metformin-treated group and the group receiving the 200 mg/kg extract of the plant.
Lipid peroxidation
According to Fig. 5, the highest TBARS level (lipid peroxidation) was observed in the diabetic group (nmol MDA eq/g tissue). Metformin and plant extract significantly (P < 0.05) decreased TBARS levels in the kidney tissue compared to the diabetic group. Also, there wasn’t a significant difference in TBARS level between the metformin-treated group and the group receiving the 200 mg/kg extract of the plant.
GSH concentration
As shown in Fig. 6, it is evident that the administration of metformin and plant extract resulted in a notable increase (p < 0.05) in the GSH level within the kidney tissues of the treated mice when compared to the diabetic group.
TNF-α and IL-6 levels
There was a significant difference (P < 0.001) in GSH level between the metformin-treated group and the group receiving the 200 mg/kg extract of the plant.
Figures 7 and 8 show IL-6 and TNF-α levels (pg/mL) in different groups. According to these figures, the highest IL-6 and TNF-α levels were observed in the diabetic group.
There was a significant difference (P < 0.05) in IL-6 and TNF-α levels in the diabetic group compared to the control group. Metformin and plant extract significantly (P < 0.05) decreased both of these inflammatory factors in the kidney tissue compared to the diabetic group. While there wasn't a significant difference in IL-6 levels between the metformin-treated group and the group receiving the 200 mg/kg extract of the plant, there was a significant difference in TNF-α levels between the two groups.
Discussion
The common long-term complications of diabetes include cardiovascular diseases, retinopathy, neuropathy, and nephropathy. Diabetic nephropathy is considered one of the leading causes of kidney failure and dialysis. Chronic hyperglycemia plays a pivotal role in the development of inflammation, apoptosis, generation of free radicals, and oxidative stress in kidney tissues, which are fundamental in the pathogenesis and progression of nephropathy29,30.
The role of oxidative stress in the pathology of diabetes complications in patients or laboratory models of diabetes has been demonstrated in previous studies. Increased blood glucose induces oxidative stress through various distinct pathways. Overall, diabetes leads to the generation of free radicals and oxidative stress, resulting in lipid, protein, and DNA oxidation31. In a previous study, systemic oxidative stress was investigated in type 2 diabetic patients with diabetic nephropathy. The results indicated increased markers of oxidative stress in these patients compared to the control group. Moreover, the study highlighted the role of oxidative stress in diabetes-related complications, including renal impairment32.
Quercetin demonstrates significant antioxidant properties, protecting retinal pigment epithelial (RPE) cells from oxidative damage and senescence in vitro in a dose-dependent manner. This protective capacity is attributed, in part, to its ability to inhibit the upregulation of caveolin-1, thereby preventing apoptotic cell death induced by cellular stress, a process implicated in early age-related macular degeneration (AMD) development. This suggests that incorporating antioxidative dietary flavonoids like quercetin could offer a promising approach for early AMD prevention33. Additionally, studies indicate that quercetin shows potential in reducing or preventing photobiologic damage induced by exposure to UVA in rats34. Moreover, quercetin's neuroprotective effects have garnered interest, with evidence suggesting its ability to counteract neurotoxic chemicals and models of neuronal injury and neurodegenerative diseases35. Our research findings align with recent studies highlighting the antioxidant activity of quercetin. These studies have consistently demonstrated the potent antioxidant properties of quercetin, affirming its antioxidant activity and its potential implications for health promotion and disease prevention.
Numerous studies have examined the level of lipid peroxidation resulting from diabetes. Serum levels of lipid peroxidation (MDA) and antioxidant enzyme in male and female diabetic patients in India, revealed elevated levels of lipid peroxidation and reduced serum glutathione in both male and female type 2 diabetic patients compared to the non-diabetic group. The conclusion drawn was that changes in these two factors may occur much earlier than the onset of secondary complications of type 2 diabetes36. The findings of the present study are in line with previous studies, demonstrating increased lipid peroxidation and decreased glutathione, thereby supporting the role of oxidative stress in diabetes-related complications37.
