Abstract
Mild sleep deprivation is widespread in many societies worldwide. Electroencephalography (EEG) microstate analysis provides information on spatial and temporal characteristics of resting brain network, serving as an indicator of neurophysiological activities at rest. This study seeks to investigate potential neural markers in EEG following mild sleep deprivation of a single night using EEG microstate analysis. Six-minute resting EEG was conducted on thirty healthy adults within 6 hours of waking in the morning and after at least 18 h of sleep deprivation. Translated and validated Malay language Karolinska Sleepiness Scale was used to assess the participants’ degree of sleepiness. Microstate characteristics analysis was conducted on the final 24 subjects based on four standard microstate maps. Microstate C shows a significant increase in mean duration, coverage and occurrence, while microstate D has significantly higher occurrence after sleep deprivation. This study demonstrates notable changes in resting state EEG microstates following mild sleep deprivation. Present findings deepen our understanding of the brain's spatiotemporal dynamics under this condition and suggest the potential utility of neural markers in this domain as components of composite markers for sleep deprivation.
Similar content being viewed by others
Introduction
Sleep plays a crucial role in human health, impacting both brain function and overall physiological well-being. Adequate sleep is of paramount importance in all age groups, as it influences crucial aspects such as cognitive processes, mood regulation, metabolism and hormone function1. Recognizing the variations in the ideal duration of sleep throughout different life stages, the American Academy of Sleep Medicine (AASM) and Sleep Research Society (SRS) guidelines advocate for adults to aim for a minimum of seven hours of sleep per night, allowing for 17 h of wakefulness each day2. Prolonged wakefulness beyond this recommended duration may lead to sleep deprivation, consequently, disrupting the balance of sleep–wake cycle.
Sleep deprivation is a state marked by a lack of adequate sleep or the inability to meet the necessary quantity or quality of sleep3,4. Mild sleep deprivation occurs when an individual experiences sleep loss of only a few hours at night5. In contrast to extreme sleep deprivation, the prevalence of mild sleep deprivation is more common, with a substantial portion of the global population routinely experiencing such conditions, particularly during the transition from standard time to daylight saving time6, during long distance air travel7 or as a consequence of shift work8,9. In real-world scenarios, the elevated risk of culpable crash involvement due to the mild sleep deprivation10 underscores the higher ecological validity of this state compared to extreme sleep deprivation6. Previous studies also indicated that mild sleep deprivation results in diminished performance11,12, deficits in emotional processing13 and reductions in decision-making capacity, as well as motor preparation and execution9.
Electroencephalography (EEG) is a non-invasive tool used to detect and measure brain electrical fields using electrodes placed on scalp. By measuring the voltage fluctuations generated by neural circuits, EEG is a suitable tool to study the functional and anatomical configuration of the brain14. Despite the well-documented impacts of mild sleep deprivation on various aspects of performance and safety, there is a limited amount of research investigating the neural markers and spatiotemporal properties of resting-state EEG following mild sleep loss. Most studies reported EEG activities of chronic or extreme sleep deprivation following prolonged periods of wakefulness15,16,17, and many others conduct EEG analysis in brain frequency domain such as spectral power analysis18,19. Furthermore, many studies also focus on specific populations such as drivers20,21,22, medical professionals23 or patients with brain pathology24,25. Therefore, specific characteristics of resting-state EEG in dynamic system domain following short duration of sleep deprivation among general population remain unclear.
The dynamic system approach to EEG analysis involves studying the spatiotemporal patterns of brain activity during periods of rest. In this approach, the state of brain activity represents the collective impact of all EEG signal variables defining the EEG system at a specific moment, while the system dynamics illustrate the changes in brain state over time26. EEG microstate analysis is one of the popular methods to study patterns in brain state and dynamics. The brain state dynamics is characterized by organised patterns of scalp potential topographies that remains stable over short period of time (80–120 ms) before rapidly switching to the next microstate26. Microstates arise from the coordinated activity of neural assemblies in the brain, generating distinct maps in a non-random fashion. Therefore, a shift in topographical map signifies a change in the distribution of underlying active dipoles that contribute to the topography27. The dynamic transitions between microstates thus indicate the sequential activation of different neural networks over time28 and reflect the combined neuronal activities across brain regions rather than activity specific to a particular frequency band, which offer insight into the overall coordination and stability of neuronal activities when the brain is at rest29.
The EEG microstate analysis is typically performed during resting state to investigate patterns of neuronal activities referred to as the resting-state network (RSN). The RSN is a spontaneous and ongoing global brain activity that occurs when the brain is at rest, characterized by highly organised spatiotemporal patterns resulting from the interplay between the network’s dynamics and structure30. It originates from the concept that functionally connected areas in brain maintain their inherent activities in an organised manner to facilitate optimal readiness for upcoming inputs31. These discrete mental operations giving rise to conscious mentation can be studied and quantified using EEG microstate analysis.
