Abstract
Acute kidney injury (AKI) is one of the most frequent and prognostic-relevant complications of cardiogenic shock (CS) complicating myocardial infarction (MI). Mechanical circulatory assist devices (MCS) like left ventricular Impella microaxial pump have increasingly been used in the last decade for stabilization of hemodynamics in those patients. Moreover, a protective effect of Impella on renal organ perfusion could recently be demonstrated. However, data identifying early risk predictors for developing AKI during Impella support in CS are rare. Data of hemodynamics and renal function from 50 Impella patients (January 2020 and February 2022) with MI-related CS (SCAI stage C), were retrospectively analyzed using e.g. multivariate logistic regression analysis as well as Kaplan–Meier curves and Cox regression analysis. 30 patients (60%) developed AKI. Central venous pressure as an indicator for venous congestion (OR 1.216, p = 0.02), GFR at admission indicating existing renal damage (OR 0.928, p = 0.002), and reduced central venous oxygen saturation (SvO2) as a marker for decreased tissue perfusion (OR 0.930, p = 0.029) were independently associated with developing an AKI. The 30-day mortality rate was significantly higher in patients with AKI stage 3 (Stage 1: 0%, Stage 2: 0%, Stage 3; 41.6%, p = 0.014) while AKI stage 3 (HR 0.095, p = 0.026) and norepinephrine dosage (HR 1.027, p = 0.008) were independent predictors for 30-day mortality. AKI as a complication of MI-related CS occurs frequently with a major impact on prognosis. Venous congestion, reduced tissue perfusion, and an already impaired renal function are independent predictors of AKI. Thus, timely diagnostics and a focused treatment of the identified factors could improve prognosis and outcome.
Similar content being viewed by others
Introduction
Cardiogenic shock (CS) is a feared complication in approximately 10% of acute myocardial infarction (MI) with a 30-day mortality of up to 40% 1. The impairment of myocardial contractility in CS is the most relevant pathophysiological problem. A reduced cardiac output (CO) results in reduced blood pressure, which in turn further impairs organ and coronary perfusion and, consequently, myocardial contractility. As part of the development of this so-called downward shock spiral, tissue and end-organ perfusion thus deteriorates considerably with corresponding consequences in terms of organ failure 2.
Acute kidney injury (AKI) is one of the most frequent complications of a reduced CO in CS with a cumulative risk of 20–35% and a significant impact on prognosis 3. Patients with AKI are accompanied by a 12 times greater 90-day mortality and the additional necessity of renal replacement therapy (RRT) further increases intrahospital mortality (62% vs. 46%) 3,4,5,6. To stabilize hemodynamics, high dosages of vasopressors are very often being substituted. However, vasoconstrictors only achieve a pseudo normalization of blood pressure while the systemic vascular resistance and hence the cardiac afterload rise, which in turn further burdens myocardial contractility and organ perfusion. During the past years, percutaneous left ventricular assist devices (pLVAD) such as the Impella micro axial pump have increasingly been used to improve CO, reduce vasopressor dosages, and thus optimize tissue and end-organ perfusion and at least function 7,8,9. While some studies have investigated the prevalence of AKI in CS, data analyzing patients with CS being supported with Impella are rare. The goal of this study was to evaluate early risk factors and define subsequent predictor characteristics for developing AKI during Impella support in CS as well as to identify potential therapeutic options during Impella support concerning the predisposing factors of AKI.
Methods
Study design
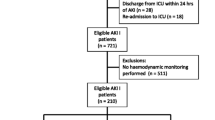
This was a retrospective data analysis of patients with CS and Impella support, admitted to the University Hospital of Marburg from January 2020 to February 2022. To be included in this analysis, patients had to present with CS due to myocardial infarction, corresponding to stage C of the SCAI classification. Data of patients with single kidney and underlying autoimmune or polycystic kidney disease were not included in this analysis.
Risk predictors for developing AKI and the influence of AKI on survival were retrospectively evaluated.
The definition of CS predisposed the presence of the following three factors: (1) impaired systolic blood pressure < 90 mmHg, longer than 30 min or necessary catecholamine support to maintain systolic blood pressure ≥ 90 mmHg, (2) onset of pulmonary congestion (3) clinical evidence of burdened end-organ perfusion (one or more of the following: serum lactate above 2.0 mmol/L, cold and wet cutis, urine output below 30 ml/h, pathological mental function), corresponding to SCAI stage C 10.