Protein carbonyl has been used as a stable marker in the assessment of oxidative stress conditions in many studies. Notably, hyperglycemia induces increased oxidative stress in type 2 diabetes patients (n = 60), and protein carbonyl can be a stable marker in type 2 diabetes, leading to the development of uncontrolled diabetes complications38. The present study is consistent with previous research, demonstrating an increase in protein carbonyl in the diabetic group.
Furthermore, numerous studies have indicated an increase in the serum levels of inflammatory cytokines such as IL-6 and TNF-α in patients with type 2 diabetes39. A study by Wong et al.40 demonstrated a significant increase in the serum levels of inflammatory cytokines IL-18, IL-6, and TNF-α in patients with type 2 diabetes who had nephropathy compared to diabetic patients without nephropathy and the control group. The present study also aligns with the previous findings mentioned, indicating an elevation in the levels of inflammatory factors IL-6 and TNF-α due to diabetes.
Various plants have been identified and studied regarding the management of diabetes, and in some cases, they have been introduced into the market as herbal remedies. Some of these herbal plants not only help in reducing blood glucose levels but also contribute to the control and improvement of diabetes-related complications, such as various inflammatory conditions8,9. In a study, the effectiveness of the plant Polygonum multiflorum in the process of glycation in diabetes was investigated and compounds of stilbene glycoside were examined. The results revealed that this plant leads to a reduction in diabetes-related complications15. Furthermore, the efficacy of diverse plants, such as Polygonum aviculare, in diabetes management has been explored. P. aviculare, known for its composition of phenolic compounds, tannins, and flavonoids, exhibited remarkable efficacy in reducing blood glucose levels41. In this study, similar to previous research, flavonoids, phenolic compounds, and antioxidants were observed in the P. hyrcanicum plant, a member of the Polygonaceae family. Furthermore, similar to earlier studies, a reduction in blood glucose levels and an improvement in diabetic nephropathy were observed in this study. Given that the compounds identified in this study align with those reported in earlier research, it is likely that the beneficial effects on diabetic complications can be attributed to the presence of flavonoids, phenolic compounds, and antioxidants.
Conclusion
In conclusion, the present study demonstrates the significant effectiveness of P. hyrcanicum extract in reducing blood glucose levels and diabetic nephropathy. Notably, the hydroalcoholic extract demonstrates favorable effects on lipid peroxidation, protein carbonyl, IL-6, TNF-a, and glutathione levels, holding promise for improved clinical outcomes and reduced long-term complications in diabetes. The study prompts critical questions: How can the observed effects be translated into clinical practice? What specific molecular pathways are involved in the extract's actions, and how can they be targeted for therapeutic purposes? These questions open avenues for future investigations, emphasizing the need for a comprehensive understanding of P. hyrcanicum extract's mechanisms and its potential application in human subjects.
Data availability
The datasets generated and analysed during the current study are not publicly available due to privacy concerns and ethical restrictions. However, they are available from the corresponding author upon reasonable request.
References
Cole, J. B. & Florez, J. C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 16, 377–390 (2020).
Taguchi, K. & Fukami, K. RAGE signaling regulates the progression of diabetic complications. Front. Pharmacol. 14, 1128872 (2023).
Calle, M. & Fernandez, M. Inflammation and type 2 diabetes. Diabetes Metab. 38, 183–191 (2012).
Sagoo, M. K. & Gnudi, L. Diabetic nephropathy: an overview. In Diabetic Nephropathy: Methods and Protocols (eds Gnudi, L. & Long, D.) 3–7 (Humana, 2020).
Sagoo, M. K. & Gnudi, L. Diabetic nephropathy: Is there a role for oxidative stress?. Free Radical Biol. Med. 116, 50–63 (2018).
Akpoveso, O.-O.P., Ubah, E. E. & Obasanmi, G. Antioxidant phytochemicals as potential therapy for diabetic complications. Antioxidants 12, 123 (2023).