To date, the evidence on EEG microstates during sleep deprivation is scarce. Back in 1999, Cantero et al. explored EEG microstates on eight male and female subjects32. The study was one of the earliest to compare EEG microstates during the state of alertness, drowsiness and in sleep. They discovered that drowsiness microstate displayed a shorter duration compared to rested wakefulness. However, the study focused mainly on brain activities during sleep, and the duration of subjects’ wakefulness was not specified. Furthermore, the microstate was not divided into topography, precluding deeper understanding of the physiology behind. In 2021, Ke et al. conducted microstate analysis on 27 healthy males after 24 h of sleep deprivation33. They found a significant decrease in presence of microstate A and increase in presence of microstate D, and correlated them to disrupted phonological information processing and altered attention resource allocation respectively. When correlated with sleepiness level, similar changes were observed in these microstates, indicating the vulnerability of brain microstates to sleepiness. On the other hand, An et al.34 conducted EEG microstate analysis on 30 males after 24 h and 36 h of sleep deprivation34. They found that the presence of microstate B increased significantly especially after 36 h of sleep deprivation, and inferred that a structure corresponding to microstate B, namely insula, underwent augmented passive activation. Despite the inconsistent findings, both of these recent studies attributed these microstate changes to compensatory mechanisms that help sustain wakefulness and mitigate the negative effects of sleep deprivation.
Present evidence has demonstrated dynamic alteration in spatiotemporal activities of brain during sleep deprivation. Nonetheless, the results were very inconsistent. The findings from recent studies also lack generalizability due to extended period of sleep deprivation and exclusion of female participants. This leaves an evidence gap for microstate changes in mild sleep deprivation among wider population. Based on the established evidence that mild sleep deprivation induces significant change to cognition and behaviour, this study hypothesizes that even mild sleep deprivation (only a few hours) of a single night would be sufficient to induce significant changes to EEG microstates. By addressing the existing gap in the literature, we aim to characterize the EEG patterns during mild sleep deprivation using dynamic system approach within a cohort of clinically validated healthy individuals recruited from general population. This study hopes to contribute additional knowledge and insights into brain dynamics among healthy population experiencing mild sleep loss.
Methodology
Study subjects
The study subjects were healthy volunteers recruited from healthy subject’s database in Clinical Research Centre, Sarawak General Hospital. 30 subjects aged between 21 to 40 years old participated the study. Six subjects were subsequently excluded due to presence of significant amount of artifact as detected by QEEG Pro software and issue with EEG format incompatibility with the software. Demographic of participants are shown in Table 1. Among the final 24 subjects, 16 (66.7%) were female. The majority of participants were of Malay descent, comprising 15 (62.5%) individuals, followed by 3 (12.5%) each of Chinese, Iban, and Bidayuh ethnicities. Their age group was categorized by decade: 13 (54.2%) were aged between 21 to 30 years old (third decade), and 11 (45.8%) were aged between 31 to 40 years old (fourth decade)35.
The health status of participants was confirmed through blood investigations involving complete blood count, renal profile, liver profile and fasting glucose level as well as vital signs assessment by attending medical doctors. Medical history and physical examination were conducted to screen out subjects with history of chronic medical illness, mental disorders, sleep disorders and brain pathology. Subjects who were smokers, consumed alcohol on regular basis, on long term medications or drug use, and had difficulty staying awake for extended period were excluded. Subjects were refrained from smoking one week prior to the experiment and from consuming food containing caffeine and nicotine 24 h prior as well as throughout the study. They were required to have minimum seven hours of sleep for at least two days prior to study.
Experiment protocol
The experiment commenced when a study participant was instructed to stay awake for at least 18 h, starting from 4 a.m. on the day of experiment. The first EEG was recorded within 6 h of waking and the second recording was carried out after 18 h of waking. During the wakefulness period, subjects were allowed to resume their routine work and activities. To ensure wakefulness during the day, subjects were required to respond to messages sent intermittently every few hours. All participants were required to report their level of sleepiness using the Malay language translated and validated Karolinska Sleepiness Scale (KSS-MAS) before and after the sleep deprivation.
Questionnaire translation and validation
Karolinska Sleepiness Scale (KSS) assesses the degree of sleepiness which best represent an individual’s psycho-physical state over the past 10 min, serving as a physiological indicator of sleepiness36,37,38. It employs a 9-point Likert scale with corresponding labels: 1 = extremely alert, 3 = alert, 5 = neither sleepy or alert, 7 = sleepy, but no difficulty remaining awake, and 9 = extremely sleepy, fighting sleep). KSS is a valuable tool to assess sleepiness due to its sensitivity to sleep deprivation and its ability to predict vigilance39,40. As the KSS was not available in Malay, the national language in Malaysia, translation and validation of Malay language KSS (KSS-MAS) were performed.
The Malay translation and validation of KSS began with content validity evaluation for contextual appropriateness, followed by independent professional forward and backward translation process and internal pilot testing involving 15 staffs from our Clinical Research Centre, with further improvement to the scale statements following the pilot test. Finally, the validation of KSS-MAS was performed with fieldwork involving 30 healthy subjects. The criterion validity was tested by having the participants complete the questionnaire at baseline and after at least 18 h of wakefulness period41,42,43.
EEG recording
Six-minute EEG recording was conducted in a quiet room in the research centre. During the procedure, subjects were placed in relaxed, sitting position, and were asked to look at a dot placed in front of them. The EEG recording was conducted using 19-channel Nicolet EEG Amplifier version 5.94. The electrode placement was carried out on the scalp according to the International 10–20 system. The Ten20 paste and Nuprep EEG skin preparation gel were used for scalp preparation. The brain electrical activity was measured in absolute power (microvolt, µV2) and hertz (frequency). The impedance was set to 5 kΩ, sampling rate was 1 kHz, low pass filter of 0.3 Hz and a high pass filter of 50 Hz and notch setting at 45–55 Hz. The electrical activity from the brain was recorded using the Average Reference Montage.
