Abstract
Dengue is a global health concern, and the host-viral interactions that regulate disease severity are largely unknown. Detrimental effects of neutrophils in this disease have been reported, but the precise mechanisms and functional properties of dengue-activated neutrophils are not fully characterised. Here, we measured the effects of dengue virus serotype 3 (DV3) on neutrophil lifespan and functions. We show that DV3 extends neutrophil survival with a significant proportion of cells surviving for 72 h post-incubation. These effects on neutrophil survival were greater than those observed by adding GM-CSF and TNF-α alone, but these cytokines enhanced survival induced by the virus. Enhanced reactive oxygen species (ROS) generation was observed following incubation with DV3 activation and this ROS production was enhanced by co-incubation with priming agents. In addition, DV triggered the enhanced IL-8 expression by the majority of neutrophils and a low percentage of cells were activated to express MCP-1 (CCL2). A low number of neutrophils showed increased co-expression of the migratory markers, CCR7 and CXCR4 which could promote their migration towards lymph nodes. DV3 significantly upregulated the BCL-XL gene at 3, 12, and 24 h, and the Mcl-1 gene at 12 h, following treatment. We also show that DV3 induces the Mcl-1 protein stabilization similar to GM-CSF. This report sheds new light on the mechanisms by which neutrophils may contribute to the pathology of dengue disease via delayed apoptosis and generation of pro-inflammatory molecules, and raises the possibility that dengue-activated neutrophils may play a role in activating cells of adaptive immunity.
Similar content being viewed by others
Introduction
Severe dengue is a major public health problem causing over 5 million infections and 5000 deaths recorded in the past two decades1. Dengue patients often have asymptomatic infections, and unidentified confounding factors, either from the host or the virus that can drive disease progression to severe dengue, characterized by extensive plasma leakage, fluid accumulation, shock, and organ failure2. However, the complexity of viral-host immune interactions that result in these severe outcomes following infection, remains to be elucidated. These diverse disease outcomes are likely to involve complex interactions between the virus and host immune cells that acquire altered functions.
Neutrophils are key innate immune cells that play a crucial role in defense against invading pathogens. These versatile cells employ diverse mechanisms including phagocytosis, degranulation, reactive oxygen species (ROS) production, cytokine release, and NET formation to eliminate pathogens, and these functions have been best characterized during bacterial and fungal infections3,4. However, there have been several recent studies reporting the role of neutrophils in viral infections, in which they can limit viral spreading, promote virus clearance, and crosstalk with other immune cells for host protection5,6. However, neutrophil activation during infections and inflammation must always be regulated in order to limit their ability to damage host tissues, e.g. by excessive ROS and cytokine production resulting in uncontrolled, persistent inflammation7. Zika virus induces a mild neutrophil response, which is in contrast to Human immunodeficiency virus-1 that strongly induces activated markers, pro-inflammatory cytokines and ROS production, similar to Influenza A virus; these mechanisms may promote neutrophil aggregation and overactivity linked to acute respiratory distress syndrome (ARDS)8,9. Mature neutrophils have a relatively short lifespan in the circulation and die by apoptosis, but this lifespan can be extended when they are exposed to agents such as cytokines, and this enhanced survival enables them to perform their function in host defense10. When their role is complete, they then undergo apoptosis to be safely cleared by tissue macrophages without releasing their cytotoxic contents10. However, delayed apoptosis can extend their ability to release cytotoxic/inflammatory molecules and this can lead to persistent inflammation with pathological consequences11,12. These pathological effects can include endothelial cell damage and increased inflammation. Activated neutrophils can migrate to lymphoid organs13 and direct (cell:cell contact) or indirect (via neutrophil-derived cytokines and chemokines) interactions with lymphocytes can result in both promoting and suppressing the functions of B and T lymphocytes14.
In dengue, the complex role of neutrophils in disease pathology includes alterations in blood circulating neutrophils, increases in their activation status and an activated secretome in severe, acute dengue patients15,16. Recruitment of neutrophils to the site of infection has been reported which may facilitate viral infection and disease development17,18. Dengue can induce neutrophil activation in a serotype-independent mechanism via interactions with C-type lectin domain family 5 member A (CLEC5A) and Toll-like receptor 2 (TLR2), that induces NLRP3 inflammasome activation and subsequent NET and inflammatory cytokine production19,20. A recent study by Rawat et al. revealed that DV2 activates neutrophils resulting in delayed apoptosis and release of an antiviral secretome, that affects the endothelial barrier function21. However, the precise mechanisms and the consequences of delayed apoptosis in dengue remain unclear. Understanding the mechanisms of dengue-delayed apoptosis might help in preventing severe infection in dengue and other viral diseases. Therefore, this study aimed to identify how dengue virus serotype three (DV3) affects neutrophil viability and function over time. Our findings reveal that DV3 prolongs neutrophil lifespan by stabilizing the anti-apoptotic protein, myeloid cell leukemia-1 protein22, and generating molecules that can recruit neutrophils and other immune cells to promote inflammation and inflammatory damage.
