Abstract
Several farmed fish species, including carps, tilapia, salmon, and catfish, have experienced significant economic losses in aquaculture due to motile Aeromonas septicemia caused by Aeromonas hydrophila. In the present study, a novel lytic bacteriophage infecting hypervirulent Aeromonas hydrophila (vAh) was isolated and characterized. This is the first report of a phage against vAh. Phage AhFM11 demonstrated lytic activity against both vAh strains and the A. hydrophila reference strain ATCC 35654. The AhFM11 genome was sequenced and assembled, comprising 168,243 bp with an average G + C content of 41.5%. The genome did not harbor any antibiotic resistance genes. Genomic information along with transmission electron microscopy revealed that phage AhFM11 belongs to the Straboviridae family. Therapeutic application of monophage AhFM11 in fish showed 100% survival in injection, 95% in immersion and 93% in oral feeding of phage top-coated feed. Fish and chicken meat spiked with A. hydrophila and phage showed significant reduction of A. hydrophila. These findings support that phage AhFM11 can be used as a biocontrol agent against vAh as an alternative to antibiotics.
Similar content being viewed by others
Introduction
Aquaculture is one food sector that continues to be a significant contributor to the global food supply with production surpassing 122.6 million tons in 20221,2. The largest contributions are from finfish which account for over 60% of the market and include carp followed by tilapia and catfish1,2,3,4. This sector plays a crucial role in food security, especially in developing nations, where it contributes nearly 25% of the total food supply and is rapidly increasing2. To answer this increasing demand for aquaculture products, the prevailing trend of aquaculture practitioners has been to grow fish at higher densities leading to higher production and profits. Unfortunately, these density increases are correlated to higher levels of disease transmission due to increased contact between the fish and higher levels of pathogens that reap nutrients from uneaten pelleted feed4,5,6,7,8,9,10. The yearly economic losses caused by these diseases range from $6–$10 billion USD annually11,12,13.
Diseases with Gram-negative bacteria as the causative agents occur at the highest frequencies in aquaculture14 and include motile Aeromonas septicemia (MAS) caused by Aeromonas hydrophila and hypervirulent A. hydrophila (vAh). MAS has been the most prevalent disease reported in freshwater aquaculture in recent years4,14,15,16,17,18,19. A. hydrophila is ubiquitous and clinical symptoms include edema, hemorrhage, scale and fin erosion, tail rot and ulcerative syndrome6,20,21,22. Amplifying the problems associated with A. hydrophila infections is the emergence of antibiotic-resistant strains which pose a significant threat to the sector's sustainability7,9,12,23,24,25,26,27. Furthermore, the recent emergence of vAh strains in carp and catfish from China and Vietnam has alarmed the aquaculture community due to their severe impact on fish health and production and has caused several million dollars of economic loss since 200915,16,28. To ameliorate these bacterial infections, feed that has been top coated with antibiotics is administered29. However, within the United States, the only readily available FDA-approved finfish antibiotic for use against vAh is florfenicol30. Six genes are already known to confer resistance to florfenicol and Aeromonas species’ have been documented to carry at least three thus emphasizing the need for antibiotic alternatives31,32,33.
Growing concerns about antibiotic resistance have spurred the search for eco-friendly and cost-effective alternatives to prevent and treat diseases in aquaculture. While vaccines offer a preventive approach, their application in juvenile fish and farmed crustaceans/mollusks is often impractical. Fortunately, numerous biological alternatives to antibiotics exist, including lytic bacteriophages (phages), endolysins, biotics, and quorum sensing mechanisms5,8,34,35,36. Among these, lytic phages are the naturally occurring predators that target and kill bacteria rapidly37. Although they are currently underutilized, their therapeutic and prophylactic potential combined with their affordability makes them a valuable addition to mitigation efforts against the prevention and spread of antibiotic resistance34,35,38,39. Investigational research on lytic phages that target A. hydrophila have been studied through the injection route in zebrafish, striped catfish, brocade carp, crucian carp, loach, eels and tilapia40,41,42,43,44; by immersion in tilapia, zebrafish, white leg shrimp, and cockles42,45,46,47; and by oral feeding in eels and tilapia. In each study, the phage treated animals exhibited better survival when challenged with bacteria40,44. Here, we describe the methods used to discover a new A. hydrophila phage, AhFM11, and investigated its ability to protect rohu (Labeo rohita) from vAh. Additionally, we explored cryopreservation methods, sequenced and annotated the genome, as well as demonstrated its ability to decontaminate A. hydrophila from meat products.
Results
Environmental isolation, host range, one step growth curve and adsorption of phage
Isolation of vAh phages was carried out by collecting river water samples from different locations in Karnataka, India. Two phages named AhFM10 and AhFM11 were isolated using vAh strains HypAh-12 and HypAh-20, respectively as host bacteria. The location, titer value, and water sample source are referenced in Table 1 and Supplemental Fig. 1. To classify the phages as broad or narrow spectrum, Aeromonas spp. and non-Aeromonas spp. were used to study their host ranges. Defining the host ranges allows for the proper selection of phages for use in different therapies. Phages AhFM11 and AhFM10 had lytic activity against 84 and 53 Aeromonas isolates, respectively (Supplemental Table 1). AhFM11 had the largest host range and was further used for phage stability tests, one step growth curves, adsorption tests, genomic characterization, and monophage therapy in fish and meat products.
One-step growth curves were performed in TSB at 28 °C to determine the latent period and burst size of phage AhFM11 (Fig. 1A). AhFM11 had a latent period of 10 min and burst size of 378 ± 49 PFU/cell. The adsorption efficiency exhibited by AhFM11 was 99.7% adsorbed within 30 min and complete adsorption (no free phages) by 40 min (Fig. 1B).
AhFM11 plaque morphology, transmission electron microscopy (TEM), and phage stability assays
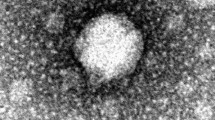
AhFM11 formed a clear zone in the soft agar overlays with a plaque diameter ranging from 1–3 mm (Fig. 2A, B, C). TEM was employed to determine morphology and revealed that AhFM11 has an icosahedral head length of 96.6 ± 8.8 nm and width of 68.7 ± 3.4 nm, a tail length of 100.2 ± 9.3 nm, a collar length of 16.5 ± 3.2 nm and a total length of 211.4 ± 11.5 nm long (Fig. 2 D). Based on the morphological analysis in combination with NCBI BLAST results of the nucleotide sequence, AhFM11 was assigned to the family of Straboviridae.
Phage (AhFM11) characteristics on agar plate, soft overlay method and transmission electron microscopy. (A) Spot tests for phage AhFM11 on hypervirulent Aeromonas hydrophila HypAh-20 and the spot 4 (black dotted rectangle) was used for further passage to get the titer value for AhFM11. (B,C) Plaque morphology of AhFM11 on TSA soft agar plates showing 1–3 mm plaque diameter. (D) Transmission electron microscopy image negatively stained with 2% uranyl acetate showing single phage AhFM11 (Bar = 50 nm) with its icosahedral head along with sheathed tail tube and its branched tail fibers.
During the 30% glycerol storage stability studies, the samples of AhFM11 stored at − 80 °C and − 20 °C significantly retained their titer values even after 60 days of storage whereas phages stored without glycerol lost their activity (Supplemental Fig. 2A). Samples stored at 0 °C and 4 °C showed a slight but significant decrease in their titer values and AhFM11 lost ~ 50% its titer value at 28 °C and ~ 75% of its titer value at 37 °C (ANOVA, p < 0.05) when compared with the phages stored with glycerol at − 80 °C and − 20 °C. The phage titer decreased over a period of 60 days when stored at 4 °C in different salinity concentrations (0.1, 0.5, 1.0, 2.0 and 3.5%). In comparison to phages stored in 1% salt, which was used as a baseline control, there was a significant difference in the 0.1 and 3.5% salinity groups whereas there were no significant differences in the rest of the salinities (Supplemental Fig. 2B). Hence, the phage was stable at wide range of salinities suggesting it will remain functional in both freshwater and estuarine waters. The phage lost its activity at acidic pH (2 and 4) and alkaline pH (10 and 12) but remained functional from pH 5–8 (Supplemental Fig. 2C). AhFM11 retained the highest titer values when stored in PBS and SM buffers followed by chloroform. However, AhFM11 significantly lost lytic functionality when exposed to phenol, propanol, ethanol, and acetone (Supplemental Fig. 2D).
Whole genome sequencing of AhFM11 and phylogenetic analysis
Whole genome sequencing, assembly, annotation, and phylogenetic tree construction was completed on AhFM11. AhFM11 has a medium-sized phage genome containing 168,243 bp with a G + C content of 41.5% (Fig. 3). We identified 265 coding sequences (CDS), and functional predictions were made for 72 ORFs and 16 tRNAs. The remaining CDS were classified as "hypothetical proteins" with unknown functions. The length of nucleic acid CDS varied from 75 to 3672 bp and encoded notable putative proteins: RNA polymerase, NAD (+)-arginine ADP-ribosyl transferase, ribonucleoside-diphosphate reductase, helicase, kinase, DNA polymerase, topoisomerase, protease, and DNA ligase. Comparative genome analysis was performed with four phages possessing > 85% similarity namely avDM14-QBC (Accession #OP380598.1), 50AhydR13PP (Accession #MK179477.1), 60AhydR15PP (Accession #MH179476.1) and Aeromonas phage phiAS4 (Accession #HM452125.1). All these phages contain similar genome organization as to our phage AhFM11 (Fig. 4 and Supplemental Table 2). At the nucleotide level, the phage genome shared the highest homology with Aeromonas phage Asfd_1 (accession number: MK577502.1) with an identity of 84.47%. Maximum-likelihood trees of major capsid proteins also indicated that the phage is closely related to the 50AhydR13PP (MK179477.1), 60AhydR15PP (MH179476.1) and Aeromonas phage phiAS4 (HM452125.1), unclassified Secunda5virus of the family Straboviridae (Fig. 5A). As shown in Fig. 5B, whole genome analysis revealed that the phage AhFM11 was closely related to the Aeromonas phage Asfd_1 (Accession #MK577502.1), forming its own grouping withing the Aeromonas phages. Separate clades were also formed between the Aeromonas phages, Enterococcus phages, and Klebsiella phages. It did not encode any known bacterial virulence-related proteins or antimicrobial resistance genes, based on the predictions by the VFDB and CARD database. The PHACTS tool predicted the phage lifestyle to be lytic. The full sequence and annotation of phage AhFM11 were submitted to the NCBI and assigned accession number ON042478.1.
Comparative analysis of four phages was conducted using Easyfig. Coding domain sequence (CDS) are shown as arrows to indicate the direction of transcription and are mentioned in the bottom legend in accordance with their predicted functions. The percentage of sequence similarity is shown as the intensity of the gray to black color.
Phylogenetic tree of (A) MCP gene of phages constructed using the Maximum-likelihood method with a bootstrap value of 1000 replicates. A total of 28 nucleotide sequences were included for the analysis including AhFM11 (1-Present study), A. hydrophila phage (11), A. salmonicida (4) and Enterobacteriaceae phages (12). (B) Phylogenetic tree of whole-genome sequences of phages were aligned using MAFFT v7.3, the tree was constructed using online software by interactive tree of life (iTOL) and the bolded ON42478.1 indicates the phage AhFM11 isolated from the present study.
In vivo experiments with phage AhFM11 against Aeromonas infection
For the in vivo efficacy tests, all "Challenged” groups received the predetermined LD50 (2.42 × 106 CFU/fish) intraperitoneal injection of vAh (HypAh-20). In the “Injection Group,” rohu carp received an injection (100 µL) containing 1.55 × 106 PFU/fish of AhFM11 2 h post infection and demonstrated 100% survival after the 15-day challenge. In the “Immersion Group,” fish were groups receiving AhFM11 had the following survival percentages: 100% when delivered by intraperitoneal injection (Fig. 6A), 95% with the immersion (Fig. 6B), and 93% with top-coated feed (Fig. 6C). These results indicated that AhFM11 can be injected or ingested by fish yet retain its lytic capacity. Based upon these results, phage AhFM11 can be delivered efficiently through injection, immersion, and oral feeding to treat A. hydrophila infections.
Food decontamination efficacy of phage AhFM11 on Aeromonas hydrophila
Fish and chicken meat samples were artificially contaminated with MDR K3 or ATCC 35654 A. hydrophila and treated with phage AhFM11 (MOI = 1000) as described in the Methods section. Significant reductions (p < 0.05) in bacterial counts were observed at all time points (24, 48, 72, and 96 h) compared to untreated controls (details provided in the Methods section). For fish meat samples contaminated with ATCC 35654, the application of phage AhFM11 resulted in reductions of the bacteria.
For the fish meat samples, the colony counts of ATCC 35654 A. hydrophila treated with phage AhFM11 resulted in 2, 1.5, 1.7 and 2.3 log10 CFU/g reductions at 24, 48, 72, 96 h when compared with the untreated control group (Supplemental Fig. 3A). The colony counts of ATCC 35654 A. hydrophila in chicken meat samples treated with AhFM11 revealed drastic reductions of 2.9, 4.2, and 3.2 log10 CFU/g at 24, 48, and 72 h; however, the 96 h timepoint was reduced below the detection limit. (Supplemental Fig. 3B).
When MDR K3 A. hydrophila was used to contaminate fish meat, AhFM11 treated samples had a 1.2 and 0.8 log10 CFU/g reduction at the 48 and 96 h timepoints. At the 24 h timepoint, there was < 1 log10 CFU/g reduction and the 72 h had a 1.5 log10 CFU/g reduction (Supplemental Fig. 3C). The chicken meat contaminated with MDR K3 and treated with AhFM11 had 0.3 log10 reduction at 24 h; 1.2 log10 CFU/g reduction at 48 h; 0.2 log10 CFU/g reduction at 72 h, and no reduction at 96 h. The positive growth control sample at 72 and 96 h was same as the phage treated (Supplemental Fig. 3D).
In the case of fish meat, ATCC 35654 A. hydrophila treated with phage AhFM11 showed a 0.72 log10 PFU/g increase in phage titer value at 24 h when compared to only phage treated fish meat sample. Later timepoints showed no significant increase in phage titers (Supplemental Fig. 4A). Chicken meat samples treated with ATCC 35654 A. hydrophila and phage AhFM11 showed a significant increase in phage titer with 0.44, 0.40, and 0.46 log10 PFU/g at 24, 48 and 96 h respectively, except 72 h (Supplemental Fig. 4B). Whereas in fish meat sample treated with MDR K3 A. hydrophila with phage AhFM11 showed a significant increase of 0.107, 0.139, 0.363 and 0.914 log10 PFU/g increase in phage titer value at 24, 48, 72 and 96 h time points, respectively (Supplemental Fig. 4C). Similarly in chicken meat samples showed an increase in phage titer value with 0.885, 0.396, 0.408 and 1.00 log10 PFU/g at 24, 48, 72 and 96 h timepoints, respectively (Supplemental Fig.4D).
Discussion
Increasing fish density practices of modern aquaculture, coupled with unregulated antibiotic administration in some countries, and climate change has led to a surge in disease outbreaks and increasing prevalence of antimicrobial resistance (AMR). A. hydrophila as a causative agent is documented as the most prevalent finfish disease and there are no commercially available vaccines against A. hydrophila in India or the United States38. Even if a protective A. hydrophila vaccine were available, their use in aquaculture is often unrealistic due to the labor costs associated with administering a vaccine by hand or the exorbitant price of automated mechanical injectors. An additional concern related to A. hydrophila is the identification of AMR and bacterial biofilm formation in recirculating aquaculture systems that compromise the effectiveness of antibiotics 18,48,49,50. These factors underscore the critical need for alternative approaches to manage A. hydrophila infections in fish that are simple, cost-effective, and environmentally friendly. Phage therapy presents a promising solution in this regard.
In the present study, phages AhFM10 and AhFM11 were isolated from river waters and showed significant lytic activity against the vAh strains HypAh-12 and HypAh-20. HypAh-12 isolated from rohu carp and HypAh-20 isolated from koi produce similar clinical symptoms (reddening, enlargement of abdomen and petechial haemorrhages) of vAh infected carps and catfishes in China and the USA7,8,21. The result of the plaque spot tests showed that phage AhFM10 and AhFM11 can lyse 53 and 84 isolates respectively, indicating a broader lytic efficacy in case of AhFM11 (Supplemental Table 1). The host range determination revealed that efficiency to lyse A. hydrophila was characterized into broader lytic efficacy and has the potential to be used for phage therapy as reported by several studies5,39,51. However, phage AhFM11 was selected due to its broader host range and to explore its potential as a therapeutant in aquaculture and food decontaminant. TEM images of AhFM11 indicated a morphology with an icosahedral head attached to a long tail that is characteristic of the Straboviridae family. Most of the lytic bacteriophages reported against Aeromonas spp. have been described as tailed phages belonging to the family Myoviridae5,6,8,32,39,51,52,53,54,55,56,57, and most recently, this has been re-classified as belonging to family Straboviridae58.
To be suitable for use in therapy and on food products, it is essential to characterize the physiochemical limitations of AhFM1147. Under the test conditions used in this study, AhFM11 is relatively stable with 30% glycerol at − 80 °C and − 20 °C for at least 60 days. Storage at 0 °C, 4 °C, and 28 °C without glycerol exhibited decreasing lytic activity with increasing temperature (Supplemental Fig. 2A). Phages completely lost their lytic capacity after 60 days of incubation without glycerol at − 80 °C, − 20 °C, and 37 °C. When evaluating the effect of salinity on AhFM11 function, phages stored at 4 °C in 0.5%, 1.0%, and 2.0% salinity retained similar lytic capacity in comparison to phages stored at low temperatures in glycerol. However, salinities of 0.1% and 3.5% showed a dramatic reduction in lytic function. There are reports of A. salmonicida phages which were stable up to 37 °C and in 3.5% salinity for a duration of 49 days47,56. AhFM11 was stable for 45 days at 37 °C and in 3.5% salinity suggesting this phage can be used for both freshwater and estuarine waters. Several factors are responsible for the natural progressions of phage infection including attachment, penetration, and multiplication; among the different environmental factors, temperature and pH are the main physiochemical parameters that play vital roles in phage therapy within an aquaculture environment41. In the present study, AhFM11 was the most functional, in terms of lytic plaque formation, at pH 6, 7, and 8. This demonstrated that AhFM11 has excellent stability around neutral pH, which is the optimum range for warm water fish farming. Generally, pH values < 5 and > 8 impair the lytic activity of phages which was also evident in the present study41,47,56. To determine whether AhFM11 contains structural lipids that are essential to its life cycle, chemical stability tests were performed with chloroform, phenol, ethanol, propanol, SM buffer, PBS and Diethyl ether. There was a 1 log reduction in the titer value after 10 min and 2-log reduction 24 h post chloroform treatment at room temperature. The results demonstrate that AhFM11 is stable in chloroform and suggests the absence of structural lipids. Several studies correlate with these results and have reported that other A. hydrophila phages are stable in PBS, SM buffer, and chloroform, and less stable with diethyl ether39,42,47,52,53,59,60.
Genomic sequencing revealed that AhFM11 has a genome size of 168,243 bp and G + C content of 41.5%. Similar results were seen for phages AhSzq-1 (112,558 bp and 43.86%), AhSzw-1 (115,739 bp and 43.82%)61; 50AhydR13PP (144,979 bp and 41.10%), 60AhydR15PP (165,795 bp and 41.2%)54, and Akh-2 (114,901 bp and 45.22%)41. Nucleotide sequence analysis revealed and absence of known virulence and antibiotic resistance genes which further increases its potential as phage therapeutic and food decontaminant. Similar results were also confirmed by Kazimierczak et al.54 when they analysed the genome of 7 A. hydrophila phages. From these seven phages, three (25AhydR2PP, 50AhydR13PP, 60AhydR15PP) were described to have potential as a phage therapeutant due to a confirmed lytic life cycle and the absence of virulence and antibiotic resistance genes.
The novel phage, AhFM11, possesses the basic characteristics for a candidate therapeutic agent. Phages delivered through injection, immersion, and oral feeding routes in this study all exhibited remarkable protection against vAh infections. In this experiment, phage AhFM11 provided protection through injection (100%), immersion (95%) and oral feeding of top-coated feed (93%) and similar results were obtained by Cao et al.52. Numerous phage therapy studies have reported on the protective effects of Aeromonas phages against A. hydrophila but none have achieved the remarkable protection rate presented here8,39,41,42,44,46,47,48,49,51,53,54,55,56,59,60,61,62,63,64,65. Jun et al.60 reported that intraperitoneal injections with phages pAh1-C (3.0 × 107 PFU/fish) and pAh6-C (1.7 × 107 PFU/fish) had cumulative mortalities of 43.33% ± 2.89% and 16.67% ± 3.82%, respectively. When the fish were fed phage top-coated feed, the cumulative mortality rates were 46.67% ± 3.82% (pAh1-C) and 26.67% ± 2.89% (pAh6-C), which indicated that injection provided better protection than phage feeding. In the present study, we likewise observed better protection via the injection route in comparison to the oral feeding route.
During the food decontamination studies, fish meat samples treated with phage AhFM11 had 1.5 and 2 log reductions in CFU/g of meat when using the AMR strain MDR K3 and 2 and 2.3 log reductions when using ATCC35654. These results are consistent with a study carried out on lettuce leaves by Islam et al.66 where the antibacterial effectiveness of phage ZPAH7 reduced A. hydrophila by 1.5 log10 CFU/cm2. The study by You et al.67, reported that when the V. parahaemolyticus strain FORC_023 was used to artificially contaminate raw fish slices and held at 5 °C without phage treatment, a marginal decrease of the pathogen load was detected. However, when phage VPT02 was applied to the fish samples, CFU/g of FORC_023 decreased dramatically up to 1.5 log. Spraying a solution of bacteriocin AS-48 on fillets of smoked salmon reduced Listeria 2, 3.4, 4.5, 4.25, and 4.25 log CFU/cm2 in respect to the untreated control at 1, 5, 10, 15, and 30 days68. Findings by Soni et al.69 showed that when P100 phage was treated to reduce L. monocytogenes on fresh catfish fillets for 10 days at 4 °C the overall reduction of L. monocytogenes on average was 1.5 log10, but the authors reported a slight increase in the bacterial count after 10 days. For only one of the bacterial isolates (MDR K3) used in the fish contamination trial, a decrease in CFU level in the bacteria-only sample was observed (Supplemental Fig. 3C). This result was unexpected. This experiment was conducted in triplicate and the same trend was observed each time. It is possible that factors other than phage predation are influencing bacterial growth in this culture and thus requires further investigation.
The results reported here for chicken meat samples contaminated with MDR K3 and treated with phage AhFM11 reached up to a 2-log reduction in CFU/g, whereas the results for ATCC 35654 were reduced below the detection limit at the 96 h timepoint. A study by Thung et al.70 evaluated the efficacy of phage CJ01 to decontaminate mutton and chicken meat contaminated with Campylobacter jejuni and achieved reductions of 1.7 and 1.68 log after 48 h. In the present study, AhFM11 was found to be more effective on chicken in comparison to fish meat when contaminated with either MDR K3 or ATCC 35654. Phage concentrations had decreased in titer values at 24 h but remained almost constant up to 96 h in both fish and chicken meat samples.
At optimum temperature, the bacterial hosts usually grow faster, which promotes the replication of phage particles. Whereas at a lower temperature, the speed of phage replication is considerably decreased or halted due to the lower growth rate of their hosts71. The efficacy of phage particles on food substrate can be affected by the type of food matrix (i.e., liquid or solid), phage immobilization due to reduction in surface water content, and the ability of certain food-associated factors that lead to the structural degradation of phage particles72.
AhFM11 contains no known virulence or AMR genes and demonstrated remarkable protective efficacy in in vivo challenge models, whereby no less than 93% protection was found using three different administrative routes. It is also notable that in all food decontamination studies described above that only AhFM11 achieved reductions in bacterial counts below detectable levels. Therefore, AhFM11 warrants consideration for regulatory approval and for future field use.
Conclusion
This is the first report on the discovery of phages possessing lytic activity against hypervirulent Aeromonas hydrophila (HypAh-12 and HypAh-20). Of these phages, AhFM11 was sequenced and found to have no known virulence or AMR genes. The results from in vivo studies demonstrated no less than 93% protection against vAh strains whereas the in vitro studies demonstrated that AhFM11 is highly effective as a food decontaminant. These results demonstrate that AhFM11 is a non-antibiotic alternative that can be safely used to treat A. hydrophila infections as well as a food decontaminant.
Methods
Environmental isolation, host range, one step growth curve and adsorption of phage
For the isolation of phages, A. hydrophila (HypAh-12 and HypAh-20) obtained from the Department of Aquatic Animal Health Management, College of Fisheries, Karnataka Veterinary, Animal and Fisheries Sciences University, Matsyanagar, Mangaluru-575002, Karnataka, India8 were grown overnight in TSB broth. Water samples were collected from several rivers in Karnataka, India, then centrifuged for 10 min at 10,000 x g. The supernatant was then filtered through a 0.45 µm membrane filter (PALL Life Sciences, Acrodisc® Syringe filter, New York). The filtrates were then put through a series of spot tests to find the lytic bacteriophages by adding 10 µL onto bacterial lawns of HypAh-12 and HypAh-20 isolates. The conventional double-layer (soft) agar overlay method was then used to confirm the presence of the lytic phages73. To get pure phage plaques, single plaques were picked repeatedly and eluted in SM buffer (50 mM Tris–HCl; 0.1 mM NaCl, 0.1% gelatin, 8 mM MgSO4, pH 7.5). Phages specific to hypervirulent A. hydrophila HypAh-12 and HypAh-20 named AhFM10 and AhFM11 were isolated. Using the soft agar overlay method, the titer values (PFU/mL) of the isolated phages were calculated. The isolated phages were stored at 4 °C, -20 °C, and -80 °C in aliquots of SM buffer supplemented with and without 30% glycerol for the long-term phage stability studies.
Bacteriophages AhFM10 and AhFM11 were evaluated for their host range against a diverse panel of 197 bacterial isolates. A total of 170 Aeromonas spp. (37 Aeromonas hydrophila obtained from the previously published paper from Dubey et al.74 and the rest were isolated in the present study) and 27 non-Aeromonas strains obtained from the Department of Aquatic Animal Health Management (AAHM) collection at the College of Fisheries, Mangaluru, Karnataka, India were utilized. The Aeromonas strains comprised A. hydrophila (105 isolates, including samples from various Indian states and two reference strains (ATCC 35654 and ATCC 7966)), and other Aeromonas species as listed in Supplemental Table 1. The 27 non-Aeromonas strains included various species of Bacillus, Chromobacterium, Citrobacter, Enterobacter, Edwardsiella, Enterococcus, Klebsiella, Escherichia, Lactococcus, Salmonella, Streptococcus, Staphylococcus, and Vibrio. Following the spot test method of Adams73, phages were assessed for host specificity based on the clarity of inhibition zones after 12 h of incubation at 28 °C. Clear zones indicated susceptibility (+), while no zones indicated resistance (−).
Based on the results obtained from host range, bacteriophage AhFM11 was selected for further characterization. The latent period and burst size of phage AhFM11 were determined using a one-step growth curve experiment. AhFM11 was separately added to A. hydrophila HypAh20 culture (OD600 = 0.8) at a multiplicity of infection (MOI) of 0.001 in 1.0 mL of sterile trypticase soy broth (TSB). After incubation at 4 °C for 30 min, the mixtures were transferred to 50 mL of sterile TSB. Samples were collected every 10 min for 120 min and immediately centrifuged (10,000 g for 2 min). As per the standard protocol, phage titers in the supernatants were determined at each time point using the soft agar overlay method. The burst size in one-step growth experiments was calculated by dividing the average phage titer during the plateau phase by the estimated number of initially infected cells. The latency period was estimated as the time between phage adsorption and the start of the exponential growth phase in the free phage titer curve. The phage AhFM11 adsorption efficacy was determined at MOI of 1, with the A. hydrophila HypAH-20 culture (OD600 = 0.6). A volume of 3 mL of bacteria culture was treated with 300 µL of AhFM11 at room temperature (RT) separately. Samples of 100 µL were removed from the mixture and immediately diluted in 900 µL of SM buffer at regular time intervals (0, 2, 4, 6, 8, 10, 15, 20, and 30 min). Samples were vortexed and then centrifuged for 2 min at 10,000 ×g at room temperature. Then the free phage titers in the supernatant were examined by the soft agar overlay method12.
Transmission electron microscopy (TEM) and phage stability assays of AhFM11
Phage AhFM11 was isolated from subsequent passages using the soft agar overlay method73. The lysate containing the phage particles was then filtered through a 0.45 µm membrane filter (PALL Life Sciences, Acrodisc® Syringe filter, New York) to remove bacterial cells and retain only the phage particles. The purified phage (AhFM11) was ultracentrifuged at 24,500 × g for 3 h at 4 °C (Hitachi, Himac CS150GXII, Japan) to obtain high titer purified phages (1012⁻14 PFU/mL). The high titer purified phages were then washed twice with 0.1 M ammonium acetate. About 10 µL of washed high titer purified phage were placed on a carbon-coated 400-mesh TEM copper grid (NisshinME, Tokyo, Japan) and then negatively stained with 2% uranyl acetate for 5 min. Imaging was performed using a Technai G2 T12 BioTwin TEM (Hillsboro, USA) at 160,000 × magnification and 120 kV at IISc, Bengaluru, Karnataka, India.
In order to check the efficacy of the phage AHFM11 at different environmental conditions, phage was stored at different temperature and salinity for 60 days; pH and chemicals for 24 h were used12. Temperature stability was assessed by storing 1 mL aliquots (1012 PFU/mL) at − 80 °C (with (WG) and without glycerol (WoG)), -20 °C (WG and WoG), 0 °C, 4 °C, 28 °C, and 37 °C for 60 days. Phage titers were determined every 15 days using the soft agar overlay method by using aliquots of phage AhFM11 (100 µL). Salinity stability was examined by storing 1 mL aliquots in varying salinity solutions (0.1%, 0.5%, 1.0%, 2.0%, 3.5%) under refrigerated conditions for 60 days. Phage titers were similarly determined every 15 days by soft agar overlay method. Stability across a pH range (2–12) and against various chemicals (acetone, chloroform, diethyl ether, ethanol, phenol, propanol) was also investigated. For pH, 100 µL phages were incubated in SM buffer (900 µL) at each pre-defined pH for 10 min, 12 h, and 24 h. Similarly, 100 µL phages were incubated with each solvent (900 µL) for the same time intervals. Surviving phages were enumerated at 10 min, 12 h and 24 h time point using the soft agar overlay method.
Whole genome sequencing of AhFM11 and phylogenetic analysis
About 50 mL of the purified phages (1011–12 PFU/mL) were concentrated by centrifugation (10,000 x g for 10 min) and again filtrated (0.45 µm). The filtrate was treated with DNase I (100 µg/mL) and RNase A (20 µg/mL) (Himedia, India) at 37 °C for 1 h to remove bacterial nucleic acids. Phages were then precipitated overnight at 4 °C with 0.4 volumes of a precipitation solution [3.3 M NaCl and 30% Polyethylene Glycol (PEG 6000)]. The overnight kept phages were centrifuged at 15,000 x g for 30 min at 4 °C. The pellet was resuspended in 500 µL phage lysis buffer (100 mM NaCl, 50 mM Tris–HCl, 0.4% Sodium dodecyl sulfate (SDS), 100 mM EDTA, pH 8.0) and treated with proteinase K (100 µg/mL) at 60 °C for 1 h. This mixture was treated with an equal volume of phenol:chloroform:isoamyl alcohol (25:24:1 v/v) twice and absolute ethanol was used to precipitate the phage nucleic acid. The nucleic acid pellet was again washed with 70% ethanol. Then again centrifuged at 10,000 × g for 10 min and the supernatant was discarded. The pellet was finally dissolved in TE buffer (1 mM EDTA, 10 mM Tris–HCl, pH 8.0). Concentration and purity of DNA was measured in a NanoDrop spectrophotometer (Thermo Fisher Scientific, USA) and using gel electrophoresis.
Isolated phage genomic DNA of AhFM11 was sequenced at Clevergene Biocorporation Private Limited located in Bengaluru, Karnataka, India. Sequencing was performed on an Illumina HiSeq using 2 × 150 bp paired-end configuration. To assess the data quality of the obtained sequence, Fast QC version 0.11.2 and Multi QC version v1.11 was used to check the quality of sequenced data. Sequencing adapter contamination and other low-quality reads were removed by using Trim Galore version 0.6.7. De novo assembly was performed using SPAdes version 3.12.0. The complete assembled sequence was aligned in NCBI using BLASTn. Prokka version 1.14.6 was used to annotate the genome and identify features of interest. A circular map of the AhFM11 whole genome was constructed using the Proksee online tool33. The presence of virulence and antibiotic resistance genes in the genome was checked by Virulence Factors of Pathogenic Bacteria Database (VFDB) and the Resistance Gene Identifier (RGI) of the Comprehensive Antibiotic Resistance Database (CARD). The genome was visualized using the CG view server. To determine the lytic or lysogenic life cycle of phage, Phage Classification Tool Set (PHACTS) software was used. Comparative genomics were conducted using the Easyfig tool75. Phylogenetic analysis of the major capsid proteins was performed using software MEGA XI version 11.034. Whole-genome sequences of phages were aligned using MAFFT v7.376; then, a phylogenetic tree was constructed using the iTOL (https://itol.embl.de/)77.
In vivo experiments with phage AhFM11 against Aeromonas infection
All experiments were conducted in the Department of Aquatic Animal Health Management wet lab, College of Fisheries, Mangaluru, Karnataka, India. The fish used in these phage therapy experiments were rohu carp (Labeo rohita) with a 7.7 to 9.8 g body mass and 6.7 cm average length, obtained from BRP, Shivamogga, Karnataka, India. The fish had not been vaccinated or exposed to disease and were healthy without any symptoms of infection. All fish were fed a basal diet during four to five weeks of acclimatization.
To establish the LD50 for vAh (HypAh-20) used in challenge models, rohu carp (Labeo rohita) were divided into challenged and unchallenged groups (n = 30) and received intraperitoneal injections of either PBS + SM buffer in the unchallenged group or vAh (HypAh-20) in challenged groups. Fish were observed for 15 days post infection (dpi). The LD50 was determined to be 2.42 × 106 CFU/mL with the final cumulative survival of 100% in the unchallenged and 43.33% in the challenged group.
During the experiment, the temperature, the level of dissolved oxygen and pH were monitored and controlled. The fish were fed a commercial fodder using automatic band feeders in an amount suitable for body weight and temperature. Physicochemical conditions were maintained at temperature (25–28 °C), dissolved oxygen (5–8 mg/L) and pH (6.5–7.5). A total of 150 fish were categorized into five groups and challenged with vAh (HypAh-20). The five groups consist of the Injection Group, Immersion Group, Oral Feeding Group, Negative Control (without phage but challenged), and Positive Control (without phage and without challenge) (Supplemental Fig. 5).
For the in vivo phage efficacy tests, bacteriophage AhFM11 with a titer value of 1010 PFU/mL was used as a stock solution and respective dilutions were made for the treatment groups. For the Injection and Immersion experimental groups, fish received an intraperitoneal injection containing an LD50 of vAh (HypAh-20) 2-h prior to phage administration. The fish in the Injection group received an intraperitoneally injection containing 1.55 × 106 PFU/fish 2-h post infection. Fish within the Immersion group were kept in water for 15 min with phage at a concentration of 1.55 × 107 PFU/mL 2 h post infection. For the Oral Feeding group, phage AhFM11 at a concentration of 1.55 × 107 PFU/g of feed pellets was thoroughly mixed in sterile 50 mL tubes by uniformly spraying on the surface and then evaporated in a sterile hot air oven cabinet for 30 min. The fish were continuously fed phage top-coated feed beginning 60-days before challenge and throughout the experiment. The control groups were treated in the same manner but with PBS in place of phage. The fish were monitored every day, and mortalities were removed immediately. Mortality was recorded daily for 15 days, and cumulative percent survival rate was calculated. The cause of mortality was confirmed by re-isolating the bacteria from the kidney of dead fish using selective Aeromonas isolation media base (AIMB).
Food decontamination efficacy of phage AhFM11 on Aeromonas hydrophila
To test the efficacy of AhFM11 as a food decontamination tool, chicken and fish meats were artificially contaminated with 106 colony forming unit (CFU)/mL of multidrug resistant (MDR) A. hydrophila (K3) or ATCC 35654 at room temperature. AhFM11 was added at an MOI = 1000 (109 PFU/mL) 30 min after the bacteria and the samples were stored at 4 °C for 4 days. The bacteria were aseptically recovered from both phage treated and untreated meat samples. Multidrug resistance (MDR) K3 A. hydrophila isolate and American Type Cell Culture 35654 (ATCC 35654) were used in this study. These two bacteria were available in the stock culture of the Department of Aquatic Animal Health Management (AAHM), College of Fisheries, Mangaluru, Karnataka, India. To prepare an A. hydrophila culture, a single colony was used to inoculate Luria Bertani broth (LB broth) and incubated at 28°C overnight.
The efficiency of phage AhFM11 was tested on fish meat and chicken meat which was artificially contaminated with MDR K3 and ATCC 35654 A. hydrophila and stored at a refrigerated storage temperature of 4 °C for 96 h incubation. Fresh raw fish and chicken fillets were procured from local markets. The samples were then placed in a cooler box containing ice packs and transported to the laboratory, Department of Aquatic Animal Health Management (AAHM) College of Fisheries, Mangaluru, Karnataka, India, and the meat was processed within an hour of collection. The samples were aseptically cut into 1 cm3 (0.5 ± 0.02 g) using sterile scissors and forceps and then transferred into sterile trays containing an average meat mass of 10 ± 0.2 g. The trays were held under UV lamps for 30 min inside a laminar flow hood to kill any contaminating bacteria.
The chicken and fish meat samples were divided into treatment groups as mentioned in Table 2. Groups within these studies containing bacteria were artificially contaminated with either MDR K3 or ATCC 35654 A. hydrophila by directly pipetting 2 mL (106 CFU/mL) of bacterial suspension onto the surface of fish and chicken meat samples in separate sterile 50 mL tubes. Samples were held at room temperature for 30 min inside a laminar flow hood to allow for bacterial attachment to the sample surface. Next, 2 mL of phage AhFM11 at a concentration of 109 PFU/mL (MOI = 1000) was pipetted directly on the meat surface and kept at room temperature inside the laminar flow hood for 30 min. For the Control groups, 2 mL of SM buffer was added in place of the phage suspension. The sample tubes were wrapped with parafilm to avoid contamination and refrigerated at 4 °C for 4 days. At timepoints 0, 24, 48, 72, and 96 h post-inoculation, 2.5 ± 0.2 g from each sample group were transferred to stomacher bags and homogenized. A 10 mL samples of homogenate were transferred into 15 mL sterile tubes and centrifuged at 12,000 ×g at 4 °C for 10 min to pellet the bacteria. Pellets were resuspended in TSB broth, pH 7.5. Tenfold serial dilutions were prepared in TSB broth, and aliquots were spread onto selective AIMB supplemented with ampicillin (HiMedia, Mumbai) for quantification of bacteria as CFU/g of meat samples.
AhFM11 replication and longevity on meat
Fish and chicken meat samples were prepared as described above and divided into groups as mentioned in Table 3. In this experiment, 2 mL of AhFM11 was added at a concentration of 109 PFU/mL to their respective groups. For negative controls, 2 mL of SM buffer was added instead of the phage suspension and 2 mL of TSB broth was added in place of bacteria. Meat samples were processed in stomacher bags and centrifuged as described above. The supernatant containing phage was collected and serially diluted in SM buffer to quantify the phage as PFU/g of meat using the soft agar overlay method described above.
Statistical analysis
One-way analysis of variance (ANOVA) was used to analyze the data along with Tukey's HSD (honestly significant difference) test using IBM SPSS Statistics 20 (SPSS, Chicago, IL, USA). The difference between the treatments was determined by an independent t-test. Statistically significant differences were considered at p < 0.05 and the data were represented as mean ± standard error of the mean using GraphPad Prism software.
Ethical statement
This study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org). The authors have adhered to all relevant international, national and/or institutional guidelines for animal care and animal utilization approved by the Institutional Animal Ethics Committee, College of Fisheries, Mangaluru, India. The experiments conducted on rohu (Labeo rohita) is a commercial fish which is not an endangered species in India.
Data availability
The datasets generated and/or analyzed during the current study are available in the NCBI repository, accession number ON042478.1.
References
FAO. Global Aquaculture Production. 02/29/2024 edn, (FAO Fisheries and Aquaculture Division, 2024).
FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation (FAO, 2022).
Sahoo, L. et al. Trajectory of 75 Years of Indian Agriculture After Independence 313–330 (Springer, 2023).
Hamod, M. A., Nithin, M. S., Shukur, Y. N., Karunasagar, I. & Karunasagar, I. Outer membrane protein K as a subunit vaccine against V. anguillarum. Aquaculture 354, 107–110 (2012).
Nithin, M. et al. Novel lytic bacteriophages (AhFM4 & AhFM5) as bio-control measures against multidrug resistant biofilm producing Aeromonas hydrophila (AhZ1K). Aquaculture 544, 737106 (2021).
Nithin, M. et al. Isolation, characterization and stability of lytic bacteriophage (AhFM22) infecting Aeromonas hydrophila. J. Exp. Zool. India 24, 749–754 (2021).
Nithin, M. et al. First evidence of extensively drug-resistant virulent Aeromonas dhakensis isolated from diseased endemic Mascara barb (Dawkinsia assimilis) in India. Aquaculture 569, 739337 (2023).
Nithin, M. S. Development of therapeutic phage consortia to manage Aeromonas hydrophila infection in aquaculture Ph.D. thesis, Karnataka Veterinary, Animal and Fisheries Sciences University (2021).
Suresh, T. et al. Largescale mortality of Oreochromis mossambicus in lakes and reservoirs of Karnataka due to coinfection of Tilapia Lake virus (TiLV) and multidrug-resistant Aeromonas veronii: An emerging fish disease in India. Aquaculture 565, 739077 (2023).
Laktuka, K., Kalnbalkite, A., Sniega, L., Logins, K. & Lauka, D. Towards the sustainable intensification of aquaculture: Exploring possible ways forward. Sustainability 15, 16952 (2023).
Brummett, R. E. et al. Reducing Disease Risk in Aquaculture (The World Bank, 2014).
Combe, M., Reverter, M., Caruso, D., Pepey, E. & Gozlan, R. E. Impact of global warming on the severity of viral diseases: A potentially alarming threat to sustainable aquaculture worldwide. Microorganisms 11, 1049 (2023).
Stentiford, G. D. et al. New paradigms to help solve the global aquaculture disease crisis. PLoS Pathog. 13, e1006160 (2017).
Hegde, A. et al. Bacterial diseases in marine fish species: Current trends and future prospects in disease management. World J. Microbiol. Biotechnol. 39, 317 (2023).
Rasmussen-Ivey, C. R. et al. Classification of a hypervirulent Aeromonas hydrophila pathotype responsible for epidemic outbreaks in warm-water fishes. Front. Microbiol. 7, 1615 (2016).
Xu, T. et al. A global survey of hypervirulent Aeromonas hydrophila (vAh) identified vAh strains in the lower Mekong river basin and diverse opportunistic pathogens from farmed fish and other environmental sources. Microbiol. Spectr. 11, e03705-03722 (2023).
Lafferty, K. D. et al. Infectious diseases affect marine fisheries and aquaculture economics. Annu. Rev. Mar. Sci. 7, 471–496 (2015).
Preena, P. G., Dharmaratnam, A., Jayadradhan Rejish Kumar, V. & Swaminathan, T. R. Plasmid-mediated antimicrobial resistance in motile aeromonads from diseased Nile tilapia (Oreochromis niloticus). Aquac. Res. 52, 237–248 (2021).
Shetty, M., Maiti, B., Shivakumar Santhosh, K., Venugopal, M. N. & Karunasagar, I. Betanodavirus of marine and freshwater fish: Distribution, genomic organization, diagnosis and control measures. Indian J. Virol. 23, 114–123 (2012).
Pu, W. et al. Three species of Aeromonas (A. dhakensis, A. hydrophila and A. jandaei) isolated from freshwater crocodiles (Crocodylus siamensis) with pneumonia and septicemia. Lett. Appl. Microbiol. 68, 212–218 (2019).
Semwal, A., Kumar, A. & Kumar, N. A review on pathogenicity of Aeromonas hydrophila and their mitigation through medicinal herbs in aquaculture. Heliyon https://doi.org/10.2139/ssrn.4298008 (2023).
Senthamarai, M. D., Rajan, M. R. & Bharathi, P. V. Current risks of microbial infections in fish and their prevention methods: A review. Microb. Pathog. 185, 106400 (2023).
Reverter, M. et al. Aquaculture at the crossroads of global warming and antimicrobial resistance. Nat. Commun. 11, 1870 (2020).
Neil, B., Cheney, G. L., Rosenzweig, J. A., Sha, J. & Chopra, A. K. Antimicrobial resistance in aeromonads and new therapies targeting quorum sensing. Appl. Microbiol. Biotechnol. 108, 1–22 (2024).
Nhinh, D. T. et al. Prevalence, virulence gene distribution and alarming the multidrug resistance of Aeromonas hydrophila associated with disease outbreaks in freshwater aquaculture. Antibiotics 10, 532 (2021).
Hossain, S. et al. Incidence of antimicrobial resistance genes and class 1 integron gene cassettes in multidrug-resistant motile Aeromonas sp. isolated from ornamental guppy (Poecilia reticulata). Lett. Appl. Microbiol. 69, 2–10 (2019).
Schar, D., Klein, E. Y., Laxminarayan, R., Gilbert, M. & Van Boeckel, T. P. Global trends in antimicrobial use in aquaculture. Sci. Rep. 10, 21878. https://doi.org/10.1038/s41598-020-78849-3 (2020).
Abdelrahman, H. A. et al. Epidemiology and economic impact of disease-related losses on commercial catfish farms: A seven-year case study from Alabama, USA. Aquaculture 566, 739206 (2023).
Trif, E. et al. Old antibiotics can learn new ways: A systematic review of florfenicol use in veterinary medicine and future perspectives using nanotechnology. Animals 13, 1695 (2023).
Administration, U. S. F. D. Approved Aquaculture Drugs (2024).
Hayatgheib, N. et al. Antimicrobial susceptibility profiles and resistance genes in genus Aeromonas spp. isolated from the environment and rainbow trout of two fish farms in France. Microorganisms https://doi.org/10.3390/microorganisms9061201 (2021).
Odeyemi, O. A. & Ahmad, A. Antibiotic resistance profiling and phenotyping of Aeromonas species isolated from aquatic sources. Saudi J. Biol. Sci. 24, 65–70. https://doi.org/10.1016/j.sjbs.2015.09.016 (2017).
Wu, C. et al. Characterization of florfenicol resistance genes in the coagulase-negative Staphylococcus (CoNS) isolates and genomic features of a multidrug-resistant Staphylococcus lentus strain H29. Antimicrob. Resist. Infect. Control 10, 9. https://doi.org/10.1186/s13756-020-00869-5 (2021).
Pérez-Sánchez, T., Mora-Sánchez, B. & Balcázar, J. L. Biological approaches for disease control in aquaculture: advantages, limitations and challenges. Trends Microbiol. 26, 896–903 (2018).
Verschuere, L., Rombaut, G., Sorgeloos, P. & Verstraete, W. Probiotic bacteria as biological control agents in aquaculture. Microbiol. Mol. Biol. Rev. 64, 655–671 (2000).
Deshotel, M. B., Dave, U. M., Farmer, B., Kemboi, D. & Nelson, D. C. Bacteriophage endolysin treatment for systemic infection of Streptococcus iniae in hybrid striped bass. Fish Shellfish Immunol. 145, 109296. https://doi.org/10.1016/j.fsi.2023.109296 (2024).
Kayansamruaj, P., Areechon, N. & Unajak, S. Development of fish vaccine in Southeast Asia: A challenge for the sustainability of SE Asia aquaculture. Fish Shellfish Immunol. 103, 73–87 (2020).
Nayak, S. K. Current prospects and challenges in fish vaccine development in India with special reference to Aeromonas hydrophila vaccine. Fish Shellfish Immunol. 100, 283–299 (2020).
Nikapitiya, C. et al. Isolation and characterization of multidrug resistance Aeromonas salmonicida subsp. salmonicida and its infecting novel phage ASP-1 from goldfish (Carassius auratus). Indian J. Microbiol. 59, 161–170 (2019).
Phumkhachorn, P. & Rattanachaikunsopon, P. Use of bacteriophage to control experimental Aeromonas hydrophila infection in Tilapia (Oreochromis niloticus). Pak. J. Biol. Sci. PJBS 23, 1659–1665 (2020).
Akmal, M., Rahimi-Midani, A., Hafeez-Ur-Rehman, M., Hussain, A.A.-O. & Choi, T. J. Isolation, characterization, and application of a bacteriophage infecting the fish pathogen Aeromonas hydrophila. Pathogens 9(3), 215. https://doi.org/10.3390/pathogens9030215 (2020).
Chandrarathna, H. P. S. U. et al. Isolation and characterization of phage AHP-1 and its combined effect with chloramphenicol to control Aeromonas hydrophila. Braz. J. Microbiol. 51, 409–416. https://doi.org/10.1007/s42770-019-00178-z (2020).
Pan, L. et al. Novel Aeromonas Phage Ahy-Yong1 and its protective effects against Aeromonas hydrophila in brocade carp (Cyprinus aka Koi). Viruses 14, 2498. https://doi.org/10.3390/v14112498 (2022).
Schulz, P., Robak, S., Dastych, J. & Siwicki, A. K. Influence of bacteriophages cocktail on European eel (Anguilla anguilla) immunity and survival after experimental challenge. Fish Shellfish Immunol. 84, 28–37 (2019).
Ghosh, S. et al. Whole genome sequence analysis of Aeromonas-infecting bacteriophage AHPMCC7, a new species of genus Ahphunavirus and its application in Litopenaeus vannamei culture. Virology 588, 109887. https://doi.org/10.1016/j.virol.2023.109887 (2023).
Easwaran, M. et al. Characterization of bacteriophage pAh-1 and its protective effects on experimental infection of Aeromonas hydrophila in Zebrafish (Danio rerio). J. Fish Dis. 40, 841–846. https://doi.org/10.1111/jfd.12536 (2017).
Duarte, J. et al. New insights on phage efficacy to control Aeromonas salmonicida in aquaculture systems: An in vitro preliminary study. Aquaculture 495, 970–982. https://doi.org/10.1016/j.aquaculture.2018.07.002 (2018).
Kabwe, M. et al. Novel bacteriophages capable of disrupting biofilms from clinical strains of Aeromonas hydrophila. Front. Microbiol. 11, 194 (2020).
Żaczek, M., Weber-Dąbrowska, B. & Górski, A. Phages as a cohesive prophylactic and therapeutic approach in aquaculture systems. Antibiotics 9, 564 (2020).
Żaczek, M., Weber-Dąbrowska, B., Międzybrodzki, R. & Górski, A. Phage prevalence in the human urinary tract—Current knowledge and therapeutic implications. Microorganisms 8, 1802 (2020).
Rai, S., Tyagi, A. & Kumar, B. N. Isolation and characterization of Aeromonas hydrophila lytic phage, and evaluation of a phage cocktail against A. hydrophila contamination in fish fillet. Food Control 145, 109460 (2023).
Cao, Y. et al. Characterization and application of a novel Aeromonas bacteriophage as treatment for pathogenic Aeromonas hydrophila infection in rainbow trout. Aquaculture 523, 735193 (2020).
Cheng, Y. et al. Characterization of novel bacteriophage AhyVDH1 and its lytic activity against Aeromonas hydrophila. Curr. Microbiol. 78, 329–337 (2021).
Kazimierczak, J. et al. Complete genome sequences of Aeromonas and Pseudomonas phages as a supportive tool for development of antibacterial treatment in aquaculture. Virol. J. 16, 1–12 (2019).
Liu, J., Gao, S., Dong, Y., Lu, C. & Liu, Y. Isolation and characterization of bacteriophages against virulent Aeromonas hydrophila. BMC Microbiol. 20, 1–13 (2020).
Pereira, C., Duarte, J., Costa, P., Braz, M. & Almeida, A. Bacteriophages in the control of Aeromonas sp. in aquaculture systems: An integrative view. Antibiotics 11, 163 (2022).
Tu, V. Q. et al. Complete genome sequence of a novel lytic phage infecting Aeromonas hydrophila, an infectious agent in striped catfish (Pangasianodon hypophthalmus). Arch. Virol. 165, 2973–2977 (2020).
Turner, D. et al. Abolishment of morphology-based taxa and change to binomial species names: 2022 taxonomy update of the ICTV bacterial viruses subcommittee. Arch. Virol. 168, 74. https://doi.org/10.1007/s00705-022-05694-2 (2023).
Wang, J.-B., Lin, N.-T., Tseng, Y.-H. & Weng, S.-F. Genomic characterization of the novel Aeromonas hydrophila Phage Ahp1 suggests the derivation of a new subgroup from phiKMV-like family. PLoS ONE 11, e0162060. https://doi.org/10.1371/journal.pone.0162060 (2016).
Jun, J. W. et al. Protective effects of the Aeromonas phages pAh1-C and pAh6-C against mass mortality of the cyprinid loach (Misgurnus anguillicaudatus) caused by Aeromonas hydrophila. Aquaculture 416–417, 289–295. https://doi.org/10.1016/j.aquaculture.2013.09.045 (2013).
Yuan, S. et al. Characterization and genomic analyses of Aeromonas hydrophila phages AhSzq-1 and AhSzw-1, isolates representing new species within the T5virus genus. Arch. Virol. 163, 1985–1988 (2018).
Kumari, R., Yadav, R., Kumar, D., Chaube, R. & Nath, G. Evaluation of bacteriophage therapy of Aeromonas hydrophila infection in a freshwater fish, Pangasius buchanani. Front. Aquac. 2, 1201466 (2023).
Le, T. S. et al. Protective effects of bacteriophages against Aeromonas hydrophila causing motile Aeromonas septicemia (MAS) in striped catfish. Antibiotics 7, 16 (2018).
Ninawe, A., Sivasankari, S., Ramasamy, P., Kiran, G. S. & Selvin, J. Bacteriophages for aquaculture disease control. Aquac. Int. 28, 1925–1938 (2020).
Rai, S. et al. Perspectives on phage therapy for health management in aquaculture. Aquac. Int. 32, 1–45 (2023).
Islam, M. S. et al. Complete genome sequence of Aeromonas Phage ZPAH7 with Halo Zones, Isolated in China. Microbiol. Resour. Announc. 8, 10–128. https://doi.org/10.1128/MRA.01678-18 (2019).
You, H. J. et al. Tackling Vibrio parahaemolyticus in ready-to-eat raw fish flesh slices using lytic phage VPT02 isolated from market oyster. Food Res. Int. 150, 110779. https://doi.org/10.1016/j.foodres.2021.110779 (2021).
Banos, A. et al. Biocontrol of Listeria monocytogenes in fish by enterocin AS-48 and Listeria lytic bacteriophage P100. LWT Food Sci. Technol. 66, 672–677. https://doi.org/10.1016/j.lwt.2015.11.025 (2016).
Soni, K. A., Nannapaneni, R. & Hagens, S. Reduction of Listeria monocytogenes on the surface of fresh channel catfish fillets by bacteriophage Listex P100. Foodborne Pathog. Dis. 7(4), 427–434 (2010).
Thung, T. Y. et al. Partial characterization and in vitro evaluation of a lytic bacteriophage for biocontrol of Campylobacter jejuni in mutton and chicken meat. J. Food Saf. 40, e12770. https://doi.org/10.1111/jfs.12770 (2020).
Hungaro, H. M., Mendonça, R. C., Gouvêa, D. M., Vanetti, M. C. & de Oliveira Pinto, C. L. Use of bacteriophages to reduce Salmonella in chicken skin in comparison with chemical agents. Food Res. Int. 52, 75–81. https://doi.org/10.1016/j.foodres.2013.02.032 (2013).
Guenther, S., Huwyler, D., Richard, S. & Loessner, M. J. Virulent bacteriophage for efficient biocontrol of Listeria monocytogenes in ready-to-eat foods. Appl. Environ. Microbiol. 75, 93–100. https://doi.org/10.1128/AEM.01711-08 (2009).
Adams, M. H. Bacteriophages (Interscience Publishers, 1959).
Dubey, S. et al. Aeromonas species obtained from different farmed aquatic species in India and Taiwan show high phenotypic relatedness despite species diversity. BMC. Res. Notes 14, 313. https://doi.org/10.1186/s13104-021-05716-3 (2021).
Sullivan, M. J., Petty, N. K. & Beatson, S. A. Easyfig: A genome comparison visualizer. Bioinformatics. 27(7), 1009–1010 (2011).
Rozewicki, J., Li, S., Amada, K. M., Standley, D. M. & Katoh, K. MAFFT-DASH: Integrated protein sequence and structural alignment. Nucleic Acids Res. 47, W5–W10. https://doi.org/10.1093/nar/gkz342 (2019).
Letunic, I.A.-O. & Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293–W296 (2021).
Acknowledgements
We are thankful to the DBT-NER-BPSC Ministry of Science and Technology, India for providing resources for this study.
Funding
This work was carried out with the funding support from the Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India, for the project entitled “Development of therapeutic phage consortia to manage Aeromonas hydrophila infection in aquaculture systems” under DBT’s Programme for NER-BPSC (Project Sanction Order No. BT/PR17014/NER/95/390/2015 dated March 30, 2015). This research was supported in part by an appointment (Research Fellowship to MSN) to the Agricultural Research Service (ARS) Research Participation Program administered by the Oak Ridge Institute for Science and education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and the U.S. Department of Agriculture (USDA). ORISE is managed by ORAU under DOE contract number DE-SC0014664. All opinions expressed in this paper are the author’s and do not necessarily reflect the policies and views of USDA, DOW, or ORAU/ORISE. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the United States Department of Agriculture. The USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Contributions
MSN., KKB., NMR., and GSK-conceptualization. MSN., KKB., NMR., and DSB-methodology. KKB., PKS., and KBK-software. MSN., KKB., and DSB-validation. MSN., KKB., NMR., and DSB-investigation. MSN., KKB., MBD., and JWA-data curation. MSN., KKB., and MBD–writing, original draft preparation. MSN., KBK., GSK., MBD., MDL., and JWA-writing, review, and editing. MSN, KKB and MBD-visualization. GSK., ST., MBD., LS., and JWA-supervision. All authors have read and agreed to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muliya Sankappa, N., Shivani Kallappa, G., Kallihosuru Boregowda, K. et al. Novel lytic bacteriophage AhFM11 as an effective therapy against hypervirulent Aeromonas hydrophila. Sci Rep 14, 16882 (2024). https://doi.org/10.1038/s41598-024-67768-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67768-2
- Springer Nature Limited