Abstract
Chronic inflammation plays a crucial role in coronary artery disease (CAD), but differences in specific cytokine profiles between acute coronary syndrome (ACS) and stable CAD remain unknown. We investigated cytokine differences between these two manifestations of CAD. The study included 308 patients with angiographically detected, hemodynamically significant CAD: 150 patients undergone angiography for ACS, 158 patients undergone angiography for stable CAD. To assess dynamic changes, 116 patients had index angiogram at least 3 months earlier. We measured the serum concentrations of 48 circulating cytokines. The ACS group had decreased interleukin (IL) 4 (p = 0.005), and increased IL-8 (p = 0.008), hepatocyte growth factor (HGF) (p < 0.001) and macrophage colony-stimulating factor (M-CSF) (p = 0.002) levels compared with the stable CAD group. Multivariable logistic regression revealed increased levels of HGF (OR 18.050 [95% CI 4.372–74.517], p < 0.001), M-CSF (OR 2.257 [1.375–3.705], p = 0.001) and IL-6 (OR 1.586 [1.131–2.224], p = 0.007), independently associated with ACS. In the post-angiography group, only diminished platelet-derived growth factor-BB levels in ACS-manifested patients were observed (OR 0.478, [0.279–0.818], p = 0.007). Cytokine profiles differ between ACS and stable CAD. Such differences seem to be mainly reversible within 3 months after ACS. Thus, targeting one or two cytokines only might not offer one-size fits all-therapeutic approach for CAD-associated inflammation.
Trial registration: NCT03444259.
Similar content being viewed by others
Introduction
Coronary artery disease (CAD) is a major cause of death globally and the incidence is increasing in Western countries1. Patients with acute coronary syndromes (ACS) have less favorable long-term prognosis compared to those with stable chronic CAD in terms of sudden cardiac death, myocardial infarction (MI) and repeated revascularization2,3. The reasons for this adverse trajectory in patients with ACS remain incompletely understood.
Atherosclerosis is a chronic inflammatory disease4. Cytokines are inflammatory modulators that have been shown to affect atherogenesis, plaque rupture, stabilization and atherothrombosis5. Therefore, cytokines play a crucial role in the manifestation of CAD. However, it is not known whether cytokines differ in patients with ACS compared to those with stable CAD. Moreover, it is not known whether cytokine profiles stabilize after treatment for ACS to levels observed in patients with stable CAD.
The aim of the present study was to determine the differences in cytokine levels between patients with ACS and those with stable CAD. Our hypothesis was that cytokine profiles differ between these two manifestations of coronary artery disease and could provide novel biomarkers to recognize patients at higher risk of adverse cardiac events.
Materials and methods
This analysis included patients from two prospective studies, the CAREBANK and the FACT studies, that enrolled patients undergoing diagnostic coronary artery angiography for clinical indications at the Heart Center, Turku University Hospital, Turku, Finland. The present study is registered in ClinicalTrials.gov (ClinicalTrials.gov Identifier: NCT03444259). Both studies were approved by the Ethical Committee of the Hospital District of South-West Finland. Studies conformed with the Declaration of Helsinki as revised in 2002. The patient cohort comprised a total of 469 patients.
Patient selection from the CAREBANK and the FACT studies for the present analysis is summarized in Fig. 1. CAREBANK is a prospective biobank of patients undergoing adult cardiac surgery (i.e., coronary artery bypass grafting, heart valve surgery, aortic surgery, and resection of cardiac tumors) at the Turku University Hospital since February 2016. Patient records were individually reviewed, and baseline clinical data were collected into a standardized structured datasheet by trained personnel6. The study has been monitored by third-party licensed data-monitor. Preoperative study blood samples (ethylenediaminetetraacetic acid treated (EDTA) -plasma and serum) were obtained at the same time with routine sampling by the accredited hospital laboratory. The database is pseudonymized and data privacy regulations have been followed during the study. Written informed consent for the study was obtained from all patients before the study enrolment. Six patients were excluded from the registry due to missing cytokine measurements or failure in ELISA analyses. The CAREBANK study population consisted of 322 patients.
Flow chart of study eligibility and formation of the study cohorts. AAA abdominal aortic aneurysm, AP angina pectoris, CAD coronary artery disease, MACCE major adverse cardio and cerebrovascular event, NSTEMI non-ST-elevation myocardial infarction, PVD peripheral vascular disease, STEMI ST-elevation myocardial infarction, UAP unstable angina pectoris.