EEG processing and analysis
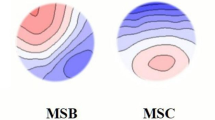
The EEG analysis in this study was limited to open-eye condition to avoid drowsiness particularly during baseline recording. The analysis was performed with QEEG Pro software. Artifact removal was conducted with Standardized Artifact Rejection Algorithm on QEEG Pro software, which uses finite impulse response (FIR) filtering functions of EEGLAB44. The montage for microstate analysis was set against average reference and EEGs were band-pass filtered between 2 and 17 Hz. The QEEG Pro software employs algorithms to fit EEG data of each subject into four standard class of microstate topographies as proposed by Koenig et al., namely microstate A – left occipital to right frontal orientation, microstate B – right occipital to left frontal, microstate C – symmetric occipital to prefrontal and microstate D – symmetric frontocentral to occipital axis orientation45.
The parameters of EEG microstate of each subject were then extracted for statistical analysis. The mean duration (in milliseconds) is the average length of time that a microstate exhibits sustained activation before transitioning to the subsequent microstate. The duration of a microstate mirrors the stability of neuronal activities at the region, with a reduction indicating an inadequacy in the resting state network46. On the other hand, coverage refers to the proportion of total recording time during which a specific microstate is dominant47, while occurrence is defined as the average number of times a microstate appears per second48. Lastly, global explained variance refers to the portion of EEG point topography that can be accounted for by the four archetypes (microstate A to D)26.
Statistical analysis
Kolmogorov–Smirnov test was used to test for normality assumption of the data. As there was no serious violation in parametric assumption, parametric test was employed in statistical analysis. Parameters of microstate were compared before and after sleep deprivation using paired sample t-test. All analyses were carried out using SPSS 19.0 (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp).
Ethics approval and informed consent
This study is registered with the National Medical Research Register (NMRR) Malaysia, with registration number NMRR-21–630-59,231. Ethical approval from Ministry of Health’s Medical Research & Ethics committee (MREC) was obtained. All methods were performed in accordance with Declaration of Helsinki and institutional guidelines. Participants were informed about the purpose of study, study procedures, potential risks and benefits of the study. Written informed consent was obtained from all participants prior to study commencement. All personal information is kept confidential.
Results
Thirty healthy adults were recruited to explore neural markers of waking EEG in mild sleep deprivation of a single night using dynamic system approach. Validation of KSS-MAS was successfully conducted on all 30 subjects. EEG microstate analysis was conducted for the final 24 subjects.
Questionnaire translation and validation
The Karolinska Sleepiness Scale was successfully translated and validated to Malay (KSS-MAS). During the pilot phase of translation process, highly satisfactory results were obtained, with only minor adjustments needed to ensure semantic alignment with the original version. All the 30 subjects underwent the fieldwork phase in validation of KSS-MAS. Their mean age was 29.1 (SD ± 5.3) years with the majority female (66.7%), and Malay (63.3%). The baseline mean score for KSS-MAS was 1.63 (SD ± 1.0) and increased to 7.80 (SD ± 1.5) following more than 18 h of sleep deprivation, with p < 0.001. This shows that the Malay-translated version of Karolinska Sleepiness Scale (KSS-MAS), is a reliable and valid scale to assess the level of subjective sleepiness in individuals.
The questionnaire scores of the 24 subjects included in the microstate analysis were examined. At baseline (within six hours of waking in the morning), 13 subjects (54.2%) rated themselves as 1 (extremely alert), 10 subjects (41.7%) rated themselves as 2, while one subject (4.1%) rated 6. Following at least 18 h of sleep deprivation, 13 subjects (54.2%) rated themselves as 8, 8 subjects (33.4%) rated themselves as 9 (extremely sleepy), 1 subject (4.1%) rated as 7 (sleepy but with no difficulty remaining awake), and 2 subjects (8.3%) rated themselves as 3 (alert).
EEG microstate
The distribution of EEG microstates’ mean duration, occurrence and coverage for all subjects before and after sleep deprivation are displayed in Fig. 1 and Supplementary Table S1.
Global explained variance
The global explained variance of our subjects was 0.69 (SD ± 0.05) at baseline and 0.68 (SD ± 0.05) after mild sleep deprivation (p = 0.497). This suggests that microstates A to D predominantly represent the microstate composition of individuals both in awake and sleep-deprived state.
Occurrence and coverage
Present results for both microstate C and D showed significant increase in occurrence after sleep deprivation: occurrence of microstate C rose from 3.09 Hz (SD ± 0.63) to 3.50 Hz (SD ± 0.69) per second, p = 0.018, while microstate D increased from 4.15 Hz (SD ± 0.51) to 4.40 Hz (SD ± 0.45), p = 0.019, implying an increased in neuro-electrical activity of the neuronal assemblies over these regions after sleep deprivation.
Similar to occurrence, coverage provides an indication of level of the neuronal activation in a microstate. In this study, coverage of microstate C exhibits a notable increase from 19.19% (SD ± 4.25) to 22.62% (SD ± 5.03), p = 0.008, following sleep deprivation. The coverage of other microstates does not show significant changes.
Mean duration
After sleep deprivation, it is found that microstate C shows a borderline significant increase in mean duration from 62.20 ms (SD ± 6.77) to 64.68 ms (SD ± 7.49), p = 0.039. The mean duration of other microstates does not reach statistical significance.
