Abstract
Statin is crucial for acute myocardial infarction (AMI) patients. However, the risk of new-onset diabetes mellitus (NODM) associated with statin is a concern. This study aimed to determine the incremental diabetogenic effects of statins according to their intensity and dose in AMI patients undergoing percutaneous coronary intervention (PCI). Among 13,104 patients enrolled in the Korea AMI Registry between 2011 and 2015, 6152 patients without diabetes mellitus (DM) who underwent PCI and received moderate-to-high-intensity atorvastatin and rosuvastatin were selected for the study. The endpoints were NODM and major adverse cardiovascular events (MACE), composite of all-cause mortality, recurrent MI, and revascularization up to 3 years. Among the participants, 3747 and 2405 received moderate- and high-intensity statins, respectively. The Kaplan–Meier curves demonstrated a higher incidence of NODM in patients with high-intensity statins than those with moderate-intensity. High-intensity statin was a significant predictor of NODM after adjusting for other co-variables (HR = 1.316, 95% CI 1.024–1.692; P < 0.032). Higher dose of rosuvastatin was associated with a higher cumulative incidence of NODM, but this dose-dependency was not apparent with atorvastatin. Cumulative incidence of MACE decreased dose-dependently only with atorvastatin. High-intensity statin was associated with a higher cumulative incidence of NODM in AMI patients, and this association was more evident in rosuvastatin. The different diabetogenic effects of the two statins provide supporting evidence for understanding the nuanced nature of statin treatment in relation to NODM.
Similar content being viewed by others
Introduction
Statins are must-have agents used in patients with acute myocardial infarction (AMI), particularly following percutaneous coronary intervention (PCI) with contemporary drug-eluting stents (DESs). The risk of cardiovascular events can be reduced not only by lowering the low-density lipoprotein (LDL) cholesterol levels but also multiple pleiotropic effects beyond lipid lowering effect with statin treatment. Statins inhibit the pathways of atherosclerosis by inhibiting HMG-CoA reductase inside of endothelial cells and vascular smooth muscle cells1. Clinical studies have shown multiple benefits of statins which are LDL-cholesterol-independent (or pleiotropic)2. Statin treatments have shown to significantly reduce not only LDL cholesterol levels but also CRP levels3, and also reduced degree of inflammation in other systemic diseases such as periodontal disease or rheumatoid arthritis4. Finally, statin treatments have significantly reduced cardiovascular mortality and morbidity in numerous previous studies5,6,7. The clinical efficacy of statins in primary and secondary prevention of atherosclerotic cardiovascular disease (ASCVD) has been proven by numerous studies8. Further, the current guidelines recommend high-intensity statins for patients with AMI9,10.
However, since the JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) trial first raised the issue of an increased risk of new-onset diabetes mellitus (NODM) associated with statin treatment3, this has been a major concern when treating AMI patients with statins. A meta-analysis of randomized trials has shown that statin therapy is associated with an approximately 10% increased risk of NODM11. Moreover, it revealed that intensive-dose statin therapy is associated with an increased risk of NODM compared with moderate-dose statin treatment12. Observational studies have also supported this finding1,13.
Although statins play a crucial role in the secondary prevention of AMI, the increasing concern for NODM associated with statin treatment is understandable due to DM being a potent risk factor for ASCVD. However, there are still unresolved questions regarding this issue. It remains uncertain whether statins genuinely contribute to the development of DM, or if patients who are subscribed statins merely belong to a high-risk group for NODM due to factors such as advanced age and numerous comorbidities, including hypertension, dyslipidemia, or obesity.
Moreover, there is ongoing argument regarding whether the diabetogenic effect of statins is a class effect. Furthermore, considering that patients with AMI need higher intensity statin treatment than the rest of the population, it is important to identify whether higher intensity statin treatment has a higher incremental diabetogenic effect. However, comprehensive understanding of this association is yet to be achieved.
To address this knowledge gap, we used the national AMI registry data and investigated the dose-dependent diabetogenic effects of atorvastatin and rosuvastatin, both of which offer various moderate- and high-intensity dosages as per the 2018 American Heart Association (AHA) guideline on the management of blood cholesterol10.
Methods
Study population
The Korea Acute Myocardial Infarction Registry (KAMIR) is a prospective multicenter national database supported by the Korean Society of Cardiology. Data on patients with AMI from 20 PCI capable tertiary or community hospitals in Korea were registered online. Patient data were gathered by well-trained study coordinators using a standardized case report form. The KAMIR registry was approved by the medical ethics committee of each participating center, and all study participants provided written informed consent. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
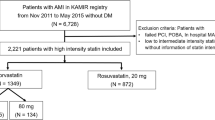
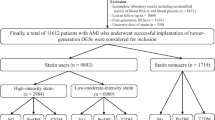
A total of 13,104 patients with AMI enrolled in the KAMIR between November 2011 and May 2015 were reviewed. Among the AMI patients without DM at enrollment and those who had undergone successful PCI with DESs, individuals taking either atorvastatin or rosuvastatin, which are available in various doses ranging from moderate to high intensity, were included in the analysis. Patients who had experienced in-hospital major adverse cardiac events (MACE) and those lacking information about statin intensity were excluded. Ultimately, a total of 6152 patients were considered in the final analysis. We investigated the cumulative incidence of NODM and MACE based on the intensity of statins, as well as the individual doses within atorvastatin and rosuvastatin group (Fig. 1).
Study schema. A total of 6152 AMI patients who were treated with moderate-to-high intensity atorvastatin and rosuvastatin were included in the final analysis. AMI acute myocardial infarction, KAMIR Korea Acute Myocardial Infarction Registry, PCI percutaneous coronary intervention; MACE major adverse cardiac events.
Study endpoints
The primary endpoint of this study was the cumulative incidence of NODM according to statin intensity during 3 years of follow-up. NODM was defined as glycated hemoglobin (HbA1c) levels ≥ 6.5% or newly treated with oral hypoglycemic agents or insulin during follow-up. The secondary endpoint was MACE which was defined as a composite of all-cause mortality, myocardial infarction (MI), and any revascularization according to statin intensity during 3 years of follow-up.
Patient clinical data were obtained through chart reviews, face-to-face interviews at the outpatient clinic, or telephone interviews.
Statistical analysis
Continuous variables are presented as mean ± standard deviation. Student’s t-test was used to analyze the differences between the two groups. Dichotomous variables are presented as percentages, and the chi-square test or Fisher’s exact test was used to analyze the differences.
The Kaplan–Meier method with log-rank test was used to compare the cumulative incidence of NODM and MACE between the two groups. Cox proportional hazard models were used to identify potential prognostic factors for NODM and MACE, and the results were presented as hazard ratios (HR) and 95% confidence intervals (CI). For multivariate analysis, variables with P-values (< 0.05) in the univariate analysis were included.
All analyses were performed using the SPSS software (version 22.0, Inc. Chicago, IL, USA). Statistical significance was set at a P-value of < 0.05.
Ethics approval and consent to participate
The KAMIR registry was approved by the medical ethics committee (Institutional Review Board) of each participating center, and all study participants provided written informed consent. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Results
Baseline characteristics
The baseline characteristics of the study participants are presented in Table 1. Among the 6152 study participants, 3747 and 2405 received moderate- and high-intensity statins, respectively. The mean age was significantly higher in the moderate-intensity statin group than in the high-intensity statin group (63.7 ± 12.8 vs. 60.5 ± 12.6; P < 0.001). The high-intensity statin group had a higher prevalence of male sex and current smokers, higher systolic and diastolic blood pressure, higher body mass index (BMI) (P < 0.001), and larger abdominal circumference (P = 0.011). The levels of total and LDL cholesterol were significantly higher in the high-intensity statin group (195.5 ± 44.6 vs. 178.2 ± 41.3; P < 0.001 and 127.8 ± 38.7 vs. 112.0 ± 38.3; P < 0.001, respectively); however, the levels of triglycerides were significantly higher in the moderate-intensity statin group (145.7 ± 126.5 vs. 123.8 ± 109.2; P < 0.001). There were no statistically significant intergroup differences in ST-segment elevation MI, non-ST segment elevation MI, high-density lipoprotein cholesterol, random blood glucose, and creatinine levels.
More than 99% of the study population was taking aspirin. The prescription rate of ticagrelor was significantly higher in the high-intensity statin group, whereas those of clopidogrel and cilostazol were higher in the moderate-intensity statin group. Prescription of β-blockers and renin–angiotensin–aldosterone system inhibitors was more frequent in the moderate-intensity group. The total number of implanted coronary stents was similar between the two groups. However, the high-intensity statin group had a slightly higher number of coronary stents than the moderate-intensity statin group, and this difference was statistically significant.
Clinical outcomes
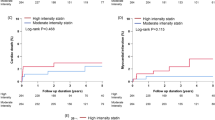
The Kaplan–Meier curve for the cumulative incidence of NODM are presented in Figs. 2 and 3, and Supplemental Table S1. The high-intensity statin group had a significantly higher cumulative incidence of NODM than the moderate-intensity statin group (7.8% vs. 5.8%; log-rank P = 0.002) (Fig. 2). Figure 3 presents the cumulative incidence of NODM according to the intensity and dose for atorvastatin and rosuvastatin. Regarding groups treated with atorvastatin, study population treated with high-intensity atorvastatin showed a significantly higher cumulative incidence of NODM than the moderate-intensity atorvastatin group (7.2% vs. 5.8%, P = 0.002) (Fig. 3A). Further, the study population treated with the highest dose (80 mg) of atorvastatin showed the highest cumulative incidence of NODM (7.7%), followed by the second highest dose (40 mg) (7.1%). On the other hand, the other two groups, treated with lower doses (20 mg and 10 mg) of atorvastatin showed a relatively lower cumulative incidence of NODM (5.8% and 5.7%), even though the association was statistically insignificant (P = 0.484) (Fig. 3B). In terms of rosuvastatin, the high-intensity rosuvastatin group showed a significantly higher cumulative incidence of NODM than moderate-intensity rosuvastatin group (8.3% vs. 5.8%, P = 0.004) (Fig. 3C). The dose dependency of diabetogenicity of rosuvastatin was more prominent than that of atorvastatin. A higher dose of rosuvastatin was significantly associated with higher cumulative incidence of NODM, which was statistically significant (20 mg vs. 10 mg vs. 5 mg: 8.3% vs. 5.5% vs. 2.8%, P = 0.008) (Fig. 3D).
Cumulative incidence of NODM according to the statin intensity and dose. (A) Cumulative incidence of NODM according to the atorvastatin intensity. Patients treated with high-intensity atorvastatin showed a significantly higher cumulative incidence of NODM compared to the moderate-intensity atorvastatin group (7.2% vs. 5.8%, P = 0.002). (B) Cumulative incidence of NODM according to the atorvastatin dose. Patients treated with the 80 mg of atorvastatin had the highest cumulative incidence of NODM, followed by those on 40 mg, 20 mg and 10 mg of atorvastatin (7.7% vs. 7.1% vs. 5.8% vs. 5.7%, respectively, P = 0.484) (C) Cumulative incidence of NODM according to the rosuvastatin intensity. Patients treated with high-intensity rosuvastatin had a significantly higher cumulative incidence of NODM compared to the moderate-intensity rosuvastatin group (8.3% vs. 5.8%, P = 0.004). (D) Cumulative incidence of NODM according to the rosuvastatin Dose. Patients treated with the 20 mg of rosuvastatin showed the highest cumulative incidence of NODM, followed by 10 mg, 5 mg of rosuvastatin (8.3% vs. 5.5% vs. 2.8%, P = 0.008). NODM new-onset diabetes mellitus.
The results of the Cox regression analysis of NODM showing the potential prognostic factors are presented in Table 2. Body mass index (BMI) and abdominal circumference were positively associated with the incidence of NODM in a univariate model. Plasma glucose and triglyceride levels were also positively associated with the incidence of NODM in a univariate model, whereas the association with the amount of smoking was not statistically significant. In the multivariate model, BMI (HR 1.071; 95% CI 1.040–1.103; P < 0.001), plasma glucose level (HR 1.007; 95% CI 1.005–1.008; P < 0.001) and triglyceride (HR 1.001; 95% CI 1.000–1.001; P = 0.035) were positively associated with the incidence of NODM. When we compared the incidence of NODM between the high- and moderate-intensity statin groups, the hazard ratios were significantly higher in the high-intensity statin group than in the moderate-intensity statin group in both univariate (HR 1.360; 95% CI 1.115–1.659; P = 0.002) and multivariate (HR 1.306; 95% CI 1.056–1.617; P = 0.014) analyses. Regarding specific statins, atorvastatin at different doses did not show a significant impact on NODM (P = 0.486). In contrast, rosuvastatin demonstrated a significant difference in NODM occurrence (P = 0.009). When comparing different doses of rosuvastatin, several dose-dependent effects were observed, with some being statistically significant in the univariable model (e.g., 10 mg vs. 20 mg with a HR 1.514; 95% CI 1.111–2.062; P = 0.009) and one remaining significant in the multivariable model (e.g., 10 mg vs. 20 mg with a HR 1.430; 95% CI 1.020–2.004; P = 0.038).
The Kaplan–Meier curve for the cumulative incidence of NODM are presented in Fig. 4 and Supplemental Fig. S1, and Supplemental Table S1. The cumulative incidence of MACE was significantly lower in the high-intensity statin group than in the moderate-intensity group (P = 0.004) (Fig. 4A). Among patients treated with atorvastatin, the study population treated with the highest dose (80 mg) showed the lowest cumulative incidence of MACE, followed by those treated with the second (40 mg) and third (20 mg) highest doses (Fig. 4B). The study population treated with the lowest dose (10 mg) of atorvastatin had the highest cumulative incidence of MACE among all groups. In patients treated with 5, 10, and 20 mg rosuvastatin, there was no dose-dependent association between the cumulative incidence of MACE and rosuvastatin (P = 0.503) (Supplemental Fig. S1). Supplemental Table S2 presents the results of the Cox regression analysis of MACE in univariate and multivariate models. When compared with the moderate-intensity statin group, the high-intensity statin group was associated with lower MACE (HR 0.809; 95% CI 0.700–0.935; P = 0.004). The multivariate model showed a similar tendency; however, the difference was not statistically significant (P = 0.252).
Cumulative incidence of MACE according to the statin intensity and dose. (A) Cumulative incidence of MACE according to the statin intensity. Patients treated with high-intensity statin showed a significantly lower cumulative incidence of MACE compared to the moderate-intensity statin group (11.6% vs. 14.1%, P = 0.004). (B) Cumulative incidence of MACE according to the atorvastatin dose. A higher dose was associated with a lower cumulative incidence of MACE (80 mg vs. 40 mg vs. 20 mg vs. 10 mg: 8.5% vs. 15.0% vs. 12.0% vs. 8.5%, respectively, P < 0.001). MACE major adverse cardiac events.
Discussion
In this prospective and multicenter study based on a national database, higher-intensity statin therapy was associated with a higher cumulative incidence of NODM and a lower cumulative incidence of MACE in patients with AMI underwent PCI with DES up to 3 years follow up. To the best of our knowledge, the current study is the first to demonstrate the dose-dependency of the diabetogenic effect of statins, especially in AMI patients with rosuvastatin.
Statins are critical agents for cardiovascular risk reduction, and their effect has been shown to be positively associated with statin intensity14,15. Therefore, current guidelines strongly recommend high-intensity or maximally tolerated-intensity statins for patients with AMI9. However, the association between statin therapy and an increased risk of NODM has always been a significant concern. As shown in the review article by Newman et al., the absolute risk of NODM with statin therapy in major trials is around 0.2% per year16. Several randomized controlled trials have suggested that the risk of NODM increases by 10% with statin therapy11,17.
Although previous studies have demonstrated the association between statin treatment and incident DM, the exact underlying mechanisms are still unclear. The hyperglycemic state associated with statins can be induced by increased insulin resistance, possibly associated with changes in free fatty acids18, harmful effects on beta cell function, or possibly a combination of the two19,20. One genetic study suggested that statin therapy is associated with an increased risk of NODM and body weight via the inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A reductase21.
Whether statins have a genuine diabetogenic effect is controversial because patients prescribed statins may already be a high-risk population for NODM because of their old age and several comorbidities. As these patients have more components of metabolic syndrome, including hypertension, dyslipidemia, or obesity, the risk of developing DM in association with statin use escalates, as demonstrated in various studies22,23. The relatively higher cumulative incidence of NODM of our study compared to other major trials16 could be attributed to the patient demographics; those with AMI are more likely to have comorbidities that inherently pose risk factors of NODM. In our study, high-intensity statin treatment exhibited a greater cumulative incidence of NODM than moderate-intensity statin treatment among patients with AMI and many comorbidities, and was a significant predictive value for NODM after adjusting other covariables. Furthermore, rosuvastatin exhibited a clear trend of dose-dependent increases in HRs compared to atorvastatin which might elucidate their diabetogenic effect. However, the lack of statistical significance can be attributed to the limited sample size of rosuvastatin 5 mg (n = 109), which may have affected the ability to detect significant associations.
Regarding whether the diabetogenic effect of statins is a class effect, unlike other statins, pravastatin and pitavastatin are not generally considered as having deleterious effects on glycemic control24,25. In our study, a dose-dependency of statins in NODM was observed in patients treated with rosuvastatin; however, this tendency was not evident in atorvastatin. This difference in the pattern of dose dependency between atorvastatin and rosuvastatin suggests varying degrees of diabetogenicity for different statins. This tendency of rosuvastatin to have the highest risk of NODM compared with other statins was also shown in the Irish Health Services Executive Primary Care Reimbursement Services national pharmacy claims database26. A randomized study also showed that rosuvastatin was associated with increased fasting insulin and HbA1c levels and decreased insulin sensitivity and plasma adiponectin levels, whereas pravastatin showed opposite effects27. It has been suggested that lipophilic statins like atorvastatin are more likely to adversely affect insulin metabolism compared to hydrophilic statins like pravastatin by crossing the blood–brain barrier27. However, even though rosuvastatin is less hydrophilic than pravastatin27, this hydrophilicity of statins cannot entirely explain rosuvastatin’s apparent dose-dependent association with NODM, especially considering the fact that atorvastatin is more lipophilic than rosuvastatin. One possible mechanism for higher diabetogenic effect of rosuvastatin is that it demonstrates a stronger bonding interaction with HMG-CoA reductase than atorvastatin19,28. Future studies are welcomed to investigate this subtle nature of different statins.
Even though the high-risk patients have developed NODM, several observational studies have demonstrated that these patients had fewer macrovascular and microvascular complications of DM while receiving statins29. Undoubtedly, the net benefit of statins for cardiovascular diseases is irreplaceable, even though DM is a major cardiovascular risk factor30,31. In our study, the cumulative incidence of MACE was significantly lower in the high-intensity statin group, and the dose-dependency of the cumulative incidence of MACE was apparent in the atorvastatin group but not the rosuvastatin group. The relatively small number of patients treated with rosuvastatin 20 mg might have contributed to this lack of association of the cumulative incidence of MACE with rosuvastatin treatment.
Study limitations
This study has several limitations. First, although the KAMIR can provide a representation of real-world clinical data due to its enrollment of patients from various hospitals across the country, the presence of selection bias is inevitable. In addition, given the clinical context of the study population being ACS, it remains unclear whether the baseline glucose levels were obtained in a fasting state or not. Second, since the study was conducted between 2011 and 2015, there were relatively small number of patients taking high-intensity statins (n = 2405 compared to 3747 patients with moderate-intensity statins). Furthermore, the absence of rosuvastatin 40 mg in the Korean market prevented the collection of data on this specific dosage, potentially contributing to the lack of an observable dose-dependent association between MACE and rosuvastatin. In contrast, atorvastatin showed an apparent dose-dependent effect on MACE, which is consistent of current understanding of statins: the lower LDL cholesterol, the better MACE outcomes. Nevertheless, the dose-dependency of rosuvastatin in NODM was still evident, which suggests the diabetogenic effect of rosuvastatin as well as its dose-dependency. The secondary analysis of LODESTAR trial that compare the rosuvastatin and atorvastatin also demonstrated higher risk of NODM in rosuvastatin group, which is consistent with our study32.
While our study highlights the relationship between dose-dependency of statins and NODM in patients with AMI, it is essential to replicate this dose-dependency effect in lower risk population. Such a demonstration becomes particularly significant when comparing the risk–benefit profile of statins in this population against higher-risk groups or patients with established ASCVD.
Conclusions
Treatment with high-intensity atorvastatin and rosuvastatin was associated with a higher incidence of NODM and a lower incidence of MACE than moderate-intensity treatment in patients with AMI underwent PCI with DES up to 3 years. In terms of the dose-dependency of each statin, a higher dose of rosuvastatin was significantly associated with a higher cumulative incidence of NODM which supports the genuine diabetogenic effect of rosuvastatin; however, this association was not observed with atorvastatin. The different characteristics of the two statins could provide supporting evidence to understand the delicate nature of statins, and this might help physicians further refine statin treatment.
Data availability
The dataset generated and/or analyzed during the current study are available from the corresponding author on a reasonable request.
Abbreviations
- AMI:
-
Acute myocardial infarction
- LDL:
-
Low-density lipoprotein
- ASCVD:
-
Atherosclerotic cardiovascular disease
- NODM:
-
New-onset diabetes mellitus
- KAMIR:
-
Korea Acute Myocardial Infarction Registry
- MACE:
-
Major adverse cardiac events
- BMI:
-
Body Mass Index
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Chung, J. et al. New onset diabetes mellitus and cardiovascular outcomes according to statin intensity in patients after drug-eluting stent implantation in Asian patients. Sci. Rep. 13, 16061. https://doi.org/10.1038/s41598-023-42277-w (2023).
Liao, J. K. & Laufs, U. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 45, 89–118. https://doi.org/10.1146/annurev.pharmtox.45.120403.095748 (2005).
Ridker, P. M. et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 359, 2195–2207. https://doi.org/10.1056/NEJMoa0807646 (2008).
Oesterle, A., Laufs, U. & Liao, J. K. Pleiotropic effects of statins on the cardiovascular system. Circ. Res. 120, 229–243. https://doi.org/10.1161/circresaha.116.308537 (2017).
Sacks, F. M. et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. New Engl. J. Med. 335, 1001–1009. https://doi.org/10.1056/nejm199610033351401 (1996).
Schwartz, G. G. et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: The MIRACL study: A randomized controlled trial. Jama 285, 1711–1718. https://doi.org/10.1001/jama.285.13.1711 (2001).
Scandinavian Simvastatin Survival. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 344, 1383–1389 (1994).
Baigent, C. et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376, 1670–1681. https://doi.org/10.1016/s0140-6736(10)61350-5 (2010).
Mach, F. et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 41, 111–188. https://doi.org/10.1093/eurheartj/ehz455 (2020).
Grundy, S. M. et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 73, e285–e350. https://doi.org/10.1016/j.jacc.2018.11.003 (2019).
Sattar, N. et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. The Lancet 375, 735–742. https://doi.org/10.1016/s0140-6736(09)61965-6 (2010).
Preiss, D. et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: A meta-analysis. Jama 305, 2556–2564. https://doi.org/10.1001/jama.2011.860 (2011).
Culver, A. L. et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch. Intern. Med. 172, 144–152. https://doi.org/10.1001/archinternmed.2011.625 (2012).
Hiro, T. et al. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: A multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J. Am. Coll. Cardiol. 54, 293–302. https://doi.org/10.1016/j.jacc.2009.04.033 (2009).
Cannon, C. P., Steinberg, B. A., Murphy, S. A., Mega, J. L. & Braunwald, E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J. Am. Coll. Cardiol. 48, 438–445. https://doi.org/10.1016/j.jacc.2006.04.070 (2006).
Newman, C. B. et al. Statin safety and associated adverse events: A scientific statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 39, e38–e81. https://doi.org/10.1161/atv.0000000000000073 (2019).
Wang, S. et al. Association between reductions in low-density lipoprotein cholesterol with statin therapy and the risk of new-onset diabetes: A meta-analysis. Sci. Rep. 7, 39982. https://doi.org/10.1038/srep39982 (2017).
Szendroedi, J. et al. Effects of high-dose simvastatin therapy on glucose metabolism and ectopic lipid deposition in nonobese type 2 diabetic patients. Diabetes Care 32, 209–214. https://doi.org/10.2337/dc08-1123 (2009).
Betteridge, D. J. & Carmena, R. The diabetogenic action of statins - mechanisms and clinical implications. Nat. Rev. Endocrinol. 12, 99–110. https://doi.org/10.1038/nrendo.2015.194 (2016).
Mach, F. et al. Adverse effects of statin therapy: Perception vs the evidence—focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur. Heart J. 39, 2526–2539. https://doi.org/10.1093/eurheartj/ehy182 (2018).
Swerdlow, D. I. et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: Evidence from genetic analysis and randomised trials. Lancet 385, 351–361. https://doi.org/10.1016/s0140-6736(14)61183-1 (2015).
Ridker, P. M., Pradhan, A., MacFadyen, J. G., Libby, P. & Glynn, R. J. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: An analysis from the JUPITER trial. The Lancet 380, 565–571. https://doi.org/10.1016/s0140-6736(12)61190-8 (2012).
Ong, K. L. et al. Effect of change in body weight on incident diabetes mellitus in patients with stable coronary artery disease treated with atorvastatin (from the treating to new targets study). Am. J. Cardiol. 113, 1593–1598. https://doi.org/10.1016/j.amjcard.2014.02.011 (2014).
Chapman, M. J. et al. Effect of high-dose pitavastatin on glucose homeostasis in patients at elevated risk of new-onset diabetes: Insights from the CAPITAIN and PREVAIL-US studies. Curr. Med. Res. Opin. 30, 775–784. https://doi.org/10.1185/03007995.2013.874989 (2014).
Baker, W. L., Talati, R., White, C. M. & Coleman, C. I. Differing effect of statins on insulin sensitivity in non-diabetics: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 87, 98–107. https://doi.org/10.1016/j.diabres.2009.10.008 (2010).
Zaharan, N. L., Williams, D. & Bennett, K. Statins and risk of treated incident diabetes in a primary care population. Br. J. Clin. Pharmacol. 75, 1118–1124. https://doi.org/10.1111/j.1365-2125.2012.04403.x (2013).
Koh, K. K. et al. Differential metabolic effects of rosuvastatin and pravastatin in hypercholesterolemic patients. Int. J. Cardiol. 166, 509–515. https://doi.org/10.1016/j.ijcard.2011.11.028 (2013).
Istvan, E. S. & Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 292, 1160–1164. https://doi.org/10.1126/science.1059344 (2001).
Nielsen, S. F. & Nordestgaard, B. G. Statin use before diabetes diagnosis and risk of microvascular disease: A nationwide nested matched study. Lancet Diabetes Endocrinol. 2, 894–900. https://doi.org/10.1016/s2213-8587(14)70173-1 (2014).
Mihaylova, B. et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 380, 581–590. https://doi.org/10.1016/s0140-6736(12)60367-5 (2012).
Kearney, P. M. et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: A meta-analysis. Lancet 371, 117–125. https://doi.org/10.1016/s0140-6736(08)60104-x (2008).
Lee, Y. J. et al. Rosuvastatin versus atorvastatin treatment in adults with coronary artery disease: Secondary analysis of the randomised LODESTAR trial. Bmj 383, e075837. https://doi.org/10.1136/bmj-2023-075837 (2023).
Acknowledgements
Database for this article were gathered from the Korea Acute Myocardial Infarction Registry (KAMIR), which is a national database supported by the Korean Society of Cardiology. This research was supported by the “National Institute of Health” research project (project No. 2016-ER6304-02) and the Korea University Grant (K2125861).
Author information
Authors and Affiliations
Consortia
Contributions
JEL and JYC contributed to the conception and design of the work, analyzed the data, and wrote the manuscript; BGC analyzed the data; YJC, SHP, DOK, EJP, JBK, SYR, JON, CUC, EJK, and CGP contributed to the acquisition of the data; MHJ, JYH, SHH, JOJ, and SKO critically revised the manuscript; SWR contributed to the acquisition and interpretation of the data, and critically revised the manuscript. All authors read and approved the final manuscript for publication. SWR has full access to the data in the study and has responsibility for the integrity of the data and accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, J., Choi, J.Y., Choi, B.G. et al. Different diabetogenic effect of statins according to intensity and dose in patients with acute myocardial infarction: a nationwide cohort study. Sci Rep 14, 19438 (2024). https://doi.org/10.1038/s41598-024-67585-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67585-7
- Springer Nature Limited