The FACT cohort included consecutive patients who had undergone a diagnostic coronary angiography between September 2015 and December 2015 at the Turku University Hospital. The FACT cohort consisted of patients in whom circulating cytokine levels were measured at least 3 months after the index coronarography. For ACS patients, this enabled the evaluation of cytokines’ dynamic changes after treatment for ACS. Overall, 267 patients fulfilling the inclusion criteria of this study were contacted and 189 of them gave written informed consent. EDTA-plasma and serum samples were taken at certified hospital laboratories. The period between recent major adverse cardiovascular event (MACCE), including nonfatal MI, nonfatal stroke and unscheduled coronary revascularization, and blood sampling was required to be ≥ 3 months. Blood sample was obtained in 152 patients. Altogether, 11 FACT patients were excluded (heart transplant: n = 1; MACCE < 3 months after index coronarography: n = 1; cytokine analyses not available: n = 9). The final FACT cohort consisted of 141 patients.
Five patients had notably high values of various markers and were excluded from the final analyses. We also excluded patients who underwent vascular endoprosthesis operation for abdominal aortic aneurysm (n = 2) or lower limb ischemia (n = 4). Indication for coronary angiography was missing in two patients and they were excluded. Overall, 308 patients had an angiographically proven CAD and were included in this study.
The diagnostic criteria for significant CAD were symptoms related to coronary artery disease and > 50% diameter stenosis in one or more coronary arteries. CAD patients were further divided into two groups: (1) the ACS group, i.e., having ST segment elevation myocardial infarction (STEMI), non-ST segment elevation myocardial infarction (NSTEMI) or unstable angina pectoris (UAP), and the stable CAD group, i.e., having stable angina pectoris. Diabetes, dyslipidemia and hypertension were defined as diseases requiring drug therapy. Based on the smoking history, the patients were classified as never-smoked, ex-smoker, or current smoker.
Blood sampling and multiplex analysis
Blood sample analyses were conducted at the MediCity Research Laboratory, University of Turku, Turku, Finland. We analyzed the levels of 48 different cytokines by using Bio-Plex panel Pro Human Cytokine Screening Panel, 48-Plex (#12007283, BioRad) according to the manufacturer’s instructions.
Statistical analysis
Continuous variables were reported as means and standard deviation as well as medians and 25th and 75th interquartile range. Categorical variables are reported as counts and percentages. The Shapiro–Wilk-test was used to examine if the cytokine concentration levels had a normal distribution. As the cytokine levels were not normally distributed, a Mann–Whitney U-test was used to examine the differences between the ACS and the stable CAD groups and between controls. The prevalence of cardiovascular risk factors, medications and other baseline characteristics between the groups were compared using the Chi-square test. Variables with a p < 0.05 in univariate analysis were entered into a logistic regression models, which were used to explore the associations between the clinical variables and cytokine levels. A p-value of < 0.05 was considered statistically significant for clinical variables, and due to nature of the study design, a stricter p-value of < 0.01 was used in cytokine analyzes. Due to small study cohort, no p-value corrections were made to avoid overcorrection. Statistical analyses were performed using the SPSS (IBM SPSS Statistics, SPSS Inc., IBM, Chicago, Illinois, USA) and the R 4.3.17 (for macOS 13.5) statistical softwares.
As the analyzes were conducted in two different study cohorts at different times, we used a Z-score to diminish the potential differences due to batch-induced variability in cytokine analyses between the CAREBANK and FACT cohorts. Z-score was calculated for each cytokine separately according to the following equation:
where \({x}_{i}\) is cytokine concentration of each patient, \(\overline{x(controls)}\) is the mean of cytokine concentrations in healthy controls in each cohort, and SD is standard deviation of cytokine concentration.
Results
Study population
The study cohort consisted of 308 patients with angiographically-confirmed CAD. The indication for coronary angiography was an ACS in 150 (48.7%) patients, whereas 158 (51.3%) patients had stable CAD. The proportions of ACS patients were 47.4% and 50.9% in the CAREBANK and FACT cohorts, respectively. Baseline characteristics of these patients are presented in Table 1. Both study cohorts showed a major prevalence of male patients. The prevalence of rheumatic disease was higher in patients with stable disease in CAREBANK cohort, but not amongst FACT patients. On the contrary, stable CAD patients had more frequently heart failure in the FACT cohort, but not in the CAREBANK cohort. Unsurprisingly, the use of aspirin and ADP receptor inhibitors was more common among patients with ACS compared to stable CAD.
Predictors for acute coronary syndrome
The levels of interleukin 2 (IL-2), IL-15, interleukin-12 subunit p40 (IL-12p40) and monocyte-chemotactic protein 3 (MCP-3) were mostly below the detection limit of the assay.
