Abstract
Non-alcoholic Fatty Liver Disease (NAFLD) and Non-alcoholic Steatohepatitis (NASH) are major metabolic diseases with increasing global prevalence and no approved therapies. There is a mounting need to develop biomarkers of diagnosis, prognosis and treatment response that can effectively replace current requirements for liver biopsies, which are invasive, error-prone and expensive. We performed SomaLogic serum proteome profiling with baseline (n = 231) and on-treatment (n = 72, Weeks 12 and 16, Placebo and 25 mg PF-05221304) samples from a Phase 2a trial (NCT03248882) with Clesacostat (PF-05221304), an acetyl coA carboxylase inhibitor (ACCi) in patients with NAFLD/NASH. SomaSignal NASH probability scores and expression data for 7000+ analytes were analyzed to identify potential biomarkers associated with baseline clinical measures of NAFLD/NASH [Magnetic Resonance Imaging-Proton Density Fat Fraction (MRI-PDFF), alanine aminotransferase (ALT) and aspartate aminotransferase (AST)] as well as biomarkers of treatment response to ACCi. SomaSignal NASH probability scores identified biopsy-proven/clinically defined NIT-based (Presumed) NASH classification of the cohort with > 70% agreement. Clesacostat-induced reduction in steatosis probability scores aligned with observed clinical reduction in hepatic steatosis based on MRI-PDFF. We identify a set of 69 analytes that robustly correlate with clinical measures of hepatic inflammation and steatosis (MRI-PDFF, ALT and AST), 27 of which were significantly reversed with ACC inhibition. Clesacostat treatment dramatically upregulated Wnt5a protein and Apolipoproteins C3 and E, with drug-induced changes significantly correlating to changes on MRI-PDFF. Our data demonstrate the utility of SomaLogic- analyte panel for diagnosis and treatment response in NAFLD/NASH and provide potential new mechanistic insights into liver steatosis reduction, inflammation and serum triglyceride elevation with ACC inhibition. (Clinical Trial Identifier: NCT03248882).
Similar content being viewed by others
Introduction
NAFLD and its more aggressive manifestation, NASH, are major risk factors for liver cirrhosis and mortality, and represent a spectrum of liver diseases with significant unmet medical need1,2. Clinical diagnosis of NASH involves histological assessment of key pathological features of progressive NAFLD including lipid accumulation and steatosis, hepatocyte ballooning, chronic inflammation and fibrosis of the liver3. Current regulatory requirements for approval of NASH therapies mandate the inclusion of liver biopsies as the only acceptable primary endpoint in registrational trials4, although they may not be necessarily the best standard. Liver biopsies are highly invasive and may require frequent sampling causing patient reluctance, technically challenging with the assessment of disease subjective, and entail significant costs. Although several soluble5,6,7 and imaging-based biomarkers8,9,10 have been explored in the context of NASH, none are in routine clinical practice and importantly, no single biomarker has been found to effectively replace biopsies. Therefore, there is considerable urgency to identify non-invasive biomarkers of NAFLD/NASH that may help in staging and progression of disease, as well as response to therapeutic interventions.
The SomaScan assay is a sensitive aptamer-based proteomic platform that can measure > 7000 protein analytes with a high degree of specificity. Validation of this platform using samples from multiple natural history and interventional studies in biopsy-proven NAFLD/NASH has identified potential biomarkers and led to the development of the SomaSignal NASH test—an algorithm-based prediction of NASH probabilities using a minimal signature of ~ 37 analytes representing the key pathologies of inflammation, ballooning, steatosis and fibrosis11,12,13. While SomaSignal test may help with disease staging, understanding of biomarker response to treatments targeting diverse mechanisms in NASH will benefit from comprehensive analyses of the total proteome signature in the panel, beyond the targeted set of analytes.
Acetyl CoA Carboxylase is a key liver-expressed enzyme involved in NAFLD pathogenesis via regulation of de novo lipogenesis and fatty acid metabolism14,15. Therapies targeting ACC have shown anti-steatotic and anti-inflammatory benefit in patients with NAFLD16,17. However, ACC inhibition is also known to cause paradoxical increase in serum triglycerides (TG) in both mice and clinical studies, which is postulated to occur through compensatory upregulation of the sterol-binding element, SREBP1c18,19. Combining an ACCi with an inhibitor of diacylglycerol acyltransferase-2 (DGAT2) has been shown to mitigate the increase in serum TG observed with ACCi alone20.