Degree of sleepiness vs EEG microstate
In order to better depict and illustrate the changes in EEG microstates between the states of alertness and drowsiness, microstate analysis was further carried out solely on participants who were alert at baseline and were sleepy after sleep deprivation (n = 21). One participant who scored 6 on KSS-MAS before sleep deprivation and two participants who scored 3 (alert) after sleep deprivation were excluded from the analysis.
The results reveal that the microstate parameters showing significant changes remain largely similar to previous findings. An increase is observed in the occurrence of microstate C and microstate D, as well as the coverage of microstate C, as shown in Supplementary Table S2. Specifically, the occurrence of microstate C rose from 3.13 Hz (SD ± 0.63) to 3.50 Hz (SD ± 0.62), p = 0.042, while the occurrence of microstate D increased from 4.17 Hz (SD ± 0.50) to 4.32 Hz (SD ± 0.37), p = 0.042. The coverage of microstate C increased from 19.31% (SD ± 3.96) to 22.62% (SD ± 4.40), p = 0.021. However, the mean duration of microstate C no longer reaches statistical significance (p = 0.054).
Discussion
Present study reveals significant changes in microstate C and D following mild sleep deprivation of one night. In order to further understand how does sleep deprivation of this duration lead to the changes observed in the microstates, it is worth considering the potential underlying brain structures and networks at each topography. Several studies have sought to establish EEG microstates with specific brain structures and networks, albeit using vastly different methods. Britz et al. (2010) utilised k-means clustering to analyse canonical four EEG microstates, and spatially correlated them with blood oxygenation level dependent (BOLD) activation patterns on functional MRI (fMRI) resting state network, and discovered that microstate C correlates to dorsal anterior cingulate cortex, bilateral inferior frontal cortices and the right insular area, while microstate D correlates with right superior and middle frontal gyrus as well as the right superior and inferior parietal lobule49. The same study also attributed distinct network to each microstate: auditory network (microstate A), visual network (microstate B), salience network (microstate C), attention network (microstate D)49. However, when Custo et al.50 extended their topography mapping to seven microstates, they discovered that although microstate D is highly concordant with the study by Btriz et al. (2010), microstate C more accurately corresponds to posterior cingulate cortex and precuneus, whilst the microstate C as reported by Britz et al. (2010) is actually microstate F. They concluded that only using four microstate maps for analysis (instead of seven) may easily cause spatially correlated microstates to be combined into one50. This finding is supported by Yuan et al.29, who utilized a data driven approach based on temporal independent component analysis in their microstate analysis, and found that each microstate is associated with at least one or more combinations of networks, implying that extension of microstate analysis into more clusters provide more accurate depiction of the underlying networks29.
Therefore, following the microstate mapping by Custo et al.50, the microstate C, which displays increased occurrence and coverage after sleep deprivation in this study, likely consists of posterior components of default mode network, namely posterior cingulate cortex and precuneus50. Default mode network is widely recognized for its high activation during rest and tendency to decrease activation below baseline levels during cognitively demanding tasks51. Of particular significance to our investigation, subregions in default mode network display a pattern of balance and synchrony in their activity, whereby posterior cingulate cortex and precuneus demonstrate higher activities in the evening/night in order to adapt and compensate for the decrease in activity of medial temporal lobe as the day goes on52. Therefore, the increase in occurrence and coverage of microstate C in this study can be explained by the enhanced activity in posterior cingulate cortex and precuneus as part of their diurnal rhythm throughout the day52.
On the other hand, as our subjects only underwent sleep loss of a few hours, heightened activity in microstate C may suggest that posterior cingulate cortex and precuneus are the primary locus of neural activity that uphold the functions of default mode network in the state of mild sleep deprivation. This hypothesis can be supported by several studies that show a decrease in functional connectivity in posterior cingulate cortex and precuneus after sleep deprivation, highlighting the impact of sleep deprivation on these areas53,54. As posterior cingulate cortex and precuneus are important in serving as a fundamental brain function in addition to their more specific roles such as memory recollection55,56, enhanced neuronal activity in these areas in response to mild sleep deprivation may indicate their greater vulnerability to sleep deprivation compared to other networks in the brain. More importantly, the susceptibility of default mode network to sleep deprivation sheds light on the potential role of sleep deprivation as one of the etiologies of neuropsychiatric disorders marked by disrupted activity in the default mode network, such as Alzheimer’s disease57. Therefore, further investigations are warranted to study the reversibility of changes in default mode network noted in this study and their possible clinical association with neuropsychiatric disorders.
Additionally, our study reveals an increase in the occurrence of microstate D following sleep deprivation, with its topography correlating to the right superior and middle frontal gyrus as well as the right superior and inferior parietal lobule49,50. These structures constitute components of the dorsal and ventral attention networks49,58. The attention network plays a crucial role in regulating a broad spectrum of cognitive functions relating to attention domain59, and it is often observed to undergo varying degrees of activation during cognitive task60. However, in this investigation, we observed an increase in neural activity of attention network during resting-state EEG following a few hours of sleep loss. This discovery is intriguing given that the subjects were not engaged in cognitive tasks during the EEG recording, thus the increased activity in attention network compared to baseline was unexpected. This finding implies that mild sleep deprivation may impose a physiological demand akin to that experienced during cognitively demanding tasks, thereby putting this network in a state of 'work' rather than 'rest'. The influence of sleep deprivation on the attention network is evident, with numerous studies noting increased activation of this network during tasks following sleep deprivation when compared to well-rested state61,62. More importantly, this sheds light on the adverse effect of sleep deprivation on the attention network, which is a crucial component for cognitive performance as reported in other studies63,64.