AKI was defined as an elevation of serum creatinine ≥ 0.3 mg/dl during the initial 48 h after admission to the hospital according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria 11. Urine output criteria were not considered since the initial administration of diuretics could represent a relevant confounder 12. For patients without any recent data regarding creatinine levels (n = 3), we assessed baseline creatinine by implementing an estimated glomerular filtration rate (eGFR) of 75 ml/min/1.73 m2 (eGFR approach) 13,14,15. Stages of chronic kidney disease (CKD) were determined by assessment of baseline (admission to the hospital) creatinine levels according to current KDIGO guidelines 16.
In all patients, the implantation of Impella and the pulmonary artery catheter (PAC) for invasive hemodynamic measurements were performed within the first 120 min after admission according to standard operating procedures in the catheterization laboratory before the PCI procedure of the culprit lesion took place (suppl. Table 1). Before implantation of the Impella, the LVEF was measured using echocardiography.
Invasive hemodynamic measurement
PAC was implemented to obtain hemodynamic parameters like cardiac output (CO), systolic arterial pulmonary pressure (sPAP), diastolic arterial pulmonary pressure (dPAP) and mean arterial pulmonary pressure (meanPAP), pulmonary capillary wedge pressure (PCWP), central venous pressure (CVP), central venous oxygen saturation (SvO2), systemic vascular resistance (SVR) and pulmonary artery pulsatility index (PAPi). Parameters were taken within the first 24 h after admission to the hospital, after the patient´s arrival on the ICU with ongoing Impella support.
Comorbidities, clinical parameters, and ICU scores
Comorbidities and clinical and treatment-related parameters, like systolic, diastolic, and mean arterial pressure, heart rate, catecholamine dosages and fluids, laboratory parameters including serum creatinine and GFR and lactate were registered and evaluated during the in-hospital stay. The common ICU scores (SAPS II, SOFA) and the Horowitz Index were documented within the first 24 h after admission.
Clinical outcomes
Primary endpoint was the development of AKI. Risk factors for the development of AKI, all-cause mortality and risk factors for mortality were defined as secondary endpoints.
Statistical analysis
This was a retrospective data analysis. Data are presented as absolute variables and percentages (%) for categorical variables and either median with interquartile range (IQR: 25th–75th percentile) or mean with standard deviation according to the distribution of the variables. Normality was assessed by using Kolmogorov–Smirnov as well as the Shapiro–Wilk test. After testing for normal distribution, the Student’s t-test or Mann–Whitney test was implemented to test for differences between various characteristics. For categorical variables, Fisher’s exact test or chi-square test was used, as appropriate. We implemented binary logistic regression analysis to identify variables associated with AKI. Considering significant associations of univariate analysis (p < 0.1 accepted for retention), multivariate logistic regression with backward elimination and probability of occurrence of 0.05 and removal of 0.10 was performed to identify independent predictors. Pearson's correlation was implemented to analyze for correlations with a p < 0.05 considered statistically significant. Kaplan–Meier curves with log-rank (Mantel-Cox) test was used to analyze 30-day survival. Cox regression analysis was performed to identify independent predictors considering variables with significant association at univariate analysis (retention p < 0.1). All analyses were made using SPSS 28 (IBM Corp., USA) and GraphPad Prism 8.0. A two-sided p-value < 0.05 was considered statistically significant.
Ethics approval and consent to participate
The data analysis was performed according to the Declaration of Helsinki and was approved by the Ethics Committee of the University Hospital of Marburg (protocol code: study 136/17, date of approval: 11 October 2019). Informed consent was acquired from all patients involved.