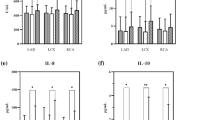
Figure 2 summarizes the levels of significant circulating cytokine levels in the CAREBANK cohort. Compared to patients with stable CAD, patients with ACS had decreased levels of IL-4 (p = 0.005) and increased levels of hepatocyte growth factor (HGF) (p < 0.001), macrophage colony-stimulating factor (M-CSF) (p = 0.002) and IL-8 (p = 0.008). The levels of interleukin-2 receptor subunit alpha (IL-2Rα) (p = 0.013), IL-6 (p = 0.018), macrophage inflammatory protein 1 alpha (MIP-1α) (p = 0.018), stem cell growth factor beta (SCGF-β) (p = 0.033) and tumor necrosis factor beta TNF-β (p = 0.035) were generally higher and level of eotaxin (p = 0.025) lower in ACS patients (p < 0.050). The results of statistical analyses are summarized in Table 2.
Cytokine levels of IL-4, IL-8, M-CSF and HGF in the CAREBANK study cohort. Data are shown as medians and 25th and 75th percentiles. The vertical axis represents cytokine concentration’s Z-scores and horizontal axis different cytokines in the acute coronary syndrome (ACS) group (orange) and stable coronary artery disease (STABLE) group (blue). Thirteen outliers were excluded from the figure. ACS acute coronary syndrome, IL interleukin, M-CSF macrophage colony-stimulating factor, HGF hepatocyte growth factor.
Multivariable logistic regressions adjusted for rheumatic disease and history of never smoking in the CAREBANK patients showed increased levels of HGF (OR 18.050, 95% CI 4.372–74.517, p < 0.001), M-CSF (OR 2.257, 95% CI 1.375–3.705, p = 0.001) and IL-6 (OR 1.586, 95% CI 1.131–2.224, p = 0.007) in ACS patients (p < 0.01). Using a significance level of p < 0.05, also IL-4 (OR 0.739, 95% CI 0.587–0.930, p = 0.010), interferon gamma (IFN-γ) (OR 0.748, 95% CI 0.593–0.944, p = 0.015), IL-8 (OR 1.591, 95% CI 1.081–2.341, p = 0.019), IL-17 (OR 0.699, 95% CI 0.516–0.945, p = 0.020), granulocyte–macrophage colony-stimulating factor (GM-CSF) (OR 0.741, 95% CI 0.560–0.981, p = 0.036), fibroblast growth factor-basic (FGFbasic) (OR 0.595, 95% CI 0.363–0.976, p = 0.040), IL-3 (OR 1.609, 95% CI 1.009–2.564, p = 0.046) and β-NGF (OR 1.434, 95% CI 1.003–2.050, p = 0.048) were independent predictors of ACS.
Comparison 3 months after the index angiogram
ACS patients in the FACT cohort represent those with most recent MACCE at least 3 months earlier. No differences were seen in the levels of cytokines between ACS and stable CAD patients. However, a logistic regression model adjusted for heart failure showed an association between ACS and diminished levels of platelet-derived growth factor B’s (PDGF-B) active form of homozygous dimer PDGF-BB (OR 0.478, 95% CI 0.279–0.818, p = 0.007), increased levels of eotaxin (OR 1.538, 95% CI, 1.089–2.172, p = 0.015) and stem cell factor (SCF) (OR 2.258, 95% CI 1.078–4.728, p = 0.031).
ACS and stable CAD patients compared to disease-free controls
There were 120 patients in CAREBANK study cohort and 22 patients in the FACT cohort with no significant CAD detectable in invasive coronary angiography. We used these patients as disease-free controls to examine how cytokine profiles differ when these two different manifestations of CAD are compared to disease-free controls. We analyzed both study cohorts separately to reduce the potential differences due to batch-induced variability in cytokine analyses. Baseline characteristics of disease-free controls are presented in Supplementary Table S1.
In the CAREBANK cohort, ACS patients had significantly higher levels of HGF (p = 0.002) compared to disease-free controls while M-CSF (p = 0.010), IL-6 (p = 0.011), TNF-α (p = 0.013) and MIP-1β (p = 0.029) achieved a p-value < 0.050 with higher levels in ACS patients. Stable patients had elevated levels of IL-4 (p < 0.001) and IL-17 (p = 0.004) compared to disease-free controls, while IFN-γ (p = 0.014), IL-10 (p = 0.022), IL-1β (p = 0.023), PDGF-BB (p = 0.035) and eotaxin (p = 0.040) were generally higher and level of stem cell growth factor-beta (SCGF-β) (p = 0.019) lower in patients with stable disease. Cytokine levels are presented in Supplementary Fig. S1 and S2.