Results
Dengue virus induces a prolonged lifespan neutrophil
Neutrophils were incubated with medium alone (control, untreated) or DV3 at an MOI of 5 before measuring cell viability: PMA was used as a positive activation control. Incubation with DV3 significantly prolonged neutrophil viability compared to the untreated control and with PMA as determined by annexin V and propidium iodide staining (Fig. 1A,B, Supplementary Fig. S2). Viable cells were detectable even after 72 h post incubation (pi.) in the presence of DV3 and significantly higher percentages of viable cells were observed in the DV3-treated group (mean 63.09%) compared with untreated controls (mean 25.11%) at 24 h pi. (p < 0.0001). This enhancement of viability was also observed at 48 h pi., p < 0.0001. In contrast, PMA resulted in significantly decreased percentages of viable cells (mean 25.81%) compared with the DV3-treated group (mean 90.91%) at 3 h pi. (p < 0.0001). This prolonged lifespan neutrophil was confirmed by an independent MTT assay. The results showed that DV3-treated neutrophils had significantly higher absorbance at 540 nm compared to untreated controls at 3, 24, and 48 h pi., (p = 0.0007, p = < 0.0001 and p = 0.0003 respectively, Fig. 1C).
Dengue virus serotype three promoted neutrophil survival. Neutrophils were cultured with medium (untreated), PMA, or DV3 (MOI of 5) to evaluate cell viability. (A) Representative dot plots indicate neutrophil cell viability measured by annexin V binding and PI staining. (B) The percentage of viable cells is shown at different times of incubation. (C) Cell vitality was confirmed using the MTT assay. Data are presented as mean ± standard deviation (SD). ****p < 0.0001, ***p < 0.001, **p < 0.01 and *p < 0.05.
During these incubations, the active virus in culture medium was quantified using Focus forming assay (FFA) and the foci of infected cells was visualized by indirect immunofluorescent staining. The results revealed that DV3 was dramatically decreased at 3 h pi. (a > tenfold decrease with a mean of 223,567 FFU, p = 0.0005, Supplementary Fig. S3A). Moreover, further decreases of DV3 FFU were observed at 24 h and 48 h (mean 447 and 5 FFU). We also determined relative levels of dengue viral RNA. Viral RNA within neutrophils was detected immediately after incubation at 0 h pi. (mean 96.8 FFU, Supplementary Fig. S3B) and then increased at 2 h pi. (mean 189.8 FFU). However, significantly lower relative intra-neutrophil viral RNA was detected at 24 h (mean 7.7 FFU) and 48 h (mean 3.0 FFU) pi. compared with 2 h pi. (p = 0.0093, p = 0.0096, respectively). These results indicated that DV3-induced delay of neutrophil apoptosis was not associated with viral replication or active virus.
Dengue virus induces functional alterations of neutrophils
To evaluate the functional properties of neutrophils after exposure to DV3, key functions such as ROS production, chemokine expression and cell migration markers were measured after 1 h and 24 h incubation with the virus. ROS are highly-reactive chemical compounds with the potential for effective viral inactivation, but it also can promote inflammation. DV3 significantly induced ROS production (mean % ROS+ 3 h 5.0%, 24 h 6.9%, and 48 h 7.0%) compared to untreated controls (p = 0.0020, 0.0003, and 0.0003, respectively, Fig. 2A). The ROS MFI of DV3-treated neutrophils were similar between 3 and 24 h pi. and then slightly decreased at 48 h pi. compared to 24 h pi. (p = 0.0169), but these levels were lower compared to PMA controls (p < 0.0001). These results show that DV3 induced low levels of ROS production in neutrophils.
Functions of neutrophils after dengue virus three incubation. Neutrophils were incubated with medium (untreated), PMA, or DV3 (MOI of 5) for different times, as indicated. (A) ROS production (% ROS+ and MFI) (B) Chemokine production (% IL8+ and % MCP1+) (C) Migratory markers (% CXCR4+ and % CCR7+). Data are presented as mean ± standard deviation (SD). ****p < 0.0001, ***p < 0.001, **p < 0.01 and *p < 0.05.
DV3 triggered intracellular IL8 production with a significantly higher percentage of IL8+ cells at 3 and 24 h pi. (mean 12.4% and 38.9%, respectively) compared with untreated controls (p = 0.0086 and 0.0001 respectively, Fig. 2B). DV3-treated neutrophils showed a similar percentage of MCP-1+ cells compared with untreated controls after 15 min and 3 h pi. (mean 0.02% and 0.05%, p = 0.3727 and 0.6309), however; a small proportion of DV3-treated neutrophils produced MCP-1 at 24 h (mean 0.43%, p < 0.0001). We measured migratory markers expressed on viable cells (Annexin V-) at 24 h pi., i.e. neutrophils with a prolonged lifespan. The results showed that DV3 significantly increased the number of CCR7+ cells (mean 0.7%) but lower numbers of CXCR4+ cells (mean 11.8%) compared with the untreated control (mean 0.2% and 30.2%, respectively) (p < 0.0001 and 0.0028, Fig. 2C). There was no statistical difference in CXCR4 MFI in DV3-treated cells compared with untreated controls (p = 0.5215, Supplementary Fig. S4A). A small proportion of DV3-treated neutrophils co-expressed both CXCR4 and CCR7 (mean 0.3%). These results suggest that DV3-delayed apoptotic neutrophils might play a role in target cell recruitment and lymph node migration.
Primed neutrophils exhibit a stronger response to dengue virus than primary cells
Neutrophil functions such as ROS production and survival can be enhanced by the cytokines, TNF-α and GM-CSF23 and both agents alone delayed apoptosis after 24 h incubation compared with untreated controls (Fig. 3A). However, cell viability was further enhanced in the presence of both the cytokines and DV3 at 24 h pi. (p < 0.0001, Fig. 3A). Moreover, DV3 alone had a greater effect on delaying apoptosis than either priming agent alone (p = 0.0005 and 0.0218). No differences in cell viability were observed in either primed or unprimed cells in the absence or presence of DV3 after 3 h pi. (Supplementary Fig. S5).