Conversely, the increased activation of the attention network following mild sleep deprivation could be a prelude to a decrease in its activation following prolonged sleep deprivation. Several studies involving participants subjected to sleep deprivation exceeding 24 h have observed that critical regions of the attention network fail to sustain their activity levels after prolonged sleep deprivation65,66, which indicate the possibility that the initial activation of the attention network observed in this study may precede a subsequent decline, likely serving as a compensatory mechanism for the network to sustain homeostatic regulation during drowsiness67. Therefore, present study illustrates the vulnerability of the attention network to sleep deprivation, even with mild sleep restriction over a single night. Hence, an in-depth exploration of the neural mechanisms responsible for the altered activation of the attention network following mild sleep deprivation warrants further research, as such exploration may facilitate a deeper comprehension of the neurophysiological alterations in this network and offer insights into the decline in cognitive performance frequently observed following sleep deprivation68,69.
The average duration of EEG microstates refers to the time spent by a group of neural assemblies in an active state and reflects the pace at which a topography transitions to a subsequent topography. Examining the temporal dimension of resting-state EEG microstates provides valuable information about the time during which diverse neural assemblies, situated in various cortical and subcortical structures, collaborate and actively coordinate to produce a unified function26. Consequently, the mean duration of a microstate serves as a metric indicating the stability of neural assemblies within a given topography28.
Present study noted a borderline significant increase in the duration of microstate C (p = 0.039). This finding was surprising, as one would expect the stability of a microstate to reduce after neuronal stress. Nonetheless, the ability of the microstates to sustain their duration indicates that mild sleep deprivation does not lead to instability of neural assemblies in serving a common function. This finding is similar to the previous studies by Ke et al.33 and An et al.34, which found that duration of microstates was not negatively affected by sleep deprivation33,34. On the other hand, the study by Cantero et al.32 observed a noteworthy reduction in microstate duration during drowsiness32. Despite the lack of details on wakefulness period and microstate subtyping, the finding suggests that more research is needed to confirm the impact of sleep deprivation on microstate duration. This is crucial as microstate duration has been identified as one of the key indicators of various neuropsychiatric conditions31. For example, a meta-analysis conducted by Rieger et al.70 revealed a significant reduction in the mean duration of microstate D among patients with schizophrenia compared to healthy controls70. More importantly, the mean duration of microstate D exhibited a noteworthy increase when healthy controls were administered a low dose of antipsychotic medication71, underscoring the influence of decreased duration in the disease mechanism and the efficacy of medications in mitigating this anomaly. Given the significance of microstate duration in brain pathology, verifying the impact of lack of sleep on microstate duration could provide valuable insights into neuronal responses under various stressful conditions.
During the state of drowsiness, it has been observed that the alpha peak frequency progressively decreases72,73, while delta and theta power increases with the level of sleepiness74,75. Since EEG microstates primarily derive from alpha waves, factors influencing alpha wave activity, such as the state of alertness and drowsiness, are likely to affect microstate parameters. Hence, additional analysis was done to focus exclusively on participants who were alert before sleep deprivation and experienced drowsiness afterward. The findings revealed an increase in the occurrence and coverage of microstate C, as well as in the occurrence of microstate D, consistent with previous observations in this study. However, the mean duration of microstate C no longer yielded significant results after excluding three subjects from the analysis, likely due to reduced sample size.
The rise in the occurrence of microstate D during drowsiness aligns with the findings by Ke et al.33, who assessed their subjects' sleepiness using the Visual Analogue Scale (VAS)33. This finding corroborates other research to indicate that the attention network is affected by sleepiness even during rest76, which may in turn explain the attention deficit observed when one is sleepy77. Aside from microstate D, Ke et al.33 discovered no change in microstate C, and instead found inverse correlation between sleepiness level and presence of microstate A (bilateral superior and middle temporal gyrus and left middle frontal gyrus)33,49. However, the evidence of correlation between drowsiness and these brain structures is still inconsistent78,79,80,81. Nonetheless, by demonstrating the effect of sleepiness on microstates, we show that our study design effectively captured subjects during the state of alertness at baseline and drowsiness post-sleep deprivation. More importantly, it underscores that sleep loss of a few hours is sufficient to induce a significant level of drowsiness and alter dynamic of neural activities in healthy adults.
Conclusion
This study employs EEG microstates to investigate the temporal and spatial characteristics of the resting brain network following mild sleep deprivation among healthy adults recruited from the general population. The findings reveal enhanced activation of microstate C (default mode network) and D (attention network) after sleep deprivation, without significant loss in the stability of any microstate. This study presents an initial exploration of the dynamic properties of neural activity in the brain following mild sleep deprivation, offering novel insights in this area. Furthermore, the alterations in EEG microstates after 18 h of sleep deprivation could serve as a suitable neural marker for identifying individuals experiencing early effects of sleep deprivation, particularly in work environments where maintaining optimal alertness is critical. Lastly, our results lay the groundwork for future investigations aimed at exploring potential composite markers of sleep deprivation that encompass various domains, including frequency, dynamics, and cognition, thus enhancing the precision and reliability of neurophysiological markers for detecting sleep deprivation.