In the FACT cohort, both ACS and stable CAD patients had higher levels of IL-18 compared to controls (p = 0.002 and 0.011, respectively). Stable CAD patients also had higher concentration of IL-9 (p = 0.005). In contrast to the CAREBANK cohort, the level of SCGF-β was elevated in stable disease compared to controls (p = 0.025). Cytokine levels are presented in Supplementary Fig. S3. Cytokine statistics are presented in more detail in Supplementary Tables S2 and S3.
In multivariable logistic regressions adjusted for age, sex, hypertension, treatment for dyslipidemia and diabetes, and in stable CAD patients also with atrial fibrillation, elevated levels of IL-4 (p = 0.003), IFN-γ (0.009) and lower level of MCP-3 (p = 0.009) and SCGF-β (p = 0.006) were independent predictors for stable CAD compared to controls in the CAREBANK cohort. In ACS patients, TNF-α reached a p-value of 0.011 and higher concentrations predicted ACS. In the FACT cohort, multivariable logistic regressions adjusted for atrial fibrillation, history of smoking, rheumatic disease and treatment for dyslipidemia, elevated levels of IL-17 (p = 0.007) and IL-18 (p = 0.003) were predictors of ACS. In a regression model adjusted with treatment for dyslipidemia, elevated level of IL-18 was also associated with stable disease. All cytokines that achieved a p-value < 0.05 are presented in Supplementary Table S4.
Atherosclerotic burden and cytokine levels
Atherosclerotic burden is a possible factor that could affect cytokine levels. The extent of atherosclerosis was not systematically assessed among the CAREBANK cohort i.e. using a SYNTAX Score. Therefore, we analyzed the extent of coronary artery disease using number of distal anastomoses as surrogate for more advanced disease in CAREBANK patients.
We divided the patients into two groups according to the number of distal anastomoses: patients with “minor” disease burden (one or two anastomoses) and “severe” disease burden (three or more). Information was available on 187 patients, of which 54 had one or two anastomoses and 133 patients had three or more anastomoses. Baseline characteristics were similar between the groups, as only use of aspirin and statin were more common in severe group (data not shown).
Amongst ACS patients, 26 patients had minor (28.6%) and 65 patients severe disease burden, and amongst stable CAD patients, 27 (29.7%) had minor and 65 severe disease burden. There was no difference in disease severity between ACS and stable groups (p = 0.908). There was no significant difference in cytokine levels between these disease burden categories in total cohort, nor in ACS or stable groups separately (data not shown).
Discussion
The study showed that patients with ACS have distinct circulating cytokine profiles compared to patients with stable CAD and that these differences diminish during the course of few months after the cardiac event. This may indicate that the majority of cytokines return to similar levels with stable CAD and new cytokines emerge within 3 months. This suggests that these cytokines have various roles in the pathobiology of an acute coronary event and plaque stability.
ACS usually results from atherosclerotic plaque rupture or plaque erosion and the following coronary artery thrombosis that obstructs blood flow leading to myocardial infarction8. Macrophages have an influence on all atherosclerosis-related processes from initiation to progression, and plaque vulnerability9. Macrophages can be divided into classical M1-phenotype and alternative M2-macrophages9. Pro-inflammatory cytokines, such as IFN-γ and IL-1β, favor M1-polarization and M1-macrophages produce inflammatory cytokines, e.g. IL-6, IL-12 and TNF-α10. M1 macrophages contribute to an increased and sustained inflammatory response and enduring M1 macrophage activation can cause tissue damage and impair wound healing9. M2-macrophages are considered anti-inflammatory and are induced by anti-inflammatory cytokines, such as IL-4, IL-13 and IL-1011. M2-polarized macrophages promote tissue repair and healing, angiogenesis, debris scavenging and production of anti-inflammatory cytokines e.g. IL-1 receptor antagonist (IL-1ra), IL-4, IL-10, IL-13 and TGF-β11,12. Histologically there is no significant difference between M1- and M2- polarized macrophages in the fibrous cap of an atherosclerotic lesion, but M1-macrophages are overrepresented in the rupture-prone shoulder lesions of the plaque13. This supports the comprehension of inflammation’s negative impact on plaque stability.