Effect of DV3 on primed neutrophil cell viability and ROS production. Neutrophils were incubated with medium (non-primed), or primed with TNF-α or GM-CSF prior to culture with or without DV3. (A) Percentage of viable cells after 24h of incubation. (B) Heatmap showing average ROS production. (C) The percentage of ROS+ cells after 15 min, 3h, and 24h. Data are presented as mean ± standard deviation (SD). ****p < 0.0001, ***p < 0.001, **p < 0.01 and *p < 0.05.
ROS production was then measured in cytokine-primed or unprimed neutrophils in the presence and absence of DV3 after 15 min (rapid), 3 h and 24 h pi. (Fig. 3B,C). In the absence of DV3, ROS produced by primed neutrophils was not different from levels observed in unprimed cells. However, DV3-treated cells had a significantly higher percentage of ROS+ cells in the presence of the priming agents at 15 min pi. (p = 0.0433 and 0.0198, for TNF-α and GM-CSF, respectively), but this increase was not observed in non-primed neutrophils (p = 0.2457). A time-dependent increase in ROS production was observed in the GM-CSF priming conditions (Fig. 3C). In addition, neutrophils treated with PMA had a significantly higher percentage of ROS+ cells (99.48%) compared to cells activated by DV3 (p < 0.0001) and the untreated control group (p < 0.0001, Supplementary Fig. S6A) and DV3 did not affect PMA-induced ROS generation compared with PMA alone at 15 min pi. (p > 0.9999). These results suggest that priming enhances the ability of neutrophils to generate ROS in the presence of DV3, compared with non-primed neutrophils.
Dengue virus induced Mcl-1 stabilization in neutrophils with delayed apoptosis
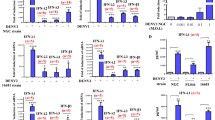
To identify the mechanisms underlying delayed apoptosis observed by dengue virus stimulation, DV3-treated neutrophils were evaluated for the expression of apoptotic-related genes at different time points. The relative fold change of the anti-apoptotic gene, BCL-XL, was significantly up-regulated at 3 h (> 3.5-fold), 12 h (> 28.1-fold), and 24 h pi. (> 31.1-fold) (p = 0.0339, 0.0002, and 0.0260, respectively, Fig. 4A). Another anti-apoptotic gene, Mcl-1, was down-regulated at 3 h pi. (0.36) and up-regulated at 6 h (1.80), 12 h (6.44), and 24 h (2.73) pi., and this increase was statistically significant at 12 h pi. (p = 0.0383) (Fig. 4B).
Regulation of anti and pro-apoptotic gene and Mcl-1 protein expression in response to DV3. (A–D) Gene expression measured by RT-qPCR. (A) BCL-XL (B) Mcl-1 (C) BAX and (D) BAK expression, corrected for the GAPDH housekeeping gene at multiple time points. (E, F) Mcl-1 protein levels were measured by western blot. (E) Representative Mcl-1 protein levels in medium (untreated), DV3, and GM-CSF after 3 and 24h. (F) Fold change of Mcl-1 protein normalized by untreated control. Data are presented as mean ± standard deviation (SD). ***p < 0.001, **p < 0.01 and *p < 0.05.
The pro-apoptotic gene BAX was significantly down-regulated at 3 h pi. (0.42, p = 0.0475). At 6 h and 12 h pi., the relative fold change of BAX gene was not different from the untreated control but was significantly up-regulated at 24 h (5.7-fold, p = 0.0025) (Fig. 4C). However, DV3 did not significantly affect gene expression of BAK (3 h, 1.05; 6 h, 0.87; 12 h, 2.94; 24 h, 0.41, Fig. 4D).
Expression of the Mcl-1 protein, which plays a central role in neutrophil survival24,25, was then determined by western blotting after treatment of neutrophils with medium (control), DV3 and GM-CSF (as a positive control) (Fig. 4E,F). Protein expression was normalized to that of untreated controls. The results showed that Mcl-1 protein expression after 3 h incubation with DV3 and GM-CSF was significantly increased above untreated control levels. After 24 h incubation, this protein was undetectable in untreated control neutrophils, but significantly elevated in cells incubated with either DV3 or GM-CSF, being elevated > 14-fold (p = 0.0208) or > ninefold (p = 0.0035) by DV3 and GM-CSF, respectively (Fig. 4F). These results indicate that DV3 slows Mcl-1 degradation during delayed apoptosis.
Discussion
The role of neutrophils in dengue virus infection is not fully defined but several studies have shown their pathological role, particularly in severe dengue19,26,27. It is important to understand how dengue virus delays neutrophil apoptosis and regulates their function, in order to predict how these cells contribute to disease progression and development of clinical symptoms. Such understanding may help in designing therapeutic strategies to limit inflammatory damage that occurs in this disease. This study utilized a dengue virus serotype 3 (DV3) isolated from a Thai dengue hemorrhagic fever (DHF) patient in 2008. This virus was shown to cause severe pathogenesis in mouse models28. Previously, Rawat et al. found that dengue virus activates neutrophils in a serotype-independent manner21 and DV2 increased neutrophil viability. This is the first report that showed delaying neutrophil apoptosis by DV3.
Several studies have investigated dengue-induced neutrophil cell death/survival at single or double time points with a maximum incubation time of 24 h pi.19,21. Our serial time point observations revealed that DV3 induces a prolonged lifespan of neutrophils, keeping a significant proportion of cells alive for up to 72 h. Previous studies have shown that DV2 delays neutrophil apoptosis, while Dengue Virus Envelope Protein Domain III (EIII) promotes cell death at early incubation time points, that was characterized by pyroptosis, necroptosis, and autophagy19. Moreover, delayed neutrophil apoptosis occurs during several virus infections, including respiratory syncytial virus (RSV), and human cytomegalovirus (HCMV) which resulted in dysregulation of neutrophil function that may exacerbate inflammation29,30.