Limitation
Firstly, sleep disorders, ideally ruled out using sleep study, are ruled out with medical history taking. Furthermore, this study does not perform cognitive assessments among participants, which could offer a measure of vigilance after mild sleep deprivation. Moreover, as participants were permitted to resume their usual activities on the day of the experiment and were tasked with responding to messages at regular intervals, the confirmation of their wakefulness was not done under direct observation and therefore remains uncertain. However, regular communication with participants likely mitigated the risk of daytime napping. Lastly, afterwork-thoughts and stress may also influence the EEG findings, which warrant further investigations in future studies.
Data availability
Raw data for the study is available at: https://github.com/khoosingyee/EEG-Microstate-After-Sleep-Deprivation/tree/main/Qeegreport.
References
Watson, N. F. et al. Joint consensus statement of the American academy of sleep medicine and sleep research society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep 38, 1161–1183 (2015).
Hirshkowitz, M. et al. National sleep foundation’s updated sleep duration recommendations: final report. Sleep Health 1, 233–243 (2015).
Bandyopadhyay, A. & Sigua, N. L. What Is sleep deprivation?. Am. J. Respir. Crit. Care Med. 199, P11–P12 (2019).
Medic, G., Wille, M. & Hemels, M. Short- and long-term health consequences of sleep disruption. Nat. Sci. Sleep. 9, 151–161 (2017).
Gibbings, A. et al. EEG and behavioural correlates of mild sleep deprivation and vigilance. Clin. Neurophys. 132, 45–55 (2021).
Gibbings, A. et al. EEG and behavioural correlates of mild sleep deprivation and vigilance. Clinic. Neurophysiol. 132, 45–55 (2021).
Weingarten, J. A. & Collop, N. A. Air travel: effects of sleep deprivation and jet lag. Chest 144, 1394–1401 (2013).
Kecklund, G. & Axelsson, J. Health consequences of shift work and insufficient sleep. BMJ 355, i5210. https://doi.org/10.1136/bmj.i5210 (2016).
Stojanoski, B. et al. Sustained vigilance is negatively affected by mild and acute sleep loss reflected by reduced capacity for decision making, motor preparation, and execution. Sleep 42(1), zsy200 (2019).
Tefft, B. C. Acute sleep deprivation and culpable motor vehicle crash involvement. Sleep 41(10), zsy144 (2018).
Cote, K. A. et al. CNS arousal and neurobehavioral performance in a short-term sleep restriction paradigm. J. Sleep Res. 18, 291–303 (2009).
Anderson, C. & Horne, J. A. Driving drowsy also worsens driver distraction. Sleep Med 14, 466–468 (2013).
Lustig, K. A. et al. Sex hormones play a role in vulnerability to sleep loss on emotion processing tasks. Neurobiol. Sleep Circadian Rhythms 5, 94–104 (2018).
Cohen, M. X. Where does EEG come from and what does It mean?. Trends Neurosci. 40, 208–218 (2017).
Cajochen, C., Brunner, D. P., Krauchi, K., Graw, P. & Wirz-Justice, A. Power density in theta/alpha frequencies of the waking EEG progressively increases during sustained wakefulness. Sleep 18, 890–894 (1995).
Hung, C.-S. et al. Local experience-dependent changes in the wake EEG after prolonged wakefulness. Sleep 36, 59–72 (2013).
Gorgoni, M. et al. Topographic electroencephalogram changes associated with psychomotor vigilance task performance after sleep deprivation. Sleep Med. 15, 1132–1139 (2014).
Forest, G. & Godbout, R. Effects of sleep deprivation on performance and EEG spectral analysis in young adults. Brain Cogn. 43, 195–200 (2000).
Henelius, A., Korpela, J. & Huotilainen, M. Individualising EEG frequency bands for sleep deprivation studies. In: 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society 6083–6086 (IEEE, 2011). https://doi.org/10.1109/IEMBS.2011.6091503.
Eoh, H. J., Chung, M. K. & Kim, S.-H. Electroencephalographic study of drowsiness in simulated driving with sleep deprivation. Int. J. Ind. Ergon. 35, 307–320 (2005).
Shoaib, Z. et al. Utilizing EEG and fNIRS for the detection of sleep-deprivation-induced fatigue and its inhibition using colored light stimulation. Sci. Rep. 13, 6465 (2023).
Papadelis, C. et al. Indicators of Sleepiness in an ambulatory EEG study of night driving. In: 2006 International Conference of the IEEE Engineering in Medicine and Biology Society 6201–6204 (IEEE, 2006). https://doi.org/10.1109/IEMBS.2006.4398875.
Su, A. T., Xavier, G. & Kuan, J. W. The measurement of mental fatigue following an overnight on-call duty among doctors using electroencephalogram. PLoS ONE 18, e0287999 (2023).
Ellingson, R. J., Wilken, K. & Bennett, D. R. Efficacy of sleep deprivation as an activation procedure in epilepsy patients. J. Clin. Neurophysiol. 1, 83–102 (1984).
Drake, M. E., Pakalnis, A., Phillips, B. B. & Denio, L. S. Sleep and sleep deprived EEG in partial and generalized epilepsy. Acta Neurol. Belg. 90, 11–19 (1990).
Khanna, A., Pascual-Leone, A., Michel, C. M. & Farzan, F. Microstates in resting-state EEG: current status and future directions. Neurosci. Biobehav. Rev. 49, 105–113 (2015).