Many cytokines affect platelet aggregation and thrombus formation, and therefore create an important connection between inflammation and coagulation14,15. Plaque rupture exposes blood and its coagulation factors to the thrombogenic material of the plaque interior, thus leading to thrombus formation16. Proinflammatory cytokines, like IL-1, IL-6 and TNF-α, activate the expression of tissue factor on blood cells and dampen the natural anticoagulant pathways, e.g. the protein C pathway, that normally limits the anticoagulation response, thereby favoring thrombus formation17,18. Activated platelets release inflammatory mediators, such as IL-1β, IL-8 and other chemokines, that in turn recruit monocytes and neutrophils to lesion site, thus enhancing the inflammation in the ruptured area19,20.
Cardiovascular disease remains as a major cause of death worldwide. The amount of ST-elevation myocardial infarction (STEMI) patients without any standard modifiable cardiovascular risk factors (SMuRFs: hypertension, diabetes mellitus, hypercholesterolemia, smoking) is increasing21. Hence, there is a clinical need to recognize these SMuRFless patient with elevated risk of ACS. Cytokines could act as biomarkers to identify these patients.
IL-6 is a widely studied cytokine with both pro-inflammatory and anti-inflammatory properties22. IL-6 has a complex role in cardiovascular system but its cumulative action results in progression of ischemic heart disease23. Similar to our study, elevated levels of circulating IL-6 have been reported in ACS patients24, and elevated levels also predict adverse events25. In our study, the level of IL-4 was decreased in ACS patients compared to those with stable disease and the decreased level was also associated with ACS in logistic regression model. IL-4 is generally considered as an anti-inflammatory cytokine, but there is also increasing evidence that IL-4 has pro-inflammatory effects on vascular endothelium26. There is limited number of studies considering circulating IL-4 levels in ACS patients. Luo et al.27 reported elevated IL-4 in STEMI patients compared to stable angina pectoris patients and Zhao et al.28 reported similar results when ACS patients were compared to healthy individuals. On the other hand, elevated level of IL-4 has also been associated with reduced risk of CVD29.
IL-8 is a proinflammatory cytokine with pro-atherogenic properties and a suspected role in plaque destabilization30. In concordance to our findings, previous studies have reported ACS patients to have higher levels of circulating IL-8 compared to stable CAD patients and healthy controls31,32. Elevated levels have been associated with greater infarct size, lower left ventricle (LV) ejection fraction and higher probability of adverse events in STEMI patients33, higher all-cause mortality in ACS patients during a 5-year follow up34 and increased risk of future CAD among apparently healthy subjects30. Due to its role in ACS and its complications, IL-8 could be a potent target for future pharmacological developments.
In addition to inflammation, also angiogenesis, neovascularization and endothelial cell apoptosis are known to have an important role in atherosclerotic plaque formation, disease progression and plaque stability8. Hepatocyte growth factor (HGF) is a pro-angiogenic growth factor, that regulates endothelial cell growth, inhibits apoptosis, promotes angiogenesis, and is expressed in atherosclerotic plaques35. Animal models have shown that HGF has cardioprotective properties36,37, and elevated levels have been reported in acute MI patients38. This has led to presumption that the increase in HGF level is a defensive response to myocardial injury39. On the other hand, elevated circulating HGF has also been associated with greater progression of atherosclerosis in 12-year follow-up40, though it might only be a biomarker of proceeding atherosclerosis, not the cause of it. Santalahti et al. discovered that high plasma levels of HGF predicted all-cause and CVD mortality in 10-year follow-up41. In our study, HGF was elevated in ACS patients in early phase but not after few months of MACCE. This is likely due to fairly short half-time, as Sato el al. have previously reported that the level of HGF decline to less than half in just over one day42.
Macrophages and monocytes are important in the pathogenesis of atherosclerosis as phagocytosis of lipids by macrophages and the following formation of foam cells initiate development of atherosclerotic lesions43. Macrophage colony-stimulating factor (M-CSF) is a growth factor that promotes the development of monocytes and macrophages from their hematopoietic pre-cursor cells44. M-CSF has an influence on atherogenesis by increasing monocyte number and macrophage proliferation, survival and differentiation into M2-polarized phenotype, monocyte migration to inflammatory sites, and lipid uptake by macrophages44. M-CSF levels have been detected to be higher in atherosclerotic arteries compared to normal arteries45. Alike to our findings, Saitoh et al. documented elevated levels of M-CSF in UAP patients compared to stable AP and also showed a positive correlation between M-CSF level and future coronary events46.