This study confirmed limited dengue virus replication in neutrophils similar to previous publications that used primary neutrophils and cell lines (HL-60)31,32. Our observations support the concept that rapid binding of dengue virus is sufficient for subsequent neutrophil activation. The binding of microbes including dengue virus, to activate neutrophils has been reported to be through CLEC5A and Toll-like receptor 2 (TLR2) which can also lead to proinflammatory cytokine production and NETosis20. We demonstrated that DV3 delays neutrophil apoptosis and this prolonged lifespan can result in extended/persistent inflammatory damage. Our investigations also showed low-level but significant ROS generation in response to dengue virus. This may contribute to ROS-dependent inflammation and tissue damage, as in several viral infections, such as SARS-CoV-2, IAV and RSV33. Viral proteins, such as influenza A virus hemagglutinin protein, RSV fusion protein, and HIV trans-activator of transcription (Tat) protein, can induce neutrophil activation and mediate ROS production, NET formation and cell death34,35,36. In addition to cell and tissue damage, ROS can also play important roles in cell signaling and infections37 and in patients may be related to dengue disease severity15. In vitro, dengue triggers an increase in ROS by various cells, including neutrophils, which correlates with lower viral viability and high viral titer38. In addition, dengue viral protein EIII can activate neutrophil ROS production in a dose-dependent manner19, but in contrast, DV2 was reported to not induce ROS and also interfered with PMA-induced ROS formation in neutrophils39. Importantly, ROS (H2O2) suppresses dengue replication in Caco-2 cells40, suggesting that ROS generated by neutrophils may interfere with viral replication and this idea is supported by our observations (Supplementary Fig. S3).
Our data showed increased IL8 and MCP-1 production in DV3-treated neutrophils compared with untreated controls. Both molecules are chemotactic factors for neutrophils and dengue-targeted cells such as monocytes and macrophages. Inflammatory mediators, derived from infiltrating neutrophils following mosquito bites, were reported to facilitate the entry and infection of viral-permissive cells, leading to increased severity of arboviral infection17. Dengue patients display elevated IL-8 levels, which correlate with neutrophil degranulation, clinical and blood parameters as well as disease severity41. MCP-1 is also highly expressed in dengue, regulating the migration of monocytes/macrophages and may promote permeability changes42. Other viruses such as CMV, HIV1, and RSV have been reported to induce IL8 and MCP-1 production, which are essential for viral replication and spreading43. It must be noted that while the majority of neutrophils incubated with DV3 expressed IL-8, only a small percentage of neutrophils stained positive for expression of MCP-1. There are several possible reasons for this observation. First, it is possible that this chemokine is only generated by a small proportion of DV3-activated neutrophils, suggesting great functional heterogeneity in responses of the circulating neutrophil pool to this virus. Second, it is possible that this low level of expression results from a non-neutrophil cell present in low numbers in our preparations. However, mRNA for this chemokine is expressed by “ultra pure” neutrophils at low levels44 and protein levels are enhanced in infections, inflammatory diseases and certain cancers45. It is possible, therefore, that this chemokine is only expressed by a small subpopulation of neutrophils in response to DV3.
Interestingly, we observed a small population of delayed apoptotic neutrophils with CXCR4 and CCR7 expression, properties that could potentially enable them to migrate to lymph nodes. Several studies have demonstrated the migration of neutrophils back to lymph nodes in response to various microbes such as Staphylococcus aureus, Pseudomonas aeruginosa, Listeria monocytogenes, Toxoplasma gondii and Vaccinia virus13,46,47,48. In S. aureus infection, CCR7 expression can mediate skin neutrophils to travel to draining lymph nodes while some studies reported that CD11b and CXCR4, but not CCR7, are required for migration46,48. In lymph nodes, neutrophils may function in antigen transport and regulate B and T cell response14. Mature neutrophils require the CXCL12-CXCR4 axis for storage in the bone marrow and aged neutrophils have been found to have upregulated CXCR4, allowing them to migrate towards CXCL12 into the bone marrow and spleen49. We found that the numbers of CXCR4+ cells positively correlated with apoptotic cells (Annexin V+) (Supplementary Fig. S4B).
We also found that DV3 enhanced cell viability and ROS production in combination with the effects of priming agents. During an infection, neutrophil functions are usually primed by pro-inflammatory cytokines, chemokines and pathogen products, and this primed state enhances their function in response to pathogens or other stimuli50. However, it is notable that the effects of DV3 alone on neutrophil apoptosis were greater than effects of cytokines alone and that functions such as ROS production were enhanced by the combined effects of DV3 and priming cytokines. Thus, during infections, increased cytokine levels can further enhance the effects of DV3 on neutrophil functions.
Our study demonstrated that DV3 increased BCL-XL gene expression and induced Mcl-1 protein stabilization in preventing neutrophil death. It must be noted, however, that while BCL-XL mRNA is expressed in purified human neutrophils and its levels can be enhanced by cytokines, this protein cannot be detected in these cells24,25. Several viruses such as IAV, RSV, HCMV, and DV2 extend neutrophil survival through the PI3K and NF-κB pathways while others result in secretion of pro-survival factors or induce anti-apoptotic protein stabilization29. Mcl-1 is a crucial moderator of neutrophil survival and its deletion or accelerated turnover leads to accelerated apoptosis22,51. Mcl-1 protein levels are subject to rapid proteasomal degradation52 and inflammatory agents stabilize this protein to prolong neutrophil survival53. In addition, neutralization of apoptosis activators like BH3-only proteins, can result in delayed apoptosis53,54.