Results
Overall cohort
Data from 50 patients were analyzed. The demographics, baseline characteristics, and relevant comorbidities of the overall cohort are displayed in Table 1. All patients presented with shock stadium C according to the SCAI classification. Only Impella MCS was used in this study cohort. None of the patients' hemodynamic situation deteriorated during Impella support and none suffered cardiac arrest. The study population had a mean age of 67 ± 13 years with 74% male individuals. 18 (36%) of the patients were accompanied by a 3-vessel coronary heart disease (CHD), 19 (38%) had a 2-vessel CHD and in 13 (26%) a 1-vessel CHD was diagnosed. Culprit lesions were in 4 patients (8%) in the left main coronary artery (LMCA), in 19 patients (38%) in the left anterior descending (LAD), in 6 patients (12%) in the LAD and first diagonal branch, in 4 patients (8%) in the LAD and in the second diagonal branch, in 6 patients (12%) in the intermediary branch, in 8 patients (16%) in the LCX, and in 3 patients (6%) in the right posterolateral branch. None of the patients presented a right ventricular infarction (Suppl. Table 1). Every patient underwent coronary angiography with appropriate percutaneous coronary intervention and implantation of left ventricular Impella (CP) under fluoroscopic control in the catheter laboratory. In all patients, arterial access was established via the femoral artery. 43 (86%) patients had a medical history of arterial hypertension (aHT), 35 (70%) were accompanied by known dyslipidemia, and in 19 (38%) patients diabetes mellitus was diagnosed. In the overall cohort, the mean LVEF was 37 ± 12% and the mean duration of Impella support was 9 ± 6 days, without any significant differences in the subgroups (AKI, Non-AKI). No relevant hemolysis (significant hemoglobine decrease, macrohematuria, bilirubin increase, free hemoglobine) could be detected. Data on renal function and hemodynamic data of the overall cohort are shown in Tables 2 and 3 respectively.
Prevalence of AKI and risk factors
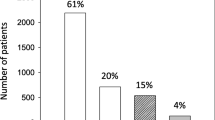
40 (80%) patients had a known medical history of chronic kidney disease (CKD). In 10 (20%) patients CKD stadium G1 had been diagnosed and 20 (40%) patients had a known CKD stadium G2 whereas 15 (30%) and 5 (10%) individuals were accompanied by CKD stadium G3a and G3b respectively. At admission, the whole cohort had a mean creatinine of 1.39 ± 0.69 mg/dl and in 13 (26%) patients AKI was diagnosed according to 2012 KDIGO criteria whereas, after the first 48 h, a total of 30 (60%) patients developed AKI. 4 (8%) of these patients were classified at AKI stage1, 2 (4%) at AKI stage 2 and 24 (48%) at AKI stage 3. In 24 (48%) patients renal replacement therapy with continuous hemodiafiltration (CVVHDF) had to be conducted (Table 2).
Comparing the no AKI and AKI subgroups patients with AKI had a significantly higher SOFA and SAPS II score (6 (6) vs. 11 (5), p = 0.001 and 36.3 ± 11.9 vs. 50.17 ± 14.51, p = 0.001 respectively) (Table 1). Furthermore, AKI patients were accompanied by a significantly lower GFR at admission (43.27 ± 17.89 ml/min vs. 70.5 ± 26.01 ml/min, p < 0.001) as well as by a significantly reduced lowest GFR level (37.23 ± 14.3 ml/min vs. 63.7 ± 20.51 ml/min, p < 0.001) (Table 2).
On the other hand, no significant differences of SAP, MAP, heart rate, and CO were documented. Interestingly CVP was significantly elevated in the AKI subgroup (12.33 ± 6.01 vs. 7.9 ± 5.1, p = 0.007) while the tissue perfusion-related values of lactate and SvO2 were reduced in AKI individuals (1.9 ± 1.96 mmol/l vs. 1.3 ± 0.68 mmol/l, p = 0.07 and 59.12 ± 12.63% vs. 66.9 ± 17.19%, p = 0.07 respectively) but p values missed slightly statistical significance. Furthermore, regarding dosages of vasopressors and inotropics, no relevant differences between the two subgroups were documented (Table 3).
In the univariate analysis, a highly significant association of chronic kidney disease (CKD) history (OR 4.846, p = 0.04), CVP at admission (OR: 1.185, p = 0–014), SvO2 at admission (OR 0.942, p < 0.001) and GFR at admission GFR at admission (OR 0.951, p = 0.002) with the development of AKI could be demonstrated. Moreover, multivariate analysis revealed CVP at admission (OR 1.216, p = 0.020), and SvO2 at admission (OR 0.93, p = 0.029) as independent predictors for the onset of AKI. Due to the collinearity of the onset of AKI and GFR at admission, GFR was not included in the multivariate analysis (Table 4).