There is a distinct difference in cytokine profiles between our two study cohorts and therefore at different time points of acute coronary syndrome. PDGF-BB was the only cytokine that was associated with ACS in the FACT cohort (MACCE over 3 months) when the level of significance was p < 0.010, with a lower concentration predicting ACS. This is in contrast to a previous study of Tahara et al., who discovered that PDGF-B was significantly elevated in AMI patients in the chronic phase (after 1–4 months after AMI) even though the levels were lower in acute phase compared to stable AP patients and controls47. Like M-CSF, PDGF-BB is also a growth factor, and it influences smooth muscle cell proliferation and migrations as well as vasoconstriction48. Animal studies with AMI models have shown that administration of exogenous PDGF-BB decreases infarct size and enhance LV ejection fraction47,49. Autopsy study of ACS patients showed that PDGF-B was highly expressed in macrophages and smooth muscle cells (SMC) at lesion sites on days 24–55 after PCI but decreased after 6 months, suggesting PDGF-BB might have a role in intima repair50.
Our study has certain limitations that we are aware of. First, the study population is small compared to the number of cytokines measured. Therefore, we decided to use a more stringent p-value < 0.010 as a level of significance. Blood samples were obtained preoperatively, not at admission to the hospital. Also, some cytokines have a short elimination time. Therefore, the most acute phase of ACS might have passed involving some ACS patients. In the FACT cohort, the disease-free control group only consisted of 22 patients, and this must be regarded when interpreting results. Especially in the CAREBANK cohort, many disease-free controls had a valvular defect as an indication for open-heart operation, which may affect the cytokine levels. Preoperative creatinine levels differed between the CAREBANK and FACT cohorts. Within these two study cohorts separately, there was no difference in creatinine levels between ACS and stable patients. It is unlikely that slightly higher creatinine levels would explain the presence of differences in cytokine profiles between ACS and stable patients only in the CAREBANK cohort. Malignancy and obesity were not registered from the whole study population, and thus, these variables were not taken into account in the analyses.
We also acknowledge that this is not a pure longitudinal study as blood samples after three months since most recent MACCE were obtained from different cohorts. However, the baseline characteristics and the proportions of ACS and stable patients were similar between these study cohorts. Thus, the most straight-forward interpretation is that the differences seen in cytokine profiles between the CAREBANK and FACT cohorts represent average cytokine trends after the most recent MACCE.
We used the number of distal anastomoses as a surrogate for disease extent as other measurements of atherosclerotic burden were not systematically collected. We understand that this is uncommon, and e.g. SYNTAX or Gensini score would be more accurate. However, cytokine profiles did not differ between minor and severe disease burden. This reinforces our findings, that differences between ACS and stable patients are due to acute coronary event rather than extent of the disease.
To summarize, the present study suggests that certain cytokines might have a pivotal role in the development of ACS and MI. Our results may imply that targeting single or only few cytokines does not offer a general therapeutic approach for the whole spectrum of atherosclerosis-related inflammation resolution. It is possible that the need and target of immunomodulation changes along with the clinical course (ACS vs. stable CAD) and other aspects of the disease. Identifying the cytokines involved in the pathophysiology of ACS could provide a novel pharmacological approach for treatment of CAD and prevention of ACS. Additional research is required to determine if cytokines could provide novel biomarkers for patients at risk of an acute coronary event.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Tsao, C. W. et al. Heart disease and stroke statistics—2022 update: A report from the American Heart Association. Circulation. 145, E153–E639 (2022).
Morrow, D. A. Cardiovascular risk prediction in patients with stable and unstable coronary heart disease. Circulation. 121, 2681–2691 (2010).
Byrne, R. A. et al. 2023 ESC guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 44, 3720–3826 (2023).
Hansson, G. K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352, 1685–1695 (2005).
Ramji, D. P. & Davies, T. S. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 26, 673–685. https://doi.org/10.1016/j.cytogfr.2015.04.003 (2015).
Lehto, J. et al. Adverse events and survival with postpericardiotomy syndrome after surgical aortic valve replacement. J. Thorac. Cardiovasc. Surg. 160, 1446–1456 (2020).
R Core Team. R: A Language and Environment for Statistical Computing. https://www.R-project.org/ (2023).
Bentzon, J. F., Otsuka, F., Virmani, R. & Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 114, 1852–1866 (2014).
Colin, S., Chinetti-Gbaguidi, G. & Staels, B. Macrophage phenotypes in atherosclerosis. Immunol. Rev. 262, 153–166 (2014).
Moreira, D. M. et al. Role of vascular inflammation in coronary artery disease: Potential of anti-inflammatory drugs in the prevention of atherothrombosis. Inflammation and anti-inflammatory drugs in coronary artery disease. Am. J. Cardiovasc. Drugs. 15, 1–11 (2015).
Peled, M. & Fisher, E. A. Dynamic aspects of macrophage polarization during atherosclerosis progression and regression. Front. Immunol. 5, 579. https://doi.org/10.3389/fimmu.2014.00579 (2014).