Wackermann, J., Lehmann, D., Michel, C. M. & Strik, W. K. Adaptive segmentation of spontaneous EEG map series into spatially defined microstates. Int. J. Psychophysiol. 14, 269–283 (1993).
Koenig, T., Studer, D., Hubl, D., Melie, L. & Strik, W. K. Brain connectivity at different time-scales measured with EEG. Philosoph. Trans. Royal Soc. B: Biol. Sci. 360, 1015–1024 (2005).
Yuan, H., Zotev, V., Phillips, R., Drevets, W. C. & Bodurka, J. Spatiotemporal dynamics of the brain at rest — Exploring EEG microstates as electrophysiological signatures of BOLD resting state networks. Neuroimage 60, 2062–2072 (2012).
Deco, G., Jirsa, V. K. & McIntosh, A. R. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat. Rev. Neurosci. 12, 43–56 (2011).
Michel, C. M. & Koenig, T. EEG microstates as a tool for studying the temporal dynamics of whole-brain neuronal networks: a review. Neuroimage 180, 577–593 (2018).
Cantero, J. L., Atienza, M., Salas, R. M. & Gómez, C. M. Brain spatial microstates of human spontaneous alpha activity in relaxed wakefulness, drowsiness period, and REM sleep. Brain Topogr. 11, 257–263 (1999).
Ke, M., Li, J. & Wang, L. Alteration in resting-state EEG microstates following 24 hours of total sleep deprivation in healthy young male subjects. Front. Human Neurosci. 12(15), 636252 (2021).
An, X. et al. Changes in electroencephalography microstates are associated with reduced levels of vigilance after sleep deprivation. Brain Res. 1825, 148729 (2024).
Taki, Y. et al. Correlations among brain gray matter volumes, age, gender, and hemisphere in healthy individuals. PLoS ONE 6, e22734 (2011).
Shahid, A., Wilkinson, K., Marcu, S. & Shapiro, C. M. Karolinska Sleepiness Scale (KSS). In: STOP, THAT and One Hundred Other Sleep Scales 209–210 (Springer New York, New York, NY, 2011). https://doi.org/10.1007/978-1-4419-9893-4_47.
Kaida, K., Åkerstedt, T., Kecklund, G., Nilsson, J. P. & Axelsson, J. Use of Subjective and physiological indicators of sleepiness to predict performance during a vigilance task. Ind. Health 45, 520–526 (2007).
Åkerstedt, T., Anund, A., Axelsson, J. & Kecklund, G. Subjective sleepiness is a sensitive indicator of insufficient sleep and impaired waking function. J. Sleep Res. 23, 242–254 (2014).
Kaida, K. et al. Validation of the Karolinska sleepiness scale against performance and EEG variables. Clin. Neurophysiol. 117, 1574–1581 (2006).
Sallinen, M. et al. The effects of sleep debt and monotonous work on sleepiness and performance during a 12-h dayshift. J. Sleep Res. 13, 285–294 (2004).
Eremenco, S. L., Cella, D. & Arnold, B. J. A comprehensive method for the translation and cross-cultural validation of health status questionnaires. Eval Health Prof 28, 212–232 (2005).
Tsang, S., Royse, C. & Terkawi, A. Guidelines for developing, translating, and validating a questionnaire in perioperative and pain medicine. Saudi J. Anaesth. 11, 80 (2017).
Bujang, A., Yoon Khee, H. & Keng Yee, L. A Step-By-Step Guide to Questionnaire Validation Research. (Zenodo, Selangor, Malaysia, 2022).
Delorme, A. & Makeig, S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21 (2004).
Koenig, T. et al. Millisecond by millisecond, year by year: normative EEG microstates and developmental stages. Neuroimage 16, 41–48 (2002).
Nishida, K. et al. EEG microstates associated with salience and frontoparietal networks in frontotemporal dementia, schizophrenia and Alzheimer’s disease. Clin. Neurophysiol. 124, 1106–1114 (2013).
Milz, P. et al. The functional significance of EEG microstates—Associations with modalities of thinking. Neuroimage 125, 643–656 (2016).
da Cruz, J. R. et al. EEG microstates are a candidate endophenotype for schizophrenia. Nat. Commun. 11, 3089 (2020).
Britz, J., Van De Ville, D. & Michel, C. M. BOLD correlates of EEG topography reveal rapid resting-state network dynamics. Neuroimage 52, 1162–1170 (2010).
Custo, A. et al. Electroencephalographic resting-state networks: source localization of microstates. Brain Connect 7, 671–682 (2017).
Raichle, M. E. et al. A default mode of brain function. Proc. Natl. Acad. Sci. 98, 676–682 (2001).
Jiang, C. et al. Diurnal variations in neural activity of healthy human brain decoded with resting-state blood oxygen level dependent fMRI. Front. Human Neurosci. 20(10), 634 (2016).
De Havas, J. A., Parimal, S., Soon, C. S. & Chee, M. W. L. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. Neuroimage 59, 1745–1751 (2012).
Wu, J. et al. Decreased resting-state alpha-band activation and functional connectivity after sleep deprivation. Sci. Rep. 11, 484 (2021).
Binder, J. R. et al. Conceptual processing during the conscious resting state: a functional MRI study. J. Cogn. Neurosci. 11, 80–93 (1999).
Vincent, J. L. et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J. Neurophysiol. 96, 3517–3531 (2006).