Analysis of survival
The 30-day mortality of the whole cohort was 22%. Patients with AKI were accompanied by a significantly higher 30-day mortality than patients without AKI (n = 10/30 (33%) vs. n = 1/20 (5%); p = 0.019). The overwhelming percentage of diseased patients was in AKI stadium 3 (n = 10/24; 41, 6%) and, consequently, this subgroup was accompanied by an even higher 30-day mortality rate (Figs. 1, 2 and 3).
In the univariate analysis, lactate at admission (HR 1.489, p = 0.004), the dosage of norepinephrine (HR 1.027, p = 0.012), and AKI stage 3 (HR 0.074, p = 0.013) were significantly associated with survival. GFR at admission was also strongly associated with 30-day mortality but the p-value did not reach statistical significance (p = 0.054). In the Cox regression analysis, AKI stage 3 (HR = 0.095, p = 0.026) and norepinephrine dosage (HR 1.027, p = 0.008) were independently associated with 30-day mortality (Table 5).
Discussion
We here defined early predictors for the occurrence of AKI during Impella support, which may be useful to optimize therapy control in CS. To our knowledge, this is the first study to analyze the occurrence of AKI according to the KDIGO criteria in CS patients during Impella support, including the evaluation of a wide range of patient characteristics, invasively obtained hemodynamics, laboratory parameters, and other indices that may influence renal function in shock.
In our Impella cohort, the prevalence of AKI was 60%, in accordance with other published data 3,17,18. The development of AKI was significantly more common in patients with CKD, possibly reflecting a reduced compensatory ability caused by the development of chronic renal organ damage. Moreover, as expected, patients developing AKI had a significantly lower GFR at admission.
Ultimately, three independent and early predictors of AKI could be defined in our cohort, namely GFR, CVP, and SvO2. While pre-existing patient-associated parameters such as CKD and thus reduced GFR on admission cannot be directly influenced, the two other hemodynamic parameters CVP and SvO2 can be improved by therapeutic methods (fluid management, catecholamines) and possibly also by MCS such as the Impella.
According to our data, hemodynamic parameters that reflect myocardial function and organ and tissue perfusion in CS seem to play an outstanding role in the occurrence of AKI and renal organ failure. However, the quality and effectiveness of the interaction between the individual treatment options used and the parameters monitored and adjusted, could ultimately determine the outcome and be decisive for the preservation of organ function in CS.
In patients with AKI, in univariate analysis, SvO2 was noteworthy lower and, correspondingly, although the p-value moderately missed statistical significance, lactate level was found to be considerably higher compared to non-AKI patients indicating an impaired tissue and end-organ perfusion as a consequence of low cardiac output. While AKI patients presented no significant differences of MAP and PCWP, CVP and sPAP on the other hand were notably elevated reflecting a substantial venous congestion and right ventricular burden. Whereas the elevated CVP and sPAP in AKI patients could pathophysiological be attributed to systemic overload in the situation of compromised hemodynamics as well as to oliguria or anuria in CS, the left ventricular unloading with the Impella seems to effectively support left ventricle function since PCWP values of patients with and without AKI presented comparable.
In addition, the vicious circle of impaired tissue and organ perfusion, which may also be caused by increased venous congestion, leads to the activation of a cascade of neuro-hormonal and inflammatory mechanisms that further deteriorate organ function 19,20,21. Moreover, in combination with oxidative stress, the so-called glycosaminoglycan (CAG) networks could be damaged 22. The CAGs, which are responsible for buffering sodium, influence endothelial function. An impaired function of the CAGs leads to an elevation of vascular resistance and imbalanced endothelial nitric oxide production. The resulting endothelial dysfunction requires an increased workload for both, the left and right myocardial ventricle, further impairing end-organ perfusion 23. Thus, in case, right ventricular filling pressure increases for example in a situation of CS complicating right ventricular MI, a backward failure may lead to a further increase of CVP. Furthermore, also left ventricular failure with consecutive congestion could cause pulmonary edema with secondary deterioration of right ventricular function and consecutive abdominal organ congestion. Among all complications of AKI, volume overload aggravating venous congestion seems to be the one with the greatest impact on mortality 24. At the same time, recent data emphasize that venous congestion and CVP are also one of the most important pathomechanisms for the development of CKD 25,26,27,28,29,30,31,32. This seems in line with to pathophysiological understanding, as a backward failure can usually also be accompanied by a forward failure with consecutive organ minder perfusion. According to the findings of this study cohort, the elevated CVP could be attributed to left ventricular systolic function impairment with consecutive post capillary congestion leading to elevated sPAP burdening right ventricular function ultimately increasing venous congestion.