Our study provides strong evidence for a potential role of delayed neutrophil apoptosis and ROS production in dengue virus infection that could contribute to disease pathology. A potential limitation of our study is that we did not use highly purified neutrophils for our chemokine/chemokine receptor measurements. However, the method that we used (using Ficoll) removes the most important contaminating cells that often compound studies measuring mRNA studies of human neutrophils55. Our investigation focused on measurements of a selective group of apoptotic genes and a key anti-apoptotic protein (Mcl-1), and future studies should focus on identifying the receptor/signaling pathways that regulate this function in order to identify potential targets for therapeutic intervention. It would also be important to identify the full range of genes including chemokines and cytokines whose expression is up-regulated by interactions with virus. Further investigations are also necessary to elucidate the impact of DV3-mediated delayed apoptotic neutrophils (that express CCR7 and MCP-1) on adaptive immune responses in this disease.
Methods
Ethics approval and blood collection
This study was approved by the Center for Ethics in Human Research, Khon Kaen University (Reference No. HE631347 and HE671102). The experiments were performed in accordance with relevant guidelines and regulations. All heparinized blood was collected from healthy donors at the Blood Bank, Srinagarind Hospital, Faculty of Medicine, Khon Kaen University, Thailand.
Neutrophil isolation
Neutrophils were isolated using density gradient centrifugation with HetaSep and Ficoll-Paque solutions (STEMCELL Technologies, #07906; Cytiva, #17544202), as described previously56. Briefly, heparinized blood was gently mixed with HetaSep solution at a ratio of 5:1 and incubated for 30 min at 37 °C. The leukocyte-rich plasma was overlaid onto Ficoll-Paque solutions at a ratio of 1:1 before centrifugation at 500 g for 30 min. Residual red blood cells (RBCs) were removed by incubation with ACK lysis buffer (Gibco, Thermo Fisher Scientific, A10492). The isolated cells were resuspended in a complete R10 medium (RPMI-1640 supplemented with 10% fetal bovine serum (FBS))(Gibco, Thermo Fisher Scientific, #31800022 and #16000044) for subsequent experiments. Live and dead cells were identified and enumerated by trypan blue exclusion assay, and the final neutrophil viability was > 95%. Flow cytometry with forward and side scatter gating was used to assess neutrophil purity as shown in Supplementary Fig. S7.
Dengue viral preparation
Dengue was propagated in the C6/36 and VERO cell lines as previously described57. Dengue virus was harvested from the cell culture medium at day 4 post-infection. Aliquots were stored at − 80 °C until use.
Focus forming assay
To quantify the infectious dengue virus particle, a focus forming assay was used as previously described57. Briefly, the virus was serially diluted and inoculated onto a monolayer of Vero cells for 2 h. The 2% Carboxymethyl Cellulose (CMC) solution supplemented with 2% FBS and 1% Penicillin Streptomycin was added to the infected cells to restrict viral spreading. After 3 days of incubation, the infected cells were visualized by indirect immunofluorescence staining using an antibody specific to the envelope protein of dengue virus and followed by goat anti-mouse IgG Alexa flour 488 (Abcam, ab150113). The viral foci were counted under a fluorescent microscope before being calculated as a focus forming units (FFU).
Neutrophil—dengue Incubation
Neutrophils were pre-incubated with medium alone (non-primed), or primed with either TNF-α (10 ng/mL, 15 min) or GM-CSF (5 ng/mL, 45 min) before incubation with DV3 at an MOI of 5. After incubation, neutrophils were analyzed for cell functions and RNA extraction. Supernatants were retained for dengue virus titration.
Flow cytometry assay
We utilized flow cytometry to evaluate neutrophil cell vitality and functionality. After incubation, neutrophils were stained with the Dead Cell Apoptosis Kit to measure cell survival and with Dihydrorhodamine (DHR) 123 to measure ROS production (all from Invitrogen™, V13242 and D23806). For measurements of cell migratory markers, neutrophils were stained with the following specific monoclonal antibodies; anti-human CXCR4-PerCP Cy5.5 (BioLegend, #306516) and anti-human CCR7-APC (R&D systems, FAB197A) or with Annexin V-CF Blue to measure apoptosis (Abcam, ab214485). For intracellular chemokine labeling, neutrophils were fixed and permeabilized using cytofix/cytoprem and perm wash buffer (BD Biosciences, # 554722), then incubated with anti-human IL8-FITC or anti-human MCP-1-APC (BioLegend, #511412 and #502612). All samples were analyzed using a BD FACSLyric Flow Cytometer (BD Biosciences) and Flowjo Software version 10 (Flowjo, LLC). The neutrophil gating strategy is shown in Supplementary Fig. S1.
Apoptotic gene and protein analysis
Total RNA of neutrophils was isolated using TRIzol Reagent (Invitrogen, #15596036) as previously described58. A first-strand cDNA synthesis kit (TransGen Biotech, AT301) was used to generate cDNA from neutrophil RNA or viral RNA. Gene expression was quantified using the Sso Advanced Universal SYBR Green Master Mix (Bio-Rad, #1725270). Specific primers (listed in Supplementary Table S1) were used to amplify homologous cDNAs for 35 cycles on an automated thermal cycler. The cycling program included denaturation (95 °C, 15 s), annealing (72 °C, 30 s) and extension (72 °C, 30 s). GAPDH expression was measured as an internal control. The relative gene expression levels were calculated by the 2−ΔΔCt equation59.