Wolfs, I. M. J., Donners, M. M. P. C. & de Winther, M. P. J. Differentiation factors and cytokines in the atherosclerotic plaque micro-environment as a trigger for macrophage polarisation. Thromb Haemost. 106, 763–771 (2011).
Stöger, J. L. et al. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 225, 461–468 (2012).
Foley, J. H. & Conway, E. M. Cross talk pathways between coagulation and inflammation. Circ. Res. 118, 1392–1408. https://doi.org/10.1161/CIRCRESAHA.116.306853 (2016).
Weber, C. Platelets and chemokines in atherosclerosis: Partners in crime. Circ. Res. 96, 612–616. https://doi.org/10.1161/01.RES.0000160077.17427.57 (2005).
Libby, P., Pasterkamp, G., Crea, F. & Jang, I. K. Reassessing the mechanisms of acute coronary syndromes: The ‘vulnerable plaque’ and superficial erosion. Circ. Res. 124, 150–160. https://doi.org/10.1161/CIRCRESAHA.118.311098 (2019).
Esmon, C. T. The impact of the inflammatory response on coagulation. Thromb. Res. 114, 321–327 (2004).
Grignani, G. & Maiolo, A. Cytokines and hemostasis. Haematologica 85, 967–972 (2000).
Davì, G. & Patrono, C. Platelet activation and atherothrombosis. N. Engl. J. Med. 357, 2482–2494 (2007).
Gawaz, M., Langer, H. & May, A. E. Platelets in inflammation and atherogenesis. J. Clin. Investig. 115, 3378–3384. https://doi.org/10.1172/JCI27196 (2005).
Vernon, S. T. et al. Increasing proportion of ST elevation myocardial infarction patients with coronary atherosclerosis poorly explained by standard modifiable risk factors. Eur. J. Prev. Cardiol. 24, 1824–1830 (2017).
Opal, S. M. & Depalo, V. A. Anti-inflammatory cytokines. Chest. 117, 1162–1172 (2000).
Nakao, T. & Libby, P. IL-6 helps weave the inflammatory web during acute coronary syndromes. J. Clin. Investig. 133, e167670. https://doi.org/10.1172/JCI167670 (2023).
Ikeda, U., Ito, T. & Shimada, K. Interleukin-6 and acute coronary syndrome. Clin. Cardiol. 24, 701–704 (2001).
Fanola, C. L. et al. Interleukin-6 and the risk of adverse outcomes in patients after an acute coronary syndrome: Observations from the SOLID-TIMI 52 (Stabilization of Plaque Using Darapladib-Thrombolysis in Myocardial Infarction 52) Trial. J. Am. Heart Assoc. 6, e005637. https://doi.org/10.1161/JAHA.117.005637 (2017).
Lee, Y. W., Kim, P. H., Lee, W. H. & Hirani, A. A. Interleukin-4, oxidative stress, vascular inflammation and atherosclerosis. Biomol. Ther. (Seoul.) 18, 135–144 (2010).
Luo, X., Zhao, C., Wang, S., Jia, H. & Yu, B. TNF-α is a novel biomarker for predicting plaque rupture in patients with ST-segment elevation myocardial infarction. J. Inflamm. Res. 15, 1889–1898 (2022).
Zhao, S. L., Mo, Z. H., He, H. H., Zhao, L. L. & Xie, Y. H. Imbalance of T-helper 1/T-helper 2 cytokines and impaired glucose tolerance among patient with acute coronary syndrome. J. Cancer Res. Ther. 14, S480–S485 (2018).
Engelbertsen, D. et al. T-helper 2 immunity is associated with reduced risk of myocardial infarction and stroke. Arterioscler. Thromb. Vasc. Biol. 33, 637–644 (2013).
Boekholdt, S. M. et al. IL-8 plasma concentrations and the risk of future coronary artery disease in apparently healthy men and women: The EPIC-Norfolk prospective population study. Arterioscler. Thromb. Vasc. Biol. 24, 1503–1508 (2004).
Hashmi, S. & Zeng, Q. T. Role of interleukin-17 and interleukin-17-induced cytokines interleukin-6 and interleukin-8 in unstable coronary artery disease. Coron. Artery Dis. 17, 699–706 (2006).
Romuk, E. et al. Selectin-P and interleukin-8 plasma levels in coronary heart disease patients. Eur. J. Clin. Investig. 32, 657–661 (2002).
Shetelig, C. et al. Association of IL-8 with infarct size and clinical outcomes in patients with STEMI. J. Am. Coll. Cardiol. 72, 187–198 (2018).