Therefore, in our opinion, the reduction of venous congestion through diuretics or early RRT while improving left ventricular function and organ perfusion, possibly by additional Impella support may be beneficial for a more favourable outcome.
As mentioned above, SvO2 at admission could be identified as an independent and early predictor for the occurrence of AKI. This association underscores the relevance of adequate organ and tissue perfusion in the situation of CS to avoid end-organ failure. The use of catecholamines and vasopressors in CS often temporarily stabilizes blood pressure at the expense of severely increasing systemic peripheral vascular resistance and cardiac afterload 33,34,35. Interestingly, as we were able to show, AKI stage 3 and norepinephrine dosages were independent predictors of 30-day mortality. Therefore, the reduction of vasopressor doses during CS should be aimed for. In this regard, the use of MCS such as the Impella is an option that may be considered in hemodynamically unstable patients, as during Impella support the CO and SvO2 increase 9.
As it is known from the current literature, in the situation of CS, the 30-day mortality remains higher than 50% without any noteworthy changes in the last two decades 3. The 30-day mortality of our cohort, however, was lower at 22%. This may be attributed to the fact that the patients of this cohort received early hemodynamic support with left ventricular Impella implemented according to standard algorithms in CS. However, the high SOFA score of this cohort of 10 (IQR:5) with an expected mortality between 33 and 50%, which is in line with the known mortality rates in the current literature contradicts this explanation at first glance 36. On the other side, one may postulate, that the interaction of myocardial function and hemodynamic support by MCS may improve the negative prognosis predicted by the scores.
In summary, early targeted monitoring of relevant parameters, as demonstrated here, should be recommended to enable timely adaptation of therapeutic approaches, in particular relieving venous congestion, increasing CO, and reducing peripheral vascular resistance to improve tissue and organ oxygenation, which in turn may reduce the prevalence of AKI 37,38,39. The use of MCS may also be supportive in this respect 40.
Limitations
The relatively small number of patients and the single center experience belong to the limitations of this study. Furthermore, the retrospective analysis also limits the interpretation of the data. However, to our knowledge, this is the first study to analyze early and subsequent predictors of AKI according to the KDIGO criteria in CS patients with Impella support using a broad spectrum of parameters and characteristics. To further evaluate the clinical relevance of these data, larger prospective randomized studies are needed that include a broader cohort of patients with different stages of CS as defined by the SCAI classification.
Conclusions
Renal function is one of the most relevant key factors regarding the prognosis of patients with CS and AKI deteriorates outcome dramatically. Increased venous congestion, reduced SvO2, and GFR on admission are early independent predictors for AKI complicating CS in patients supported with Impella, while catecholamine dosages and the onset of AKI are independent predictors of mortality. The reduction of catecholamine dosages during ongoing support with pVLAD like the Impella and the decrease of venous congestion by additional volume restriction and if indicated the early use of RRT may improve prognosis.
Data availability
Data will be made available upon individual request (contact: corresponding author).
References
Samsky, M. D. et al. Cardiogenic shock after acute myocardial infarction: A review. JAMA 326(18), 1840–1850 (2021).
Thiele, H., Ohman, E. M., de Waha-Thiele, S., Zeymer, U. & Desch, S. Management of cardiogenic shock complicating myocardial infarction: An update 2019. Eur. Heart J. 40(32), 2671–2683 (2019).
Tarvasmäki, T. et al. Acute kidney injury in cardiogenic shock: Definitions, incidence, haemodynamic alterations, and mortality. Eur. J. Heart Fail. 20(3), 572–581 (2018).