Darenskaya, M., Kolesnikov, S., Semenova, N. & Kolesnikova, L. Diabetic nephropathy: Significance of determining oxidative stress and opportunities for antioxidant therapies. Int. J. Mol. Sci. 24, 12378 (2023).
Rahmatullah, M. et al. Medicinal plants used for treatment of diabetes by the Marakh sect of the Garo tribe living in Mymensingh district, Bangladesh. Afr. J. Tradit. Complement. Altern. Med. 9, 380–385 (2012).
Patel, D., Prasad, S. K., Kumar, R. & Hemalatha, S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2, 320–330 (2012).
Moradi-Afrapoli, F. et al. In vitro α-glucosidase inhibitory activity of phenolic constituents from aerial parts of Polygonum hyrcanicum. DARU J. Pharm. Sci. 20, 1–6 (2012).
Ghanim, H. et al. An antiinflammatory and reactive oxygen species suppressive effects of an extract of Polygonum cuspidatum containing resveratrol. J. Clin. Endocrinol. Metab. 95, E1–E8 (2010).
Smolarz, H. D. Chromatographical analysis of phenolic acids in some species of Polygonum L. genus. Quantitative determination of the major components by high performance liquid chromatography (HPLC). Acta Soc. Bot. Pol. 69, 21–23 (2000).
Mozaffarian, V. A Dictionary of Iranian Plant Name (Farhang Moaser Publ. Co., 2007).
Avicenna, A. (A. Sharafkandi (Trans.). Sorush Press, 2010).
Lv, L. et al. Stilbene glucoside from Polygonum multiflorum Thunb.: A novel natural inhibitor of advanced glycation end product formation by trapping of methylglyoxal. J. Agric. Food Chem. 58, 2239–2245 (2010).
Raafat, K., Aboul-Ela, M. & El-Lakany, A. Alloxan-induced diabetic thermal hyperalgesia, prophylaxis and phytotherapeutic effects of Rheum ribes L. in mouse model. Arch. Pharmacal Res. 44, 1–10 (2021).
Sharma, S. S., Kumar, A., Arora, M. & Kaundal, R. K. Neuroprotective potential of combination of resveratrol and 4-amino 1, 8 naphthalimide in experimental diabetic neuropathy: Focus on functional, sensorimotor and biochemical changes. Free Radic. Res. 43, 400–408 (2009).
Woldekidan, S. et al. Evaluation of antihyperglycemic effect of extract of Moringa stenopetala (Baker f.) aqueous leaves on alloxan-induced diabetic rats. Diabetes Metab. Syndr. Obes. 14, 185–192 (2021).
De Broe, M. E., Kajbaf, F. & Lalau, J.-D. Renoprotective effects of metformin. Nephron 138, 261–274 (2018).
Nikolova, M. Screening of radical scavenging activity and polyphenol content of Bulgarian plant species. Pharmacogn. Res. 3, 256 (2011).
Hagerman, A. Quantification of tannins in tree foliage: A laboratory manual for the FAO/IAEA co-ordinated research project on “The use of nuclear and related techniques to develop simple tannin assays for predicting and improving the safety and efficiency of feeding ruminants on tanninferous tree foliage. http://www.iaea.org/programmes/nafa/d3/crp/pubd31022manual-tannin.pdf (2000).
Amerifar, M., Arabnozari, H., Shokrzadeh, M. & Habibi, E. Evaluation of antioxidant properties and cytotoxicity of brown algae (Nizamuddinia zanardinii) in uterine (hela) and pancreatic cancer cell lines (paca-2). Hum. Exp. Toxicol. 43, 09603271241227228 (2024).
Benzie, I. F. & Strain, J. J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 239, 70–76 (1996).
Hensley, K. & Floyd, R. A. Methods in Biological Oxidative Stress (Springer, 2008).
Liepinsh, E. et al. Protective effects of mildronate in an experimental model of type 2 diabetes in Goto-Kakizaki rats. Br. J. Pharmacol. 157, 1549–1556 (2009).