Simultaneously with RNA isolation, neutrophil proteins were precipitated from TRIzol organic phase using isopropanol and guanidine hydrochloride solution60. Total protein was quantified with the Pierce BCA protein assay kit (Thermo Scientific™, #23225). The samples were denatured in loading buffer (250 mM Tris–HCl, pH 6.8, 4% SDS, 20% glycerol, and 0.01% bromophenol blue) at 95 °C for 5 min. Eighty micrograms of protein were loaded onto a 12% SDS–polyacrylamide gel, electrophoresed, and transferred to a polyvinylidene membrane by a semi-dry method. The membrane was incubated with monoclonal antibodies including anti-human Mcl-1 (1:2000) and anti-human GAPDH (1:5000) (BD Pharmingen, #559027 and BioLegend, #607903). Enzyme-conjugated goat anti-mouse IgG (1:5000, Abcam, ab97023) was used as a secondary antibody. The blot was visualized with a chemiluminescence substrate (Thermo Scientific™, #34577) and captured with an ImageQuant 800 system (GE Healthcare).
Statistical analysis
Values are presented as mean ± standard deviation (SD). All statistical analyses were performed using GraphPad Prism Software version 9.5.1 (GraphPad Software, Inc., San Diego, CA, USA). A comparison of DV3 treated and control was evaluated using the Student’s t-test. One-way ANOVA was applied to test for differences in multi-time point data. A statistically-significant difference is considered at *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
Data availability
All data in this article are available in the paper or the supplementary material.
References
World Health Organization. Disease Outbreak News; Dengue–Global Situation, <https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON498> (2023).
Khan, M. B. et al. Dengue overview: An updated systemic review. J Infect Public Health 16, 1625–1642. https://doi.org/10.1016/j.jiph.2023.08.001 (2023).
Burn, G. L., Foti, A., Marsman, G., Patel, D. F. & Zychlinsky, A. The neutrophil. Immunity 54, 1377–1391. https://doi.org/10.1016/j.immuni.2021.06.006 (2021).
Wei, Y., Kim, J., Ernits, H. & Remick, D. The septic neutrophil-friend or foe. Shock 55, 147–155. https://doi.org/10.1097/SHK.0000000000001620 (2021).
Ma, Y., Zhang, Y. & Zhu, L. Role of neutrophils in acute viral infection. Immun Inflamm Dis 9, 1186–1196. https://doi.org/10.1002/iid3.500 (2021).
Galani, I. E. & Andreakos, E. Neutrophils in viral infections: Current concepts and caveats. J Leukoc Biol 98, 557–564. https://doi.org/10.1189/jlb.4VMR1114-555R (2015).
Mittal, M., Siddiqui, M. R., Tran, K., Reddy, S. P. & Malik, A. B. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20, 1126–1167. https://doi.org/10.1089/ars.2012.5149 (2014).
George, S. T. et al. Neutrophils and influenza: A thin line between helpful and harmful. Vaccines (Basel) 9, 597. https://doi.org/10.3390/vaccines9060597 (2021).
Aggio, J. B., Porto, B. N., Duarte Dos Santos, C. N., Mosimann, A. L. P. & Wowk, P. F. Human neutrophils present mild activation by Zika virus but reduce the infection of susceptible cells. Front Immunol 13, 784443. https://doi.org/10.3389/fimmu.2022.784443 (2022).
Akgul, C., Moulding, D. A. & Edwards, S. W. Molecular control of neutrophil apoptosis. FEBS Lett 487, 318–322. https://doi.org/10.1016/s0014-5793(00)02324-3 (2001).
Wright, H. L., Moots, R. J., Bucknall, R. C. & Edwards, S. W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford) 49, 1618–1631. https://doi.org/10.1093/rheumatology/keq045 (2010).
Wright, H. L., Moots, R. J. & Edwards, S. W. The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol 10, 593–601. https://doi.org/10.1038/nrrheum.2014.80 (2014).
Chtanova, T. et al. Dynamics of neutrophil migration in lymph nodes during infection. Immunity 29, 487–496. https://doi.org/10.1016/j.immuni.2008.07.012 (2008).
Lok, L. S. C. & Clatworthy, M. R. Neutrophils in secondary lymphoid organs. Immunology 164, 677–688. https://doi.org/10.1111/imm.13406 (2021).
Garishah, F. M. et al. Neutrophil extracellular traps in dengue are mainly generated NOX-independently. Front Immunol 12, 629167. https://doi.org/10.3389/fimmu.2021.629167 (2021).
Anwar, F. et al. Epidemiological and hematological investigation of dengue virus infection. Microbiol Immunol 66, 426–432. https://doi.org/10.1111/1348-0421.13018 (2022).
Pingen, M. et al. Host inflammatory response to mosquito bites enhances the severity of arbovirus infection. Immunity 44, 1455–1469. https://doi.org/10.1016/j.immuni.2016.06.002 (2016).
Wang, Z. et al. A mosquito salivary protein-driven influx of myeloid cells facilitates flavivirus transmission. EMBO J https://doi.org/10.1038/s44318-024-00056-x (2024).
Lien, T. S., Sun, D. S., Hung, S. C., Wu, W. S. & Chang, H. H. Dengue virus envelope protein domain III induces Nlrp3 inflammasome-dependent NETosis-mediated inflammation in mice. Front Immunol 12, 618577. https://doi.org/10.3389/fimmu.2021.618577 (2021).
Sung, P. S., Huang, T. F. & Hsieh, S. L. Extracellular vesicles from CLEC2-activated platelets enhance dengue virus-induced lethality via CLEC5A/TLR2. Nat Commun 10, 2402. https://doi.org/10.1038/s41467-019-10360-4 (2019).