Mezhonov, E. M., Vialkina, I. A., Vakulchik, K. A. & Shalaev, S. V. Acute kidney injury in patients with ST-segment elevation acute myocardial infarction: Predictors and outcomes. Saudi J. Kidney Dis. Transpl. 32(2), 318–327 (2021).
Cosentino, N. et al. Acute kidney injury and in-hospital mortality in patients with ST-elevation myocardial infarction of different age groups. Int. J. Cardiol. 344, 8–12 (2021).
Vallabhajosyula, S. et al. Temporal trends, predictors, and outcomes of acute kidney injury and hemodialysis use in acute myocardial infarction-related cardiogenic shock. PLoS ONE 14(9), e0222894 (2019).
Karatolios, K. et al. Impella support compared to medical treatment for post-cardiac arrest shock after out of hospital cardiac arrest. Resuscitation 126, 104–110 (2018).
Lüsebrink, E. et al. Percutaneous transvalvular microaxial flow pump support in cardiology. Circulation 145(16), 1254–1284 (2022).
Patsalis, N. et al. Renal protection and hemodynamic improvement by impella microaxial pump in patients with cardiogenic shock. J. Clin. Med. 11(22), 6817 (2022).
Naidu, S. S. et al. SCAI SHOCK stage classification expert consensus update: A review and incorporation of validation studies: This statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association (AHA), European Society of Cardiology (ESC) Association for Acute Cardiovascular Care (ACVC), International Society for Heart and Lung Transplantation (ISHLT), Society of Critical Care Medicine (SCCM), and Society of Thoracic Surgeons (STS) in December 2021. J. Am. Coll. Cardiol. 79(9), 933–946 (2022).
Kidney Disease, Improving Global Outcomes (KDIGO). Kidney Int. Suppl. 2, 19–36 (2012).
Sims, A. J., Hussein, H. K., Prabhu, M. & Kanagasundaram, N. S. Are surrogate assumptions and use of diuretics associated with diagnosis and staging of acute kidney injury after cardiac surgery?. Clin. J. Am. SocNephrol. 7(1), 15–23 (2012).
Siew, E. D. & Matheny, M. E. Choice of reference serum creatinine in defining acute kidney injury. Nephron 131(2), 107–112 (2015).
Bellomo, R., Ronco, C., Kellum, J. A., Mehta, R. L. & Palevsky, P. Acute Dialysis Quality Initiative Workgroup: Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit. Care 8, R204–R212 (2004).
National Kidney Foundation K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease. Evaluation, classification and stratification. Am. J. Kidney Dis. 39(2 Suppl 1), S76–S92 (2002).
Kidney International Supplements (2013) 3, 4.
Fuernau, G. et al. Prognostic impact of established and novel renal function biomarkers in myocardial infarction with cardiogenic shock: A biomarker substudy of the IABP-SHOCK II-trial. Int. J. Cardiol. 191, 159–166 (2015).
Werdan, K., Ruß, M., Buerke, M., Delle-Karth, G., Geppert, A., Schöndube, F. A., German Cardiac Society, German Society of Intensive Care and Emergency Medicine, German Society for Thoracic and Cardiovascular Surgery (Austrian Society of Internal and General Intensive Care Medicine; German Interdisciplinary Association of Intensive Care and Emergency Medicine; Austrian Society of Cardiology; German Society of Anaesthesiology and Intensive Care Medicine; German Society of Preventive Medicine and Rehabilitation. Cardiogenic shock due to myocardial infarction: diagnosis, monitoring and treatment: a German-Austrian S3 Guideline. DtschArztebl Int. 109(19), 343–351 (2012).
Harjola, V. P. et al. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 19(7), 821–836 (2017).
Calfee, C. S. & Matthay, M. A. Clinical immunology: Culprits with evolutionary ties. Nature 464(7285), 41–42 (2010).
Rudiger, A. Understanding cardiogenic shock. Eur. J. Heart Fail. 17(5), 466–467 (2015). https://doi.org/10.1002/ejhf.265. Erratum in: Eur. J. Heart Fail. 17(6), 639 (2015).
Nijst, P. et al. The pathophysiological role of interstitial sodium in heart failure. J. Am. Coll. Cardiol. 65(4), 378–388 (2015).