Greicius, M. D., Srivastava, G., Reiss, A. L. & Menon, V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc. Natl. Acad. Sci. 101, 4637–4642 (2004).
Fox, M. D., Corbetta, M., Snyder, A. Z., Vincent, J. L. & Raichle, M. E. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. 103, 10046–10051 (2006).
Scolari, M., Seidl-Rathkopf, K. N. & Kastner, S. Functions of the human frontoparietal attention network: evidence from neuroimaging. Curr. Opin Behav. Sci. 1, 32–39 (2015).
Banich, M. T. et al. fMRI Studies of stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. J. Cogn. Neurosci. 12, 988–1000 (2000).
Klumpers, U. M. H. et al. Neurophysiological effects of sleep deprivation in healthy adults, a pilot study. PLoS ONE 10, e0116906 (2015).
Drummond, S. P. A., Gillin, J. C. & Brown, G. G. Increased cerebral response during a divided attention task following sleep deprivation. J. Sleep Res. 10, 85–92 (2001).
Wehrens, S. M. T., Hampton, S. M., Kerkhofs, M. & Skene, D. J. Mood, alertness, and performance in response to sleep deprivation and recovery sleep in experienced shiftworkers versus non-shiftworkers. Chronobiol. Int. 29, 537–548 (2012).
Doran, S. M., Van Dongen, H. P. & Dinges, D. F. Sustained attention performance during sleep deprivation: evidence of state instability. Arch. Ital. Biol. 139, 253–267 (2001).
Drummond, S. P. A. et al. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. Neuroreport 10, 3745–3748 (1999).
Thomas, M. L. et al. Neural basis of alertness and cognitive performance impairments during sleepiness II. Effects of 48 and 72 h of sleep deprivation on waking human regional brain activity. Thalamus Relat. Syst. 2, 199 (2003).
Dang-Vu, T. T. Imaging Sleep and Sleep Deprivation. In Sleep Deprivation and Disease 33–43 (Springer New York, New York, NY, 2014). https://doi.org/10.1007/978-1-4614-9087-6_4.
Kaliyaperumal, D. Effects of sleep deprivation on the cognitive performance of nurses working in shift. J. Clin. Diagn. Res. https://doi.org/10.7860/JCDR/2017/26029.10324 (2017).
Lo, J. C. et al. Effects of partial and acute total sleep deprivation on performance across cognitive domains, Individuals and Circadian Phase. PLoS ONE 7, e45987 (2012).
Rieger, K., Diaz Hernandez, L., Baenninger, A. & Koenig, T. 15 years of microstate research in schizophrenia–where are we? A meta-analysis. Front. Psych. 26(7), 22 (2016).
Yoshimura, M. et al. A pharmaco-EEG study on antipsychotic drugs in healthy volunteers. Psychopharmacology (Berl) 191, 995–1004 (2007).
Gennaro, L. D. et al. Antero-posterior functional coupling at sleep onset: changes as a function of increased sleep pressure. Brain Res. Bull. 65, 133–140 (2005).
Krylova, M. et al. Evidence for modulation of EEG microstate sequence by vigilance level. Neuroimage 224, 117393 (2021).
Olbrich, S. et al. EEG-vigilance and BOLD effect during simultaneous EEG/fMRI measurement. Neuroimage 45, 319–332 (2009).
Strijkstra, A. M., Beersma, D. G. M., Drayer, B., Halbesma, N. & Daan, S. Subjective sleepiness correlates negatively with global alpha (8–12 Hz) and positively with central frontal theta (4–8 Hz) frequencies in the human resting awake electroencephalogram. Neurosci. Lett. 340, 17–20 (2003).
Thomas, M. et al. Neural basis of alertness and cognitive performance impairments during sleepiness I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J. Sleep Res. 9, 335–352 (2000).
Yun, C.-H. et al. Daytime sleepiness associated with poor sustained attention in middle and late adulthood. Sleep Med. 16, 143–151 (2015).
Zhang, L. et al. Decreased information replacement of working memory after sleep deprivation: evidence from an event-related potential study. Front. Neurosci. 26(13), 408 (2019).
Drummond, S. P. A. et al. Altered brain response to verbal learning following sleep deprivation. Nature 403, 655–657 (2000).
Yeo, B. T. T., Tandi, J. & Chee, M. W. L. Functional connectivity during rested wakefulness predicts vulnerability to sleep deprivation. Neuroimage 111, 147–158 (2015).
Kaufmann, T. et al. The brain functional connectome is robustly altered by lack of sleep. Neuroimage 127, 324–332 (2016).
Acknowledgements
We would like to thank the Director General of Health Malaysia for his permission to publish this article.
Funding
Funding was provided by Ministry of Health Research Grant (MRG) Malaysia.
Author information
Authors and Affiliations
Contributions
Study was designed and conceived by SAT, FAYY, LWH, KSY; translation and validation of Karolinska Sleepiness Scale by MAB, KSY, OSH and OYY; EEG data processing by KSY, LWC and NBHS, statistical analysis by KSY and MAB; manuscript writing by KSY, LWH, OSH and OYY; overall study supervised by SAT and FAYY.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Khoo, S., Lai, W., On, S. et al. Resting-state electroencephalography (EEG) microstates of healthy individuals following mild sleep deprivation. Sci Rep 14, 16820 (2024). https://doi.org/10.1038/s41598-024-67902-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67902-0
- Springer Nature Limited