Rawat, S., Kumar, S., Duggal, S. & Banerjee, A. Phenotypic alteration by dengue virus serotype 2 delays neutrophil apoptosis and stimulates the release of prosurvival secretome with immunomodulatory functions. J Leukoc Biol 115, 276–292. https://doi.org/10.1093/jleuko/qiad133 (2024).
Fontaine, M. A. C. et al. Low human and murine Mcl-1 expression leads to a pro-apoptotic plaque phenotype enriched in giant-cells. Sci Rep 9, 14547. https://doi.org/10.1038/s41598-019-51020-3 (2019).
Cross, A., Moots, R. J. & Edwards, S. W. The dual effects of TNFalpha on neutrophil apoptosis are mediated via differential effects on expression of Mcl-1 and Bfl-1. Blood 111, 878–884. https://doi.org/10.1182/blood-2007-05-087833 (2008).
Moulding, D. A., Quayle, J. A., Hart, C. A. & Edwards, S. W. Mcl-1 expression in human neutrophils: Regulation by cytokines and correlation with cell survival. Blood 92, 2495–2502 (1998).
Moulding, D. A., Akgul, C., Derouet, M., White, M. R. & Edwards, S. W. BCL-2 family expression in human neutrophils during delayed and accelerated apoptosis. J Leukoc Biol 70, 783–792 (2001).
Chia, P. Y., Teo, A. & Yeo, T. W. Association of neutrophil mediators with dengue disease severity and cardiac impairment in adults. J Infect Dis 226, 1974–1984. https://doi.org/10.1093/infdis/jiac383 (2022).
Schulz, C., Gabriel, G. & von Kockritz-Blickwede, M. Detrimental role of neutrophil extracellular traps during dengue virus infection. Trends Immunol 41, 3–6. https://doi.org/10.1016/j.it.2019.11.010 (2020).
Phanthanawiboon, S. et al. Acute systemic infection with dengue virus leads to vascular leakage and death through tumor necrosis factor-alpha and Tie2/angiopoietin signaling in mice lacking type I and II interferon receptors. PLoS One 11, e0148564. https://doi.org/10.1371/journal.pone.0148564 (2016).
Pocock, J. M. et al. Human cytomegalovirus delays neutrophil apoptosis and stimulates the release of a prosurvival secretome. Front Immunol 8, 1185. https://doi.org/10.3389/fimmu.2017.01185 (2017).
Lindemans, C. A. et al. Respiratory syncytial virus inhibits granulocyte apoptosis through a phosphatidylinositol 3-kinase and NF-kappaB-dependent mechanism. J Immunol 176, 5529–5537. https://doi.org/10.4049/jimmunol.176.9.5529 (2006).
Theofilopoulos, A. N., Brandt, W. E., Russell, P. K. & Dixon, F. T. Replication of dengue-2 virus in cultured human lymphoblastoid cells and subpopulations of human peripheral leukocytes. J Immunol 117, 953–961 (1976).
Duggal, S. et al. Dengue virus infection in mice induces bone marrow myeloid cell differentiation and generates Ly6Glow immature neutrophils with modulated functions. J Leukoc Biol 115, 130–148. https://doi.org/10.1093/jleuko/qiad099 (2024).
Reshi, M. L., Su, Y. C. & Hong, J. R. RNA viruses: ROS-mediated cell death. Int J Cell Biol 2014, 467452. https://doi.org/10.1155/2014/467452 (2014).
Rothwell, S. W. & Wright, D. G. Characterization of influenza A virus binding sites on human neutrophils. J Immunol 152, 2358–2367 (1994).
Funchal, G. A. et al. Respiratory syncytial virus fusion protein promotes TLR-4-dependent neutrophil extracellular trap formation by human neutrophils. PLoS One 10, e0124082. https://doi.org/10.1371/journal.pone.0124082 (2015).
Madzime, M., Rossouw, T. M., Theron, A. J., Anderson, R. & Steel, H. C. Interactions of HIV and antiretroviral therapy with neutrophils and platelets. Front Immunol 12, 634386. https://doi.org/10.3389/fimmu.2021.634386 (2021).
Foo, J., Bellot, G., Pervaiz, S. & Alonso, S. Mitochondria-mediated oxidative stress during viral infection. Trends Microbiol 30, 679–692. https://doi.org/10.1016/j.tim.2021.12.011 (2022).
Suwanprinya, L. et al. Dengue virus-induced reactive oxygen species production in rat microglial cells. Jpn J Infect Dis 70, 383–387. https://doi.org/10.7883/yoken.JJID.2016.236 (2017).
Moreno-Altamirano, M. M., Rodriguez-Espinosa, O., Rojas-Espinosa, O., Pliego-Rivero, B. & Sanchez-Garcia, F. J. Dengue virus serotype-2 interferes with the formation of neutrophil extracellular traps. Intervirology 58, 250–259. https://doi.org/10.1159/000440723 (2015).
Khan, N. A. et al. Oxidative stress specifically inhibits replication of dengue virus. J Gen Virol 102, 001596. https://doi.org/10.1099/jgv.0.001596 (2021).
Ralapanawa, U. et al. Value of peripheral blood count for dengue severity prediction. BMC Res Notes 11, 400. https://doi.org/10.1186/s13104-018-3505-4 (2018).
Lee, Y. R. et al. MCP-1, a highly expressed chemokine in dengue haemorrhagic fever/dengue shock syndrome patients, may cause permeability change, possibly through reduced tight junctions of vascular endothelium cells. J Gen Virol 87, 3623–3630. https://doi.org/10.1099/vir.0.82093-0 (2006).