Cavusoglu, E. et al. Elevated baseline plasma IL-8 levels are an independent predictor of long-term all-cause mortality in patients with acute coronary syndrome. Atherosclerosis. 242, 589–594 (2015).
Ma, H., Calderon, T. M., Fallon, J. T. & Berman, J. W. Hepatocyte growth factor is a survival factor for endothelial cells and is expressed in human atherosclerotic plaques. Atherosclerosis. 164, 79–87 (2002).
Ueda, H. et al. A potential cardioprotective role of hepatocyte growth factor in myocardial infarction in rats. Cardiovasc. Res. 51, 41–50 (2001).
Nakamura, T. et al. Myocardial protection from ischemia/reperfusion injury by endogenous and exogenous HGF. J. Clin. Investig. 106, 1511–1519 (2000).
Soeki, T. et al. Serum hepatocyte growth factor predicts ventricular remodeling following myocardial infarction. Circ. J. 66, 1003–1007 (2002).
Madonna, R., Cevik, C., Nasser, M. & de Caterina, R. Hepatocyte growth factor: molecular biomarker and player in cardioprotection and cardiovascular regeneration. Thromb. Haemost. 107, 656–661 (2012).
Bell, E. J. et al. Hepatocyte growth factor is associated with progression of atherosclerosis: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 272, 162–167 (2018).
Santalahti, K. et al. Plasma levels of hepatocyte growth factor and placental growth factor predict mortality in a general population: A prospective cohort study. J. Intern. Med. 282, 340–352 (2017).
Sato, T. et al. Hepatocyte growth factor (HGF): A new biochemical marker for acute myocardial infarction. Heart Vessels. 2, 241–246 (1997).
Bäck, M., Yurdagul, A., Tabas, I., Öörni, K. & Kovanen, P. T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 16, 389–406. https://doi.org/10.1038/s41569-019-0169-2 (2019).
Singhal, A. & Subramanian, M. Colony stimulating factors (CSFs): Complex roles in atherosclerosis. Cytokine. 122, 154190. https://doi.org/10.1016/j.cyto.2017.10.012 (2019).
Clinton, S. K. et al. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am. J. Pathol. 140, 301–316 (1992).
Saitoh, T. et al. Clinical significance of increased plasma concentration of macrophage colony-stimulating factor in patients with angina pectoris. J. Am. Coll. Cardiol. 35, 655–665 (2000).
Tahara, A. et al. Plasma levels of platelet-derived growth factor in normal subjects and patients with ischemic heart disease. Am. Heart J. 122, 986–992 (1991).
Lu, Y. et al. Correlation between platelet-derived growth factor-B gene polymorphism and coronary heart disease. J. Clin. Lab. Anal. 36, e24683. https://doi.org/10.1002/jcla.24683 (2022).
Hsieh, P. C. H., Macgillivray, C., Lee, R. T., Davis, M. E. & Gannon, J. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J. Clin. Investig. 116, 237–248 (2006).
Nakagawa, M. et al. A decline in platelet activation and inflammatory cell infiltration is associated with the phenotypic redifferentiation of neointimal smooth muscle cells after bare-metal stent implantation in acute coronary syndrome. J. Atheroscler. Thromb. 17, 675–687 (2010).
Funding
This study was funded by Finnish Medical Foundation, Finnish Foundation for Cardiovascular Research, State Research Funding (Hospital District of Southwest Finland). Funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
E.M.: Investigation, data curation, formal analysis, visualization, writing—original draft, S.S.: Investigation, data curation, J.J.: Conceptualization, resources, T.V.: Investigation, data curation, writing—review and editing, F.B.: Writing—review and editing, J.G.: Conceptualization, S.J.: Conceptualization, resources, writing—review and editing, K.E.J.A.: Conceptualization, writing—review and editing, M.H.: Conceptualization, resources, T.K.: Conceptualization, project administration, supervision, funding acquisition, writing—review and editing.
Corresponding authors
Ethics declarations
Competing interests
Dr. K.E. J. Airaksinen has received speaker fees from Bayer, Pfizer, and Boehringer Ingelheim, and has been a member of the advisory boards of Bayer, Pfizer, and Astra-Zeneca. Other authors declare no potential conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maaniitty, E., Jalkanen, J., Sinisilta, S. et al. Differential circulating cytokine profiles in acute coronary syndrome versus stable coronary artery disease. Sci Rep 14, 17269 (2024). https://doi.org/10.1038/s41598-024-68333-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68333-7
- Springer Nature Limited