Marti, C. N. et al. Endothelial dysfunction, arterial stiffness, and heart failure. J. Am. Coll. Cardiol. 60(16), 1455–1469 (2012).
Payen, D. et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit. Care 12(3), 74 (2008).
Sheikh, O., Nguyen, T., Bansal, S. & Prasad, A. Acute kidney injury in cardiogenic shock: A comprehensive review. Catheter Cardiovasc. Interv. 98, E91–E105 (2021).
McCallum, W. & Sarnak, M. J. Cardiorenal syndrome in the hospital. Clin. J. Am. Soc. Nephrol. 18(7), 933–945 (2023).
Andrei, S., Bahr, P. A., Nguyen, M., Bouhemad, B. & Guinot, P. G. Prevalence of systemic venous congestion assessed by Venous Excess Ultrasound Grading System (VExUS) and association with acute kidney injury in a general ICU cohort: a prospective multicentric study. Crit. Care 27(1), 224 (2023).
D’Marco, L. Congestive nephropathy. Int. J. Environ. Res. Public Health 19(5), 2499 (2022).
Palazzuoli, A. et al. Chronic kidney disease and worsening renal function in acute heart failure: Different phenotypes with similar prognostic impact?. Eur. Heart J. Acute Cardiovasc. Care 5(8), 534–548 (2016).
van den Akker, J. P. C., Bakker, J., Groeneveld, A. B. J. & den Uil, C. A. Risk indicators for acute kidney injury in cardiogenic shock. J. Crit. Care 50, 11–16 (2019).
Legrand, M., Mebazaa, A., Ronco, C. & Januzzi, J. L. Jr. When cardiac failure, kidney dysfunction, and kidney injury intersect in acute conditions: The case of cardiorenal syndrome. Crit. Care Med. 42(9), 2109–2117 (2014).
Mullens, W. et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J. Am. Coll. Cardiol. 53(7), 589–596 (2009).
Lüsebrink, E. et al. Percutaneous transvalvular microaxial flow pump support in cardiology. Circulation 145(16), 1254–1284 (2022).
Upadhyaya, V. D. et al. Outcomes of renal function in cardiogenic shock patients with or without mechanical circulatory support. J. Clin. Med. Res. 13(5), 283–292 (2021).
Flaherty, M. P. et al. Hemodynamic support with a microaxial percutaneous left ventricular assist device (Impella) protects against acute kidney injury in patients undergoing high-risk percutaneous coronary intervention. Circ. Res. 120(4), 692–700 (2017).
Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315(8), 801–810 (2016).
Nohria, A. et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J. Am. Coll. Cardiol. 41(10), 1797–1804 (2003).
Mullens, W. & Nijst, P. Cardiac output and renal dysfunction: Definitely more than impaired flow. J. Am. Coll. Cardiol. 67, 2209–2212 (2016).
Hanberg, J. S. et al. Reduced cardiac index is not the dominant driver of renal dysfunction in heart failure. J. Am. Coll. Cardiol. 67, 2199–2208 (2016).
Chatzis, G. et al. Early Impella support in postcardiac arrest cardiogenic shock complicating acute myocardial infarction improves short- and long-term survival. Crit. Care Med. 49(6), 943–955 (2021).
Funding
Open Access funding enabled and organized by Projekt DEAL. This study acquired no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, NP, BS, and BM; Data curation, NP, GC, MC, JK; Data analysis, NP, GC, MC, JK; Methodology, NP, BS, and BM; Project administration, SS; Resources, BS and BM; Software, NP, JK, and GC; Supervision, BS; Validation, NP, GC, BS, and BM; Visualization, NP, JK, GC, and SS; Writing – original draft, NP and BM; Writing – review & editing, GC, SS, MC, BS, and BM.
Corresponding author
Ethics declarations
Competing interests
B.M. received research funding from Abiomed; J.K., G.C., B.S., and B.M. receive speaker’s honoraria from Abiomed; no other of the remaining authors declared any disclosures.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Patsalis, N., Kreutz, J., Chatzis, G. et al. Early risk predictors of acute kidney injury and short-term survival during Impella support in cardiogenic shock. Sci Rep 14, 17484 (2024). https://doi.org/10.1038/s41598-024-68376-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68376-w
- Springer Nature Limited