Hamilton, S. T., Scott, G. M., Naing, Z. & Rawlinson, W. D. Human cytomegalovirus directly modulates expression of chemokine CCL2 (MCP-1) during viral replication. J Gen Virol 94, 2495–2503. https://doi.org/10.1099/vir.0.052878-0 (2013).
Thomas, H. B., Moots, R. J., Edwards, S. W. & Wright, H. L. Whose gene is it anyway? The effect of preparation purity on neutrophil transcriptome studies. PLoS One 10, e0138982. https://doi.org/10.1371/journal.pone.0138982 (2015).
Tecchio, C. & Cassatella, M. A. Neutrophil-derived chemokines on the road to immunity. Semin Immunol 28, 119–128. https://doi.org/10.1016/j.smim.2016.04.003 (2016).
Hampton, H. R., Bailey, J., Tomura, M., Brink, R. & Chtanova, T. Microbe-dependent lymphatic migration of neutrophils modulates lymphocyte proliferation in lymph nodes. Nat Commun 6, 7139. https://doi.org/10.1038/ncomms8139 (2015).
McMinn, P. H., Ahmed, A., Huttenlocher, A., Beebe, D. J. & Kerr, S. C. The lymphatic endothelium-derived follistatin: Activin A axis regulates neutrophil motility in response to Pseudomonas aeruginosa. Integr Biol (Camb) 15, zyad003. https://doi.org/10.1093/intbio/zyad003 (2023).
Ozcan, A. et al. CCR7-guided neutrophil redirection to skin-draining lymph nodes regulates cutaneous inflammation and infection. Sci Immunol 7, eabi9126. https://doi.org/10.1126/sciimmunol.abi9126 (2022).
Strydom, N. & Rankin, S. M. Regulation of circulating neutrophil numbers under homeostasis and in disease. J Innate Immun 5, 304–314. https://doi.org/10.1159/000350282 (2013).
Miralda, I., Uriarte, S. M. & McLeish, K. R. Multiple phenotypic changes define neutrophil priming. Front Cell Infect Microbiol 7, 217. https://doi.org/10.3389/fcimb.2017.00217 (2017).
Derouet, M. et al. Sodium salicylate promotes neutrophil apoptosis by stimulating caspase-dependent turnover of Mcl-1. J Immunol 176, 957–965. https://doi.org/10.4049/jimmunol.176.2.957 (2006).
Thomas, L. W., Lam, C. & Edwards, S. W. Mcl-1; the molecular regulation of protein function. FEBS Lett 584, 2981–2989. https://doi.org/10.1016/j.febslet.2010.05.061 (2010).
Derouet, M., Thomas, L., Cross, A., Moots, R. J. & Edwards, S. W. Granulocyte macrophage colony-stimulating factor signaling and proteasome inhibition delay neutrophil apoptosis by increasing the stability of Mcl-1. J Biol Chem 279, 26915–26921. https://doi.org/10.1074/jbc.M313875200 (2004).
Lee, C. H., Chuah, S. K., Tai, W. C., Chang, C. C. & Chen, F. J. Delay in human neutrophil constitutive apoptosis after infection with Klebsiella pneumoniae serotype K1. Front Cell Infect Microbiol 7, 87. https://doi.org/10.3389/fcimb.2017.00087 (2017).
Calzetti, F., Tamassia, N., Arruda-Silva, F., Gasperini, S. & Cassatella, M. A. The importance of being “pure” neutrophils. J Allergy Clin Immunol 139, 352–355. https://doi.org/10.1016/j.jaci.2016.06.025 (2017).
Chokesuwattanaskul, S., Phelan, M. M., Edwards, S. W. & Wright, H. L. A robust intracellular metabolite extraction protocol for human neutrophil metabolic profiling. PLoS One 13, e0209270. https://doi.org/10.1371/journal.pone.0209270 (2018).
Medina, F. et al. Dengue virus: Isolation, propagation, quantification, and storage. Curr Protoc Microbiol Chapter 15, Unit 15D 12. https://doi.org/10.1002/9780471729259.mc15d02s27 (2012).
Rio, D. C., Ares, M. Jr., Hannon, G. J. & Nilsen, T. W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb Protoc 2010, pdbprot439. https://doi.org/10.1101/pdb.prot5439 (2010).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25, 402–408. https://doi.org/10.1006/meth.2001.1262 (2001).
Hummon, A. B., Lim, S. R., Difilippantonio, M. J. & Ried, T. Isolation and solubilization of proteins after TRIzol extraction of RNA and DNA from patient material following prolonged storage. Biotechniques 42, 467–470. https://doi.org/10.2144/000112401 (2007).
Acknowledgements
Chatcharin Kamsom was supported by a Postgraduate Study Support Grant of Faculty of Medicine, Khon Kaen University. This study was partly supported by Faculty of Medicine, Khon Kaen University, Thailand (Grant Number IN67053).
Author information
Authors and Affiliations
Contributions
C.K., C.P., T.K. and S. Phanthanawiboon conceptualized the research work. C.K., C.T. and S. Papalee designed and performed experiments. C.K. analyzed, interpreted and drafted the manuscript. C.K., S.W.E. and S. Phanthanawiboon reviewed and revised the submitted version. All authors who contributed to the article have approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kamsom, C., Edwards, S.W., Thaosing, J. et al. Altered neutrophil responses to dengue virus serotype three: delayed apoptosis is regulated by stabilisation of Mcl-1. Sci Rep 14, 18414 (2024). https://doi.org/10.1038/s41598-024-68642-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68642-x
- Springer Nature Limited