Zhang, F., Xu, Z., Gao, J., Xu, B. & Deng, Y. In vitro effect of manganese chloride exposure on energy metabolism and oxidative damage of mitochondria isolated from rat brain. Environ. Toxicol. Pharmacol. 26, 232–236 (2008).
Arab-Nozari, M. et al. Ginkgo biloba attenuated hepatotoxicity induced by combined exposure to cadmium and fluoride via modulating the redox imbalance, Bax/Bcl-2 and NF-kB signaling pathways in male rats. Mol. Biol. Rep. 47, 6961–6972 (2020).
Emsley, H. C. A. et al. Clinical outcome following acute ischaemic stroke relates to both activation and autoregulatory inhibition of cytokine production. BMC Neurol. 7, 5. https://doi.org/10.1186/1471-2377-7-5 (2007).
Elmarakby, A. A. & Sullivan, J. C. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc. Ther. 30, 49–59 (2012).
Murrow, J. R. et al. The differential effect of statins on oxidative stress and endothelial function: Atorvastatin versus pravastatin. J. Clin. Lipidol. 6, 42–49 (2012).
Jw, B. Role of oxidative stress in development of complication in diabetes. Diabetes 40, 405–412 (1991).
Calabrese, V. et al. Oxidative stress and cellular stress response in diabetic nephropathy. Cell Stress Chaperones 12, 299 (2007).
Kook, D. et al. The protective effect of quercetin against oxidative stress in the human RPE in vitro. Investig. Ophthalmol. Vis. Sci. 49, 1712–1720 (2008).
Erden Inal, M., Kahraman, A. & Köken, T. Beneficial effects of quercetin on oxidative stress induced by ultraviolet A. Clin. Exp. Dermatol. 26, 536–539 (2001).
Costa, L. G., Garrick, J. M., Roquè, P. J. & Pellacani, C. Mechanisms of neuroprotection by quercetin: Counteracting oxidative stress and more. Oxidative Med. Cell. Longev. 2016, 1–10 (2016).
Mahboob, M., Rahman, M. & Grover, P. Serum lipid peroxidation and antioxidant enzyme levels in male and female diabetic patients. Singap. Med. J. 46, 322 (2005).
Ceriello, A. Oxidative stress and diabetes-associated complications. Endocr. Pract. 12, 60–62 (2006).
Dayanand, C., Vegi, P. K. & Kutty, A. Protein carbonyl content as a stable oxidative stress marker in type II diabetes. Int. J. Biol. Med. Res. 3, 2362–2365 (2012).
Pickup, J. C., Chusney, G. D., Thomas, S. M. & Burt, D. Plasma interleukin-6, tumour necrosis factor α and blood cytokine production in type 2 diabetes. Life Sci. 67, 291–300 (2000).
Wong, C. et al. Aberrant activation profile of cytokines and mitogen-activated protein kinases in type 2 diabetic patients with nephropathy. Clin. Exp. Immunol. 149, 123–131 (2007).
Baharvand-Ahmadi, B., Bahmani, M., Tajeddini, P., Naghdi, N. & Rafieian-Kopaei, M. An ethno-medicinal study of medicinal plants used for the treatment of diabetes. J. Nephropathol. 5, 44 (2016).
Acknowledgements
The authors are grateful to Mazandaran University of Medical Sciences for their financial support.
Author information
Authors and Affiliations
Contributions
H.A. Drafting the article and analysis of data, F.S. Concept and design of study and interpretation of data, A.N. Acquisition of data and analysis and interpretation of data, F.S.A. design of article and data analysis, S.S. article writing and data analysis, E.H. Final approval of the version to be published and L.N. article writing and data analysis. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Arabnozari, H., Shaki, F., Najjari, A. et al. The effect of Polygonum hyrcanicum Rech. f. hydroalcoholic extract on oxidative stress and nephropathy in alloxan-induced diabetic mice. Sci Rep 14, 18117 (2024). https://doi.org/10.1038/s41598-024-69220-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69220-x
- Springer Nature Limited