Abstract
Orthostatic intolerance (OI) is a key symptom of long COVID; however, the pathophysiology remains unknown. Among 688 long COVID patients who visited our clinic during the period from February 2021 to April 2023, 86 patients who were suspected of having OI and who underwent an active standing test (ST) were investigated to elucidate the clinical characteristics of OI in patients with long COVID. Of the 86 patients, 33 patients (38%) were ST-positive. Nausea and tachycardia in daily life were frequent complaints in the ST-positive group. The increase in heart rate (HR) during the ST was significantly greater during a 10-min period after standing in the ST-positive group (+ 30 bpm) than in the ST-negative group (+ 16 bpm). The initial increase in diastolic blood pressure (DBP) just after standing was significantly greater in the ST-positive group (+ 14 mmHg) than in the ST-negative group (+ 9 mmHg). Serum cortisol levels in the ST-positive patients aged over 20 years were higher and growth hormone levels in the patients under 20 years of age were lower than those in the ST-negative group. Autonomous nervous symptoms, transient DBP rise with increasing HR after standing, and endocrine dysfunctions are helpful for detecting OI related to long COVID.
Similar content being viewed by others
Introduction
A relatively large percentage of patients with COVID-19 suffer from long COVID and post-COVID-19 condition, and appropriate treatments for the remaining symptoms and conditions have become important issues1. The medical problem lies not only in the symptoms experienced during the infection but also in the significant after-effects. The cumulative prevalence of long COVID was reported to range from 9 to 63%, which is up to sixfold higher than that of similar post conditions observed after other viral infections2, although the definitions of post-COVID-19 condition have differed in real-world studies3.
There are various symptoms of long COVID including general fatigue, headaches, fever, taste disorders, olfactory disturbances, and tachypnea4. We previously reported that general fatigue is the most common symptom in Japanese patients with long COVID5. We also reported that general fatigue in some long COVID patients may develop into the prolonged condition of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)6 and that increased serum ferritin level and lowered insulin-like growth factor (IGF)-I level can be biomarkers for detecting the transition to an ME/CFS condition7. Moreover, some patients with long COVID complain of palpitations and syncope just after standing up, and autonomic nervous system dysfunction is involved in these symptoms8,9. Symptoms upon standing such as syncope and palpitations that are chronic in nature are defined as orthostatic intolerance (OI)10, which in most cases can be linked to autonomic nervous system dysfunction. It has been reported that a substantial number of long COVID patients experience palpitations and that many of those patients have OI8.
OI has been recognized as a condition associated with abnormal cardiovascular autonomic response to gravitational stress10. It has also been suggested that the autonomic disturbances in long COVID patients may lead to complications related to OI8. The diagnosis of OI is established through active-standing and head-up tilt tests. Recent studies on long COVID have shown the prevalence of OI and predictors of OI11,12 including age, gender, fatigue and depressive symptoms, inability of daily living, and peak heart rate (HR) after the highest suppression of blood pressure immediately after until one minute after taking the orthostatic position12.
Although autonomic neuropathy and endocrine abnormalities have been implicated in the pathophysiology of OI13, there has been no clear evidence regarding the interrelationships between clinical/laboratory characteristics and occurrence of OI in patients with long COVID. The aim of the present study was to determine the prevalence of a positive standing test (ST) and accompanying autonomic manifestations in long COVID patients with features suggestive of OI and their pathophysiologic features.
Results
First, to evaluate the clinical features of OI associated with long COVID, we performed an active standing test (ST) in 86 patients who were suspected of having OI among 688 patients with long COVID, and 33 patients (38%) were positive for the ST.
The median age of ST-positive patients was 20 years, which was significantly younger (**P < 0.01) than the median age of 40 years for ST-negative patients (Table 1). In addition, 48.5% of the ST-positive patients were aged under 20 years of age, while 79.2% of the ST-negative patients were older than 20 years of age (**P < 0.01). The gender ratios in the two groups were not significantly different: 57.6% of the patients in the ST-positive group were male and 56.6% of the ST-negative patients were female (Table 1).
There were no statistically significant differences between ST-positive patients and ST-negative patients in background factors including body mass index (BMI), habits of smoking and drinking, severity of COVID-19, viral variants, and vaccination history (Table 1). Also, evaluation of data for blood cells and biochemical laboratory data showed no notable differences in peripheral blood counts, liver and kidney functions, electrolytes, and inflammatory and nutritious factors between the ST-positive and ST-negative groups (Table 2).
The most frequent daily symptoms in the long COVID patients who underwent an ST were fatigue, headache, sleep disturbance, dizziness, dyspnea, nausea, and poor concentration (Fig. 1). There were no significant differences in the clinical symptoms between the ST-positive and ST-negative patients with long COVID. Only a tendency for accompanying symptoms including nausea (P = 0.06) and tachycardia (P = 0.07) was seen in the ST-positive patients, though the differences between the two groups were not statistically significant (Fig. 1).
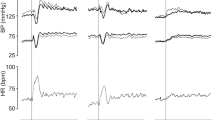
As shown in Fig. 2, recording of HR during the ST showed that both the ST-positive and ST-negative patients had significant increases (**P < 0.01) in HR after standing up from the supine position (Fig. 2A), though the initial increment of HR (ΔHR) from basal HR (at − 5 min) in the ST-positive patients (25 bpm) was significantly higher (##P < 0.01) than that in the ST-negative patients (14 bpm; Fig. 2B). Moreover, the ST-positive patients showed a significantly greater ΔHR (#P < 0.05, ##P < 0.01: highest ΔHR of 30 bpm) during a 10-min period after standing than ΔHR (highest ΔHR of 16 bpm) in the ST-negative patients at the corresponding time points (Fig. 2B).
Changes in heart rate (HR) during the active standing test in long COVID patients. (A) The results of individual HR (upper panel) and mean HR ± standard deviation (SD; lower panel) are shown for a 5-min recumbent position and for a subsequent 10-min upright position after active-standing stimulation. The data were analyzed by Student’s t-test; **P < 0.01 vs. basal HR at − 5 min. (B) Changes from baseline HR (ΔHR) were plotted as mean ΔHR ± SD for 10 min during the standing test (ST). The data were analyzed by Student’s t-test; #P < 0.05 and ##P < 0.01 vs. ΔHR of the ST-negative patients at the corresponding time points. “n” indicates the number of patients.
On the other hand, as shown in Fig. 3, systolic blood pressure (SBP) showed moderate but significant increases (**P < 0.01) in both groups (Fig. 3A). In the ST-positive group, SBP showed transient increases at the time points of 0 min and + 8 min after standing, while SBP in the ST-negative group constantly increased during the 10-min period after standing. The increments of SBP (ΔSBP) from basal HR at − 5 min were not significantly different between the two groups: ΔSBP increased up to + 7 mmHg just after standing and then gradually decreased during the 10-min standing period without significant differences between the two groups (Fig. 3B).
Changes in systolic blood pressure (SBP) during the active standing test in long COVID patients. (A) The results of individual SBP (upper panel) and mean SBP ± standard deviation (SD; lower panel) are shown for a 5-min recumbent position and for a subsequent 10-min upright position after active-standing stimulation. The data were analyzed by Student’s t-test; **P < 0.01 vs. basal SBP at − 5 min. (B) Changes from baseline SBP (ΔSBP) were plotted as mean ΔSBP ± SD for 10 min during the standing test. The data were analyzed by Student’s t-test. “n” indicates the number of patients.
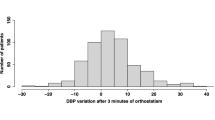
Diastolic blood pressure (DBP) also showed significant increases (**P < 0.01) in both groups (Fig. 4A). However, the increment of DBP (ΔDBP) from basal DBP at − 5 min in the ST-positive group (highest ΔDBP of + 14 mmHg) was significantly higher (##P < 0.01) than that in the ST-negative group (highest ΔDBP of + 9 mmHg) just after standing (Fig. 4B). In the ST-positive group, the increase in ΔDBP persisted during the 10-min period with a transient decrease to + 11 mmHg at 2 min after standing, whereas ΔDBP in the ST-negative group showed a gradual increase from + 9 to + 12 mmHg during the 10-min standing period (Fig. 4B), indicating that an acute and transient rise of ΔDBP just after standing is characteristic for ST-positive patients.
Changes in diastolic blood pressure (DBP) during the active standing test in long COVID patients. (A) The results of individual DBP (upper panel) and mean DBP ± standard deviation (SD; lower panel) are shown for a 5-min recumbent position and for a subsequent 10-min upright position after active-standing stimulation. The data were analyzed by Student’s t-test; **P < 0.01 vs. basal DBP at − 5 min. (B) Changes from baseline DBP (ΔDBP) were plotted as mean ΔDBP ± SD for 10 min during the standing test (ST). The data were analyzed by Student’s t-test; ##P < 0.01 vs. ΔDBP of the ST-negative patients at the corresponding time points. “n” indicates the number of patients.
Finally, to evaluate the differences in endocrine characteristics between the ST-positive and ST-negative groups, the patients were divided into two age groups: under 20 years of age and 20 years of age or older. The major symptoms of long COVID in the ST-positive patients aged 20 years or older were general fatigue, sleep disturbance, dizziness, and headache, which were also common symptoms in patients in the ST-positive group who were aged under 20 years (Supplementary Fig. 1). As shown in Table 3, there were no significant differences in thyroid hormones (free thyroxine (FT4), thyrotropin (TSH), and ratio of TSH/FT4) and adrenocortical hormones (cortisol, adrenocorticotropin (ACTH), and ratio of ACTH/cortisol) between patients under 20 years of age in the ST-positive and ST-negative groups. Serum growth hormone (GH) level, but not IGF-I standard deviation (SD) level, was significantly lower (*P < 0.05) in the ST-positive group than in the ST-negative group for patients aged under 20 years (0.92 ng/mL vs. 2.68 ng/mL; Table 3). However, for patients aged 20 years or older, serum cortisol level was significantly higher (*P < 0.05) in the ST-positive group than in the ST-negative group (11.2 μg/dL vs. 8.4 μg/dL; Table 3).
Discussion
In the present study, the clinical characteristics of long COVID patients who were suspected of having OI were elucidated. In our database of 86 patients with long COVID, 33 patients (38%) were found to be ST-positive. The prevalence of OI in the present study was quite high compared to the prevalence of 14.2% previously reported by another group in Japan9. The criteria for diagnosis of long COVID and the method for performing the ST were almost the same in our study and that previous study. However, the composition of viral variants of SARS-CoV-2 based on the infection phase of COVID-19 might be different.
In our study performed during the period from February 2021 to April 2023, the majority of long COVID patients with suspected OI were infected during the Omicron phase (70%). The previous study conducted in Japan by another group might have included many long COVID patients who were infected in the Preceding and Delta-dominant phases9. It is thus possible that variant-dependent changes in OI symptoms due to long COVID occurred and that the population of long COVID patients with suspected OI has been increasing during the Omicron phase.
In our study, the proportion of patients aged under 20 years in the ST-positive patients was 48.5%, while the proportion of patients aged under 20 years in the ST-negative patients was only 20.8%. There was no significant difference in the gender ratio between the ST-positive and ST-negative groups: 42.4% of the ST-positive patients were female patients, while 56.6% of the ST-negative patients were female patients.
In a related study performed by Monaghan et al.12, OI during active standing tests like ours was observed in 66% of 85 long COVID patients with a mean age of 46 years including 74% women, with 56 women (66%) being diagnosed with OI. Of interest, OI in 32% of the patients in that study was detected as orthostatic hypotension12. In that study, the proportion of patients who fulfilled the postural orthostatic tachycardia syndrome (POTS) criteria was only 2% of the participants who completed a 10-min tilt test12. It is possible that age-dependent issues, namely younger generation, were involved in the occurrence of POTS detected in long COVID patients in our study, whereas female gender might be related to the manifestation of orthostatic hypotension in long COVID patients in the study by Monaghan et al.12.
In the present study, the symptomatic frequencies of nausea and tachycardia were relatively high in the ST-positive patients with long COVID despite the statistical fact that there were no clinical differences in COVID-19-related issues in the ST-positive group. In general, patients with long COVID have a variety of symptoms including dizziness, shortness of breath, and general fatigue14. Among these symptoms, nausea and tachycardia as subjective symptoms could be related to the pathophysiology that affects the neurovascular system via direct cytotoxic and/or indirect pro-inflammatory mechanisms, leading to dysfunction of the autonomic nervous system in long COVID15,16.
In the present study, the rate of increase in HR during the 10-min standing period was high with a median maximum ΔHR of 30 bpm in the ST-positive group, which was two-times greater than that in the ST-negative group. Although SBP showed similar modest increases in both groups during the standing test, DBP was significantly higher in the ST-positive group just after standing. Of note, the initial increase in DBP just after standing was significantly greater in patients in the ST-positive group than in patients in the ST-negative group (ΔDBP: 14 vs. 9 mmHg). The physiological mechanism of the DBP rise in our patients with POTS is not known. However, since this phenomenon of an initial DBP rise was also found in Monaghan’s study in which one third of the participants had orthostatic hypotension12, the elevated DBP may reflect a compensative and physiologic response to rapidly preserve the cardiac output in addition to a rapid increase of HR after the stress of an upright position. In this regard, Tsuchida and colleagues9 reported that treatment with a small dose of a beta-blocker was effective for ST-positive long COVID patients with POTS, suggesting that the manifestation of OI symptoms of POTS could be, at least in part, originated from the increase of HR in long COVID patients.
We previously revealed that endocrine dysfunction can be a factor contributing to general fatigue in long COVID patients17,18,19, in whom serum cortisol levels were affected by the depressive condition in long COVID and were significantly decreased in patients with dysosmia and dysgeusia associated with long COVID17. A recent study further demonstrated that serum cortisol levels were significantly lowered despite inappropriately low levels of ACTH in patients with severe long COVID, indicating the possibly of impaired function in the hypothalamic–pituitary–adrenal (HPA) axis20.
Adrenocortical responses vary depending on the impact and duration of each stress21,22,23. Stress-induced hypercortisolemia occurs due to prolonged cortisol secretion leading to glucocorticoid resistance24. In the chronic phase of prolonged stress, switching from a hypercortisolemic phase to a hypocortisolemic phase may occur24, suggesting the existence of adrenal exhaustion related to degenerating mitochondrial functions, leading to a reduction of glucocorticoid synthesis as seen in chronic stress-related disorders such as ME/CFS25. In the present study, endocrine data were evaluated in two age groups because of the inclusion of a large proportion of younger patients in the ST-positive group. A significant increase in cortisol was found in patients aged 20 years or older but not in patients aged under 20 years in the ST-positive group. These results suggest that in long COVID patients aged under 20 years, the HPA-responsiveness in response to physical stress may be rather impaired or exhausted21,24 for compensation to the autonomous nervous dysfunction, which might affect the daily lives of teenage patients26.
The lowered levels of serum GH in patients under 20 years of age in the ST-positive group was also an interesting finding in our study. In this regard, an interrelationship between lowered GH and occurrence of POTS has recently been pointed out in patients unrelated to COVID-19. Johansson and colleagues investigated serum GH levels in patients with POTS and age-matched healthy controls and found that plasma GH levels were significantly lower in patients with POTS and that GH levels were functionally associated with the severity of impairment of daily activity in patients with POTS27. Although the underlying mechanism involved in the lowered GH and pathophysiology of POTS has yet to be elucidated27, it is possible that a low GH level is a predictor of the occurrence of POTS in patients with long COVID manifestations.
This study has several limitations. First, this study included patients with long COVID presenting to an outpatient clinic for long COVID at a single center in Japan, although the patients were referred widely from 19 out of the 47 prefectures in Japan. Second, this study is a descriptive study, and its goal is to summarize the characteristics of the long COVID patients who visited our outpatient clinic over a given period of time and who had results for ST. Therefore, no sample size was calculated for this study. Third, the suspicion of a neuro-modulatory disorder and the decision to perform an ST were left to the judgment of the physician in charge, and it is possible that patients with OI were not completely detected15. Fourth, OI was examined only by an active standing test, and tests were therefore not performed in patients with severe long COVID in this study. In addition, the tilt-test was not performed in the present study. Fifth, detailed endocrine tests including pituitary and adrenal stimulating tests were not performed in this study, although two endocrine specialists included in our clinical team carefully checked all of the endocrine data.
In conclusion, we determined the prevalence of OI and its characteristic features in patients with long COVID. There was a high ST-positive rate of 38% in patients suspected of having OI. Symptoms related to autonomous nerve imbalance, such as nausea and tachycardia, and an acute rise of DBP with increasing HR during active standing were characteristics for the ST-positive patients with long COVID. Thus, long COVID is likely to encompass heterogenous pathologies of OI and accompanying endocrine alteration involving the HPA axis and GH due to the psychosomatic stress triggered by COVID-19. Ongoing research is crucial for the early detection and effective treatment of these patients.
Patients and methods
Enrollment of long COVID patients
This descriptive study was conducted in a single facility. Patients who visited a COVID-19 aftercare outpatient clinic (CAC), which was established on February 15 in 2021 in the Department of General Medicine, Okayama University Hospital, a tertiary hospital with 865 beds located in the western area of Japan, were enrolled in this study. In the CAC, we have been treating patients who have experienced various symptoms related to the post-COVID-19 condition for longer than 4 weeks (1 month) following the onset of COVID-19. Most of the consulting patients were referred from other affiliated hospitals and clinics.
Inclusion and exclusion criteria
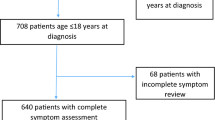
Clinical information on the patients who visited the CAC was obtained retrospectively. A total of 691 patients who visited the CAC between February 2021 and April 2023 were diagnosed as having long COVID, and 3 of those patients who were younger than 10 years of age were excluded in advance. Then the medical records of 688 long COVID patients were carefully reviewed. Long COVID patients who had undergone a standing test (ST) due to the symptoms of orthostatic intolerance (OI) such as palpitation and syncope were included in this study. As a result, 86 long COVID patients who had undergone an ST due to suspicion of OI were included in this study and their clinical data were analyzed.
Collection of clinical data
The medical records of long COVID patients whose ST data were documented were carefully investigated. The term “long COVID” refers to symptoms that last longer than 1 month after the onset of COVID-194,28,29. Information on age, gender, BMI, smoking/alcohol drinking habits, severity of the acute phase of COVID-19, phase of viral variants, duration after the onset of COVID-19 to visiting the CAC, history of COVID-19 vaccination, and clinical symptoms of long COVID as well as results of the ST were obtained from medical records. Through the physician’s face-to-face examinations, all of the patients were investigated to detect and exclude any underlying diseases. In addition, the patients who had undergone an ST were new patients in our clinic who had not received any drug for treatment of symptoms of long COVID.
Active standing test and positive definition
The active standing test (ST) was performed when the physicians clinically suspected positional changes in blood pressure or pulse rate through an examination. In the ST, the individual lies supine for 5 min to yield a steady baseline and then stands up actively for 10 min. In detail, all of the patients laid on their backs for 5 min, during which time their baseline blood pressure and HR were measured, and then they were instructed to stand for 10 min. SBP, DBP and HR were monitored every 2 min for up to 10 min in the standing position to observe symptoms that occurred during the test with careful observation to avoid the risk of syncope and injury30,31. The individual is instructed to avoid moving, shifting his or her weight, or engaging in leg and other muscle contraction maneuvers. Symptoms such as fatigue, lightheadedness, nausea, warmth, shortness of breath, headache, pain, and impaired concentration/mental fogginess are recorded while the individual is in the supine position and every 1–2 min while in the standing position. Individuals are also requested to report changes in symptoms as they occur. An increase in HR from basal HR (at the time point of − 5 min) of 30 bpm or more, an increase to 120 bpm or more over the same period, and a decrease of 25 mmHg or more in SBP were all considered positive indicators for the ST.
Laboratory examinations
We reviewed each patient’s laboratory results for blood cells and biochemistry examinations and the following hormones at the time of the first visit. Selection of the laboratory examination for the long COVID patients was decided by each physician. Blood samples were collected at approximately the same time each day after the initial patient interview and physical examination, from late morning to early afternoon, with the patients in a seated position with resting for 10 min, by experienced blood sampling technicians to avoid stress and pain. The auto-analyzer system in the central laboratory of our facility was used to analyze regular blood samples, and Cobas 8000 (F. Hoffmann-La Roche AG, Basel, Switzerland) was used for measuring hormone levels. As we reported previously17, for assessment of thyroid function, assays for FT4 and TSH were performed by using Elecsys FT4 III and TSH kits, respectively. For assessment of adrenocortical function, plasma ACTH and serum cortisol levels were measured by an electro-chemiluminescence immunoassay (ECLIA) using Elecsys ACTH and Elecsys Cortisol II kits (F. Hoffmann-La Roche AG). For assessment of the GH axis, serum GH and IGF-I levels were measured by using Elecsys GH and IGF-I kits (F. Hoffmann-La Roche AG), in which IGF-I levels were converted to SD values32.
Statistical analyses
All statistical analyses were performed using Stata/SE 18.0 (StataCorp, 4905 Lakeway Dr, College Station, TX 77845, USA). The characteristics of ST-positive patients and ST-negative patients were compared using the Mann–Whitney U test or Student’s t-test for continuous variables and Fisher's exact test or Pearson’s chi-square test for categorical variables. A P value of less than 0.05 was considered statistically significant.
Ethical approval
Information on this study was shown on the website of our hospital. The need for informed consent from individual patients was waived because of anonymization of the data, whereas patients who wished to opt out were offered the opportunity (Ethics Committee of Okayama University Hospital). The committee granted approval for this study (No. 2105-030) and the study followed the Declaration of Helsinki.
Data availability
Detailed data will be available if requested to the corresponding author.
Abbreviations
- ACTH:
-
Adrenocorticotropin
- BMI:
-
Body mass index
- COVID-19:
-
Coronavirus disease 2019
- CAC:
-
COVID-19 aftercare outpatient clinic
- FT4:
-
Free thyroxine
- GH:
-
Growth hormone
- IGF:
-
Insulin-like growth factor
- ME/CFS:
-
Myalgic encephalomyelitis/chronic fatigue syndrome
- OI:
-
Orthostatic intolerance
- POTS:
-
Postural orthostatic tachycardia syndrome
- ST:
-
Standing test
- TSH:
-
Thyrotropin
References
Nuzzo, J. B. & Gostin, L. O. The first 2 years of COVID-19: Lessons to improve preparedness for the next pandemic. JAMA 327, 217–218. https://doi.org/10.1001/jama.2021.24394 (2022).
Lippi, G., Sanchis-Gomar, F. & Henry, B. M. COVID-19 and its long-term sequelae: What do we know in 2023?. Pol. Arch. Intern. Med. https://doi.org/10.20452/pamw.16402 (2023).
Yang, J. et al. Definition and measurement of post-COVID-19 conditions in real-world practice: A global systematic literature review. BMJ Open 14, e077886. https://doi.org/10.1136/bmjopen-2023-077886 (2024).
Shah, W., Hillman, T., Playford, E. D. & Hishmeh, L. Managing the long term effects of COVID-19: Summary of NICE, SIGN, and RCGP rapid guideline. BMJ 372, n136. https://doi.org/10.1136/bmj.n136 (2021).
Otsuka, Y. et al. Clinical characteristics of Japanese patients who visited a COVID-19 aftercare clinic for post-acute sequelae of COVID-19/long COVID. Cureus 13, e18568. https://doi.org/10.7759/cureus.18568 (2021).
Tokumasu, K. et al. Clinical characteristics of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) diagnosed in patients with long COVID. Medicina (Kaunas) https://doi.org/10.3390/medicina58070850 (2022).
Yamamoto, Y. et al. Utility of serum ferritin for predicting myalgic encephalomyelitis/chronic fatigue syndrome in patients with long COVID. J. Clin. Med. https://doi.org/10.3390/jcm12144737 (2023).
Dani, M. et al. Autonomic dysfunction in ‘long COVID’: Rationale, physiology and management strategies. Clin. Med. (Lond.) 21, e63–e67. https://doi.org/10.7861/clinmed.2020-0896 (2021).
Tsuchida, T. et al. Treatment of long COVID complicated by postural orthostatic tachycardia syndrome—Case series research. J. Gen. Fam. Med. 25, 53–61. https://doi.org/10.1002/jgf2.670 (2024).
Hamrefors, V. et al. Syndromes of orthostatic intolerance and syncope in young adults. Open Heart 4, e000585. https://doi.org/10.1136/openhrt-2016-000585 (2017).
Ladlow, P. et al. Dysautonomia following COVID-19 is not associated with subjective limitations or symptoms but is associated with objective functional limitations. Heart Rhythm 19, 613–620. https://doi.org/10.1016/j.hrthm.2021.12.005 (2022).
Monaghan, A. et al. Orthostatic intolerance in adults reporting long COVID symptoms was not associated with postural orthostatic tachycardia syndrome. Front. Physiol. 13, 833650. https://doi.org/10.3389/fphys.2022.833650 (2022).
Benarroch, E. E. Postural tachycardia syndrome: A heterogeneous and multifactorial disorder. Mayo Clin. Proc. 87, 1214–1225. https://doi.org/10.1016/j.mayocp.2012.08.013 (2012).
Cabrera Martimbianco, A. L., Pacheco, R. L., Bagattini, A. M. & Riera, R. Frequency, signs and symptoms, and criteria adopted for long COVID-19: A systematic review. Int. J. Clin. Pract. 75, e14357. https://doi.org/10.1111/ijcp.14357 (2021).
Raj, S. R. et al. Long-COVID postural tachycardia syndrome: An American Autonomic Society statement. Clin. Auton. Res. 31, 365–368. https://doi.org/10.1007/s10286-021-00798-2 (2021).
Khosravani, H. The dysfunction is in the details: Neurovascular changes in COVID-19. Can. J. Neurol. Sci. 48, 1–2. https://doi.org/10.1017/cjn.2020.150 (2021).
Sunada, N. et al. Hormonal trends in patients suffering from long COVID symptoms. Endocr. J. 69, 1173–1181. https://doi.org/10.1507/endocrj.EJ22-0093 (2022).
Yamamoto, Y. et al. Detection of male hypogonadism in patients with post COVID-19 condition. J. Clin. Med. https://doi.org/10.3390/jcm11071955 (2022).
Otsuka, Y. & Otsuka, F. Possibility of endocrine dysfunction in post coronavirus disease 2019 (COVID-19) condition. Endocr. J. 69, 1357. https://doi.org/10.1507/endocrj.EJ22-0459 (2022).
Klein, J. et al. Distinguishing features of long COVID identified through immune profiling. Nature 623, 139–148. https://doi.org/10.1038/s41586-023-06651-y (2023).
Aizpurua-Perez, I., Arregi, A., Labaka, A., Martinez-Villar, A. & Perez-Tejada, J. Psychological resilience and cortisol levels in adults: A systematic review. Am. J. Hum. Biol. 35, e23954. https://doi.org/10.1002/ajhb.23954 (2023).
Juruena, M. F., Bocharova, M., Agustini, B. & Young, A. H. Atypical depression and non-atypical depression: Is HPA axis function a biomarker? A systematic review. J. Affect. Disord. 233, 45–67. https://doi.org/10.1016/j.jad.2017.09.052 (2018).
Miller, G. E., Chen, E. & Zhou, E. S. If it goes up, must it come down? Chronic stress and the hypothalamic–pituitary–adrenocortical axis in humans. Psychol. Bull. 133, 25–45. https://doi.org/10.1037/0033-2909.133.1.25 (2007).
de Kloet, E. R. Glucocorticoid feedback paradox: A homage to Mary Dallman. Stress 26, 2247090. https://doi.org/10.1080/10253890.2023.2247090 (2023).
Agorastos, A. & Chrousos, G. P. The neuroendocrinology of stress: The stress-related continuum of chronic disease development. Mol. Psychiatry 27, 502–513. https://doi.org/10.1038/s41380-021-01224-9 (2022).
Sakurada, Y. et al. Trends in long COVID symptoms in Japanese teenage patients. Medicina (Kaunas) https://doi.org/10.3390/medicina59020261 (2023).
Johansson, M. et al. Circulating levels of growth hormone in postural orthostatic tachycardia syndrome. Sci. Rep. 11, 8575. https://doi.org/10.1038/s41598-021-87983-5 (2021).
Carfi, A., Bernabei, R., Landi, F., Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA 324, 603–605. https://doi.org/10.1001/jama.2020.12603 (2020).
Soriano, J. B. et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 22, e102–e107. https://doi.org/10.1016/S1473-3099(21)00703-9 (2022).
in Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness The National Academies Collection: Reports funded by National Institutes of Health (2015).
Rowe, P. C. et al. Orthostatic intolerance and chronic fatigue syndrome associated with Ehlers–Danlos syndrome. J. Pediatr. 135, 494–499. https://doi.org/10.1016/s0022-3476(99)70173-3 (1999).
Isojima, T. et al. Standardized centile curves and reference intervals of serum insulin-like growth factor-I (IGF-I) levels in a normal Japanese population using the LMS method. Endocr. J. 59, 771–780. https://doi.org/10.1507/endocrj.ej12-0110 (2012).
Acknowledgements
We are sincerely grateful to all the of clinical and office staff at the Department of General Medicine who contributed to this work.
Funding
This work was supported by AMED (22fk0108517h0001 and 23fk0108585h0001).
Author information
Authors and Affiliations
Contributions
A.K., K.T. and F.O. conceived and designed the study; N.S., Y.N., Y.S., Y.M., T.H., R.T. and Y.O. performed data collection; A.K., K.T., K.Y. H.H. and K.U. analyzed the data; A.K., K.T., and F.O. wrote the paper; and F.O. revised the paper. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kato, A., Tokumasu, K., Yamamoto, K. et al. Clinical and endocrine features of orthostatic intolerance detected in patients with long COVID. Sci Rep 14, 17025 (2024). https://doi.org/10.1038/s41598-024-67815-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67815-y
- Springer Nature Limited