Using samples from a Phase 2 trial with Clesacostat (an ACC inhibitor) in patients with NAFLD/NASH, we sought to a. assess the utility of the SomaSignal NASH test in predicting baseline NAFLD vs NASH status and clinical response of steatosis reduction and b. perform a SomaScan deep proteome analyses to identify potential predictive biomarkers of disease pathology and treatment response to ACCi and inform the forward strategy with an ACC/DGAT2 dual inhibition.
Our data reveal a distinct set of potential biomarkers in NASH with utility as surrogate measures of steatosis and inflammation, and treatment response to ACCi in NAFLD/NASH.
Materials and methods
Patient cohort and study design
Serum samples for this retrospective analysis were sourced from a Phase 2a clinical trial investigating liver-directed ACC1 inhibition by QD dosing of PF-05221304 (clesacostat) in adults with NASH and/or NAFLD (NCT03248882)20. Patient disposition and clinical characteristics of the study have been previously described20. This study was conducted in accordance with International Ethical Guidelines for Biomedical Research Involving Human Subjects, the International Conference on Harmonization Guideline for Good Clinical Practice and the principles of the Declaration of Helsinki. It was registered on clinicaltrials.gov (NCT03248882). Study protocols, informed consent forms and other appropriate study-related documents were reviewed and approved by local independent ethics committees and institutional review boards (IRBs) [BRANY IRB, Lake Success, NY, USA; Copernicus Group IRB, Durham, NC, USA; Copernicus Group IRB, Cary, NC, USA; Henry Ford Health Systems Institutional Review Board, Detroit, MI, USA; Mayo Clinic Institutional Review Board, Rochester, MN, USA]20. Informed consent was obtained from all participants or, if participants were under 16, from a parent and/or legal guardian.
In the current analysis, we initially used pre-treatment (Week 0) samples from n = 231 patients to assess the predictive power of the SomaSignal probability scores to classify NAFLD and NASH, as well as to determine baseline association of analytes to clinical measures of NASH. Subsequently, we included baseline (week 0) and on-treatment (week 12 or week 16) samples from Placebo (n = 40) or 25 mg PF-05221304 (n = 32) patients (Fig. 1). Table 1 describes the sample sizes, timepoints and randomized arms used for the SomaLogic analyses and clinical measures for inclusion in the analyses. The choice of the single dose-level of clesacostat (25 mg QD) is in line with the maximum dose currently in development (10 mg BID)21.
SomaScan Assay
Serum samples were analyzed on the SomaScan V4.1 multiplex proteomic assay (SomaLogic, Boulder CO)—an aptamer (slow off rate modified SOMAmer)-based quantitative proteomic biomarker discovery platform22 which simultaneously measures 7289 protein analytes expressed over a wide concentration range. The performance of this assay platform has been extensively and systematically validated23. The assay covers a broad range of proteins associated with disease physiology and biological functions, including cytokines, kinases, growth factors, proteases and their inhibitors, receptors, hormones and structural proteins. Samples were distributed randomly in 96-well microtiter plates and the assay operators were blinded to the identity of all samples. Inter-plate and inter-run calibration were achieved by applying a multiplicative scaling coefficient to each SOMAmer. These scaling factors were calculated using previously established reference calibrators on each plate. Sample data were first normalized to remove hybridization variation within a run followed by median normalization across all samples to remove other assay biases within the run and finally calibrated to remove assay differences between runs. Assay results were reported in normalized relative fluorescence units (RFU).
The SomaSignal NASH test provides an algorithm-derived composite measure of NASH probability through the targeted measurement of 37 analytes within the SomaScan panel that fall under the broad NASH pathological categories of inflammation, ballooning, steatosis and fibrosis. The test was developed through extensive analyses of training and validation datasets derived from 2800 samples of natural history (NASH-CRN) and interventional (FLINT and PIVENS trials) cohorts of biopsy-proven NASH patients11. In the current study, we adjusted the existing algorithm to derive a modified probability score and NAFLD/NASH classification based on the non-fibrotic components of the SomaSignal NASH test (Supplementary Table 1).
QC analyses of SomaScan data
To ensure batch normalization did not impact treatment effects, a Wilcoxon rank-sum test was conducted between treatment and placebo samples for each of the normalization factors. No significant differences were detected between treatment and control, affirming the normalization of batch effects were consistent across treatment arms (Supplementary Fig. S1).
Statistical analyses
R version 4.2.1 was used for statistical analyses and figure generation. A list of R packages utilized is provided in the Supplementary File.
The 95% confidence intervals for agreement between NASH classifications were calculated using the Clopper-Pearson method. Prior to analysis of the full panel, 37 analytes showing significant baseline differences between treatment arms after multiplicity adjustments were filtered out as patients were randomized and no statistical differences at baseline were expected The Wilcoxon rank-sum test was used to compare analyte levels between treatment arms at baseline and the Wilcoxon signed-rank test was used to compare analyte levels between paired samples across time points. To account for multiple comparisons, all p-values were adjusted using the Benjamini–Hochberg method24. For each patient, analyte precent changes from baseline was calculated as:
Analyte correlations to clinical endpoints (liver fat as assessed using MRI-PDFF, and laboratory parameters ALT, and AST) at baseline and between percent change from baseline were assessed using Spearman’s correlation. The placebo-adjusted log2 fold change between treatment arms for each analyte was calculated using the least squared means from a linear model. Differential expressions of proteins between timepoints were calculated by paired t-test, and hallmark gene set enrichment analysis25 was performed.
Pathway analysis of baseline analytes correlated to clinical measures was accomplished with KEGG pathway analyses (via String db—STRING: functional protein association networks (string-db.org)).
Results
Alignment of SomaSignal-derived and clinically stratified NAFLD and NASH classification
The SomaSignal NASH test uses an algorithm-derived composite probability score from a subset of 37 analytes spanning multiple components of NASH pathology (Inflammation, Ballooning, Steatosis and Fibrosis) to predict NASH status of a sample. Our phase 2 clinical study20 included a sequential three-step eligibility process to stratify the study population as NIT-based (Presumed) NASH (those with either a documented liver biopsy within 24 months of screening or meet the initial screening criteria of liver stiffness of ≥ 7 kPa and ALT levels between 1.25X and 5X upper limit of normal) or NAFLD with minimal inflammation (those that do not meet the screening criteria for NASH).
We first used the SomaSignal data from baseline samples (n = 231) to determine the alignment between SomaSignal-derived and clinically-derived NASH classification. Given that the study cohort was a primarily steatotic population, we updated the SomaScan algorithm to derive a modified score multiplying the probabilities of the non-fibrosis components (i.e., inflammation, ballooning and steatosis) of the SomaSignal output. Accordingly, samples with a composite probability score of ≥ 0.125 (0.53) were classified as NASH. There was 70% agreement between SomaSignal and clinical classification of NASH in the entire population using this modified algorithm (Fig. 2a). When analyzed by separating the samples based on SomaSignal fibrosis probability scores, the degree of agreement with clinical classification reduced to 64% in the fibrosis-negative (Soma fibrosis probability < 0.5) population (Fig. 2b) and increased to 80% in the fibrosis-positive (Soma fibrosis probability > 0.5) population (Fig. 2c), indicating that the SomaSignal-predicted classifications were more accurate in patients with increased fibrosis.
Contingency table of alignment between SomaSignal-derived versus clinically defined NASH and NAFLD classification in entire analyses cohort (a), patients with SomaSignal fibrosis score ≤ 0.5 (b) and ≥ 0.5 (c). Supplemental Table 1 contains details on algorithm for SomaSignal NASH classification, and clinical NASH classification of study cohort is described in Calle et al.20.
We next analyzed the SomaSignal scores from a smaller batch of baseline (week 0) and post treatment [week 12 or week 16, placebo (n = 40) and 25 mg PF-05221304 (n = 32)] samples. These treatment arms and time points were chosen based on observed robust anti-inflammatory and anti-steatotic effects in the clinical study. The reduction in steatosis probability scores aligned with the observed reduction in liver fat assessed using MRI-PDFF (Fig. 3a). As shown in Fig 3b, the reduction in steatosis probability scores decreased from baseline with cleasocostat treatment compared to placebo, although the magnitude of change was less pronounced. When separated by weeks of treatment, the reduction in steatosis probability scores were larger than with placebo at both 12 and 16 weeks, although the change at 16 weeks did not meet significance (p = 0.088, Supplementary Fig. S2a&b). When stratified by NAFLD and NASH status, the changes showed stronger significance in the NASH group compared to NAFLD, primarily due to increased number of samples in the NASH group (Supplementary Fig. S2c&d). The SomaSignal scores for inflammation and ballooning however did not change significantly with drug treatment (data not shown).
These initial data suggested that the SomaSignal NASH test based on the 37-analyte panel was reasonably effective in predicting NASH status and treatment-induced effects on steatosis, but not sensitive enough to identify or predict treatment induced changes in inflammation and ballooning, at least in the context of ACC inhibition.
SomaScan analytes associated with NASH indices of steatosis and inflammation
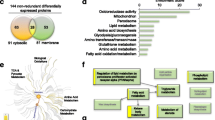
We next analyzed the total data on the 7000+ analytes from the panel with a goal to identify a more robust panel of biomarkers of disease and treatment response to ACCi. Baseline analyses using an adjusted p-value cutoff of 0.05 revealed a set of 69 analytes that were positively correlated to all clinical measures of NASH-ALT, AST and MRI-PDFF (Fig. 4a, Table 2). Notably, most of the analytes were involved in NASH-relevant pathways such as fatty acid degradation, amino acid metabolism, fructose metabolism, glycolysis/gluconeogenesis and retinol biosynthesis (Fig. 4b, Supplementary Table 2, KEGG pathway enrichment). Additionally, we found that the expressions of these analytes were significantly higher in the NASH group versus NAFLD group (unpaired t-test). Baseline measures of MRI-PDFF, ALT and AST were also higher in the NASH group (Supplementary Fig. S3).
Identification of SomaScan analytes (serum proteins) significantly correlated to baseline clinical measures. (a) Venn diagram showing common analytes correlated to liver fat assessed using MRI-PDFF, ALT and AST; (b) KEGG pathway analyses of common analytes from (a); (c) Venn diagram comparing baseline correlated analytes with those changing with clesacostat treatment; (d) KEGG pathway analyses of analytes correlated at baseline as well as changing with clesacostat treatment. (Spearman's correlation with adjusted p-value cutoff of < 0.05 for baseline correlation and unadjusted p-value cutoff of < 0.05 for treatment response).
Of the analytes correlated at baseline to NASH-related variables, 27 were significantly reduced with clesacostat (Fig. 4c, Table 3) compared to placebo, with an unadjusted p value cutoff of 0.05 (Wilcoxon test). This set of analytes also showed enrichment of many of the same pathways as observed with the baseline correlated analytes suggesting treatment impact on these pathways (Fig. 4d, Supplementary Table 3). Stratification of drug response by post-treatment timepoint or NASH status did not reveal statistically significant differences from placebo comparison although drug-induced reduction in expression was much greater than with placebo.
These initial analyses helped us identify a set of analytes that may be used as surrogate measures of steatosis and inflammation and their changes in response to ACCi dosing in NASH.
Clesacostat treatment dramatically upregulates Wnt5a and Apolipoproteins C and E
We next analyzed the biomarker signature of clesacostat treatment in the cohort across the entire dataset (independent of baseline association of analytes to clinical measures) using an adjusted p value threshold of 0.05. Figure 5 shows volcano plots of placebo-adjusted changes in analyte expression from baseline, with drug treatment. Top analytes changing with dosing of ACCi are indicated in Tables 4 and 5 (see Supplementary Table 4 for change from baseline for all analytes). We observed a dramatic increase in the Wnt ligand, Wnt5a as well as Apolipoproteins C3 and E with clesacostat dosing compared to placebo (Fig. 6a–c). These changes were significant in both NAFLD and NASH subgroups within the cohort for Wnt5a (Supplementary Fig. S4a). For ApoC3 and ApoE, the differences did not reach significance in the NAFLD group, although a larger increase with clesacostat compared to placebo was observed (Supplementary Fig. S4b,c). We also found that the inflammatory marker, CHI3Li (YKL-40) was upregulated by clesacostat treatment, perhaps a consequence of SREBP1c transactivation that is known to increase monocytic differentiation (Fig. 6d). In addition, drug induced changes in the expression of Wnt5a, ApoC3 and ApoE significantly (and negatively) correlated with drug-induced changes in MRI-PDFF confirming that the changes in these analytes may be clinically relevant (Fig. 7).
Finally, we performed Hallmark pathway analyses with the list of analytes significantly altered with clesacostat treatment using placebo-adjusted fold changes. Hallmark gene sets are derived from founder gene sets that define specific biological states and processes and provide a more accurate and less redundant method for gene set enrichment analyses26. Clesacostat treatment resulted in a potent downregulation of fatty acid metabolism, adipogenesis and inflammatory pathways consistent with the mechanism of action of ACC, while upregulating hedgehog signaling and heme metabolism pathways (Fig. 8).
Discussion
Clinical studies in patients with biopsy-confirmed NASH are very challenging due to sampling errors27,28, reader-to-reader variability in interpretation including amongst highly specialized pathologists29, procedure-related morbidity and mortality30, patient reluctance and cost. Therefore, there is a global effort in identifying biomarkers of diagnosis and treatment response in NASH, that can potentially replace biopsies and/or serve as early surrogates of prognosis and efficacy. The LITMUS consortium for NASH biomarkers investigated multiple non-invasive tests (NITs) with a goal to identify the best biomarker(s) capable of replacing biopsies13,31,32. Although no single biomarker met the predefined performance threshold, the study determined that the SomaSignal NASH test provided the most acceptable accuracy in predicting advanced fibrosis13. We therefore sought to determine the clinical utility of the SomaSignal test and the larger panel of SomaScan biomarkers using samples from a phase 2 steatotic NAFLD/NASH cohort with clesacostat, an ACC inhibitor.
Comparison of NAFLD and NASH classification of our clinical cohort with that derived from the non-fibrotic components of SomaSignal NASH scores revealed only a 70% alignment indicating the targeted panel was only moderately sensitive to predict NASH status, although the alignment slightly improved to 80% in patients with higher fibrosis scores. These findings align with published data describing the development of the SomaScan NASH assay that also suggests a better separation of predictive probabilities (higher AUC) with the fibrosis-based model compared to models based on steatosis, ballooning and inflammation11. The SomaSignal NASH panel was developed through analyses of biopsy-proven natural history and select interventional (Obeticholic acid and Vitamin E) NASH cohorts11. In the absence of extensive biopsies and given that our cohort was primarily a steatotic population, we compared the treatment induced changes in the steatosis component scores to observed clinical fat reduction through MRI-PDFF and found a good alignment. We did observe that clesacostat-induced reduction in steatosis probability score was more pronounced at week 12 when stratified by treatment timepoint, suggesting that analytes within this model could be early indicators of treatment response. Notably, SomaLogic proteomic analyses on samples from the FALCON phase 2 trial with pegbelfermin, an FGF-19 agonist, also showed most consistent effects on steatosis-related biomarkers compared to inflammatory and tissue injury markers12. We reasoned that the targeted panel of 37 analytes in the SomaSignal test may not provide the best surrogacy for the entire spectrum of disease and multiple mechanisms targeted by other interventions and hypothesized that perusing the larger data from 7000+ analytes will provide a more optimal biomarker signature in the context of anti-steatotic intervention.
To identify the most robust and clinically relevant biomarkers, we looked for analytes that correlated to multiple key clinical variables of inflammation and steatosis—MRI-PDFF, ALT and AST. These were also endpoints that were significantly reduced with clesacostat treatment in the study. These analyses revealed a set of 69 analytes, most of which were involved in fatty acid metabolism, cholesterol production, amino acid and pyruvate metabolism, glycolysis and fructose metabolism. In addition, most of these analytes were significantly increased in the NASH sub-group compared to NAFLD. FBP1, and ALDOB are key enzymes involved in fructose metabolism. These, along with ALDOC and ASL have been shown to be progressively increased with severity of NASH33. Additionally, many of the identified markers have been shown to be associated with NASH severity in the LITMUS study comparing the liver transcriptome and circulating proteome of NAFLD patients34. These markers could therefore possibly serve as early indicators of steatotic changes in NASH. It is important to note that a majority of the 37 analytes from the existing SomaSignal NASH test were not represented in the panel of markers that we identified from the larger dataset, indicating that the biomarker signature would be influenced by the study cohort and disease status.
Our data show a remarkable increase in Wnt5a protein with clesacostat treatment. Although Wnt5a is a member of the non-canonical Wnt family known to be involved in promoting pro-inflammatory and pro-fibrotic signaling, it has also been shown to play a major role in facilitating cholesterol egress and maintaining lipid homeostasis through canonical pathways35,36,37. The low-density lipoprotein receptor, LRP1, is a critical regulator of lipid homeostasis and hepatic inflammation, with markedly distinct functions in adipocytes, liver and macrophage compartments. LRP1 drives increases in Wnt5a that limits cholesterol accumulation in adipocytes, and disruption of this axis causes defective fatty acid uptake in adipose and its redistribution to liver resulting in pathological hepatic steatosis35. Intriguingly, loss of LRP1 activity also results in the reduction of ACC activity in adipose tissue. In the liver, loss of LRP1 in hepatocytes exacerbates palmitate-induced steatosis and increased hepatic TG38. Macrophage LRP1 regulates diet-induced hepatic inflammation and metabolic dysfunction through modulation of Wnt signaling39,40. Thus, although the direct impact of ACC inhibition on De Novo Lipogenesis (DNL) has been established before, these new data provide an intriguing possibility that additional mechanisms may be at play involving Wnt5a, resulting in steatosis reduction in the liver while possibly also impacting components of inflammation.
While our clinical data shows reduction in ALT suggesting anti-inflammatory activity in the liver, we also found an increase in CHI3L1 (aka YKL-40) with clesacostat treatment. CHI3L1 is a known proinflammatory marker of differentiated M2 macrophages and an established biomarker that associates with NASH and fibrosis41,42. Monocyte differentiation to phagocytic macrophages depends on fatty acid synthesis driven by an increase in SREBP-1c activation and subsequent expression of CHI3L143. During this process, SREBP2 signaling is impaired causing a switch in intracellular lipid profile from cholesterol to phosphatidyl choline43. Blockade of SREBP-1c with small interfering RNA attenuates the CHI3L1 increase during monocyte differentiation to macrophages. We speculate that SREBP-1c driven by ACC inhibition18,19 may potentially account for the observed increases in CHI3L1 with clesacostat treatment. Our studies also reconfirmed the earlier observations of increases in APOC3 with clesacostat20, potentially contributing to the increased serum TG, again through the activation of SREBP-1c. It is worthwhile to note that APOC3 mediated systemic TG regulation is also dependent on LRP144. In addition, we also found an increase in ApoE with clesacostat treatment. Preclinical evidence indicates that mice lacking APoE show increased hepatic TG, cholesterol and steatosis at baseline and under high fat diet conditions45, implying that an increase in ApoE could work in favor of steatosis reduction. It is to be noted that, with the current data, it would not be possible to determine if the observed analyte changes are a cause for or an effect of ACC inhibition. Further mechanistic/preclinical studies are likely needed to gain a deeper understanding of the implication of these markers.
The biomarker findings in our study are further strengthened by the fact that the increases with treatment clearly (and negatively) correlate with observed reduction in steatosis through MRI-PDFF, suggesting that these changes may be clinically relevant. Based on our biomarker findings, we provide a proposed working hypothesis linking LRP1, Wnt5a and SREBP-1c to speculate how ACC inhibition may affect multiple mechanisms including cholesterol homeostasis, TG regulation, and hepatic/systemic inflammation (Fig. 9). It is possible that these mechanisms may work in conjunction with the known core mechanism of ACC inhibition mediated through effects on de novo lipogenesis.
Proposed mechanism by which ACC inhibition modulates steatosis, TG homeostasis and inflammation. ACC Inhibition is well known to reduce steatosis primarily through inhibition of “de novo lipogenesis". This is also known to be accompanied by elevated SREBP-1c that increases ApoC3 and elevates serum TG. It is possible that compartmental activation of LRP1 could drive the Wnt5a increase and impact lipid homeostasis in liver, while possibly modulating inflammatory endpoints through its activity in macrophages. Increased SREBP-1c activity may also potentiate the modulation of macrophage maturation and expression of CHI3L1. impacting some aspects of systemic inflammation. APOE increase may also potentially contribute to liver steatosis reduction.
Our study is not without limitations. First, only a handful of the patients in the cohort had a liver biopsy at screening and the clinical classification of NAFLD and NASH in most patients was based on predefined liver stiffness (assessed using FibroScan) and ALT levels20. This precluded us from benchmarking biomarker findings against histology which could have helped us determine how systemic changes measured through soluble biomarkers correlate to established pathology in the diseased tissue. Next, the study was run in two tiers with a larger number of baseline samples analyzed initially and a smaller subset of post-treatment samples subsequently. In addition, we only included samples from one dose level (25 mg QD) of the drug where we observed near 100% clinical efficacy, which prevented us from assessing dose response for the analytes. Notwithstanding these limitations, our work identifies a unique set of potential biomarkers that relate to steatosis and inflammation in NAFLD/NASH and provide novel insights into pathways through which ACC inhibition may exert its clinical effect in the liver. The study also generates new hypothesis to explain the safety-related limitations of ACC inhibition on steatosis, inflammation and systemic TG regulation. Future studies are needed to explore the broader utility of the identified markers to stage disease, select patients, and evaluate drug response of therapies to treat NASH.
Data availability
All data as part of the described work have been included in the manuscript or as Supplementary Files. Upon request, and subject to review, Pfizer will provide additional data that supports the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
Serfaty, L. Management of patients with non-alcoholic steatohepatitis (NASH) in real life. Liver Int. 38(Suppl 1), 52–55 (2018).
Traussnigg, S. et al. Challenges and management of liver cirrhosis: practical issues in the therapy of patients with cirrhosis due to NAFLD and NASH. Dig. Dis. 33(4), 598–607 (2015).
Giashuddin, S. & Alawad, M. Histopathological diagnosis of nonalcoholic steatohepatitis (NASH). Methods Mol. Biol. 2455, 1–18 (2022).
Tong, X. F. et al. Histological assessment based on liver biopsy: The value and challenges in NASH drug development. Acta Pharmacol. Sin. 43(5), 1200–1209 (2022).
Contreras, D. et al. Diagnostic accuracy of blood biomarkers and non-invasive scores for the diagnosis of NAFLD and NASH: Systematic review and meta-analysis. Ann. Hepatol. 28(1), 100873 (2023).
Govaere, O. et al. A proteo-transcriptomic map of non-alcoholic fatty liver disease signatures. Nat. Metab. 5(4), 572–578 (2023).
Liu, Y., et al. A multi-omics analysis of NASH-related prognostic biomarkers associated with drug sensitivity and immune infiltration in hepatocellular carcinoma. J. Clin. Med. 12(4) (2023).
Harrison, S. A. et al. Utility and variability of three non-invasive liver fibrosis imaging modalities to evaluate efficacy of GR-MD-02 in subjects with NASH and bridging fibrosis during a phase-2 randomized clinical trial. PLoS One 13(9), e0203054 (2018).
Ajmera, V. & Loomba, R. Imaging biomarkers of NAFLD, NASH, and fibrosis. Mol. Metab. 50, 101167 (2021).
Troelstra, M. A. et al. Assessment of imaging modalities against liver biopsy in nonalcoholic fatty liver disease: The Amsterdam NAFLD-NASH Cohort. J. Magn. Reson. Imaging 54(6), 1937–1949 (2021).
Sanyal, A. J. et al. Defining the serum proteomic signature of hepatic steatosis, inflammation, ballooning and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 78(4), 693–703 (2023).
Brown, E. A. et al. Effect of pegbelfermin on NASH and fibrosis-related biomarkers and correlation with histological response in the FALCON 1 trial. JHEP Rep. 5(4), 100661 (2023).
Vali, Y. et al. Biomarkers for staging fibrosis and non-alcoholic steatohepatitis in non-alcoholic fatty liver disease (the LITMUS project): A comparative diagnostic accuracy study. Lancet Gastroenterol. Hepatol. 8(8), 714–725 (2023).
Ross, T. T. et al. Acetyl-CoA carboxylase inhibition improves multiple dimensions of NASH pathogenesis in model systems. Cell Mol. Gastroenterol. Hepatol. 10(4), 829–851 (2020).
Lambrecht, J. & Tacke, F. Acetyl-CoA carboxylase inhibition as a therapeutic tool in the battle against NASH: Hitting more than just one mechanism?. Cell Mol. Gastroenterol. Hepatol. 10(4), 859–861 (2020).
Alkhouri, N. et al. GS-0976 (Firsocostat): An investigational liver-directed acetyl-CoA carboxylase (ACC) inhibitor for the treatment of non-alcoholic steatohepatitis (NASH). Expert Opin. Investig. Drugs 29(2), 135–141 (2020).
Bergman, A. et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of a liver-targeting Acetyl-CoA Carboxylase Inhibitor (PF-05221304): A three-part randomized phase 1 study. Clin. Pharmacol. Drug Dev. 9(4), 514–526 (2020).
Kim, C. W. et al. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: A bedside to bench investigation. Cell Metab. 26(2), 394-406.e6 (2017).
Goedeke, L. et al. Acetyl-CoA carboxylase inhibition reverses NAFLD and hepatic insulin resistance but promotes hypertriglyceridemia in rodents. Hepatology 68(6), 2197–2211 (2018).
Calle, R. A. et al. ACC inhibitor alone or co-administered with a DGAT2 inhibitor in patients with non-alcoholic fatty liver disease: Two parallel, placebo-controlled, randomized phase 2a trials. Nat. Med. 27(10), 1836–1848 (2021).
Amin, N. B. et al. Efficacy and safety of an orally administered DGAT2 inhibitor alone or coadministered with a liver-targeted ACC inhibitor in adults with non-alcoholic steatohepatitis (NASH): Rationale and design of the phase II, dose-ranging, dose-finding, randomised, placebo-controlled MIRNA (Metabolic Interventions to Resolve NASH with fibrosis) study. BMJ Open 12(3), e056159 (2022).
Mehan, M. R. et al. Highly multiplexed proteomic platform for biomarker discovery, diagnostics, and therapeutics. Adv. Exp. Med. Biol. 735, 283–300 (2013).
Candia, J. et al. Assessment of variability in the plasma 7k SomaScan proteomics assay. Sci. Rep. 12(1), 17147 (2022).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J. Royal Stat. Society Series B-Stat. Methodol. 57(1), 289–300 (1995).
Subramanian, A. et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102(43), 15545–15550 (2005).
Liberzon, A. et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1(6), 417–425 (2015).
Ratziu, V. et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 128(7), 1898–1906 (2005).
Merat, S. et al. Sampling error in histopathology findings of nonalcoholic fatty liver disease: A post mortem liver histology study. Arch Iran Med. 15(7), 418–421 (2012).
Kleiner, D. E. et al. Association of histologic disease activity with progression of nonalcoholic fatty liver disease. JAMA Netw. Open 2(10), e1912565 (2019).
Thomaides-Brears, H. B. et al. Incidence of complications from percutaneous biopsy in chronic liver disease: A systematic review and meta-analysis. Dig. Dis. Sci. 67(7), 3366–3394 (2022).
Pavlides, M. et al. Liver investigation: Testing Marker Utility in Steatohepatitis (LITMUS): Assessment & validation of imaging modality performance across the NAFLD spectrum in a prospectively recruited cohort study (the LITMUS imaging study): Study protocol. Contemp. Clin. Trials 134, 107352 (2023).
Rasmussen, D. G. K. et al. NAFLD and NASH biomarker qualification in the LITMUS consortium—Lessons learned. J. Hepatol. 78(4), 852–865 (2023).
Corey, K. E. et al. ADAMTSL2 protein and a soluble biomarker signature identify at-risk non-alcoholic steatohepatitis and fibrosis in adults with NAFLD. J. Hepatol. 76(1), 25–33 (2022).
Govaere, O., et al. Transcriptomic profiling across the nonalcoholic fatty liver disease spectrum reveals gene signatures for steatohepatitis and fibrosis. Sci. Transl. Med. (2020). 12(572).
Terrand, J. et al. LRP1 controls intracellular cholesterol storage and fatty acid synthesis through modulation of Wnt signaling. J. Biol. Chem. 284(1), 381–388 (2009).
Awan, S. et al. Wnt5a promotes lysosomal cholesterol egress and protects against atherosclerosis. Circ. Res. 130(2), 184–199 (2022).
Qin, L. et al. The novel role and underlying mechanism of Wnt5a in regulating cellular cholesterol accumulation. Clin. Exp. Pharmacol. Physiol. 41(9), 671–678 (2014).
Hamlin, A. N. et al. LRP1 protein deficiency exacerbates palmitate-induced steatosis and toxicity in hepatocytes. J. Biol. Chem. 291(32), 16610–16619 (2016).
Lillis, A. P. et al. LDL receptor-related protein-1 (LRP1) regulates cholesterol accumulation in macrophages. PLoS One 10(6), e0128903 (2015).
Au, D. T. et al. Macrophage LRP1 promotes diet-induced hepatic inflammation and metabolic dysfunction by modulating Wnt signaling. Mediators Inflamm. 2018, 7902841 (2018).
Kumagai, E. et al. Serum YKL-40 as a marker of liver fibrosis in patients with non-alcoholic fatty liver disease. Sci. Rep. 6, 35282 (2016).
Harrison, S. A. et al. A blood-based biomarker panel (NIS4) for non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis: A prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 5(11), 970–985 (2020).
Ecker, J. et al. Induction of fatty acid synthesis is a key requirement for phagocytic differentiation of human monocytes. Proc. Natl. Acad. Sci. USA 107(17), 7817–7822 (2010).
Gordts, P. L. et al. ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J. Clin. Invest. 126(8), 2855–2866 (2016).
Schierwagen, R. et al. Seven weeks of Western diet in apolipoprotein-E-deficient mice induce metabolic syndrome and non-alcoholic steatohepatitis with liver fibrosis. Sci. Rep. 5, 12931 (2015).
Acknowledgements
The authors wish to thank William Esler and Gregory Tesz (Pfizer Research and Development, Cambridge, MA, USA) as well as Will Schwarzmann and Clare Paterson (SomaLogic Inc., Boulder, CO) for their critical input into this manuscript. This study was sponsored by Pfizer Inc.
Author information
Authors and Affiliations
Contributions
NBA, LK and PS designed the study. PS and MS analyzed the data and PS led the efforts in writing the manuscript. MS and DR performed all the statistical analyses. All authors provided input into manuscript preparation and have read and approved the final submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors were Pfizer employees at the time of the work and shareholders of Pfizer Inc.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sivakumar, P., Saul, M., Robinson, D. et al. SomaLogic proteomics reveals new biomarkers and provides mechanistic, clinical insights into Acetyl coA Carboxylase (ACC) inhibition in Non-alcoholic Steatohepatitis (NASH). Sci Rep 14, 17072 (2024). https://doi.org/10.1038/s41598-024-67843-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67843-8
- Springer Nature Limited