Abstract
The aim of this study is to assess the effectiveness of conventional and two additional functional markers derived from standard cardiac magnetic resonance (CMR) images in detecting the occurrence of late gadolinium enhancement (LGE) in patients with secondary cardiac amyloidosis (CA) related to multiple myeloma (MM). This study retrospectively included 32 patients with preserved ejection fraction (EF) who had MM-CA diagnosed consecutively. Conventional left ventricular (LV) function markers and two additional functional markers, namely myocardial contraction fraction (MCF) and LV long-axis strain (LAS), were obtained using commercial cardiac post-processing software. Logistic regression analyses and receiver operating characteristic (ROC) analysis were performed to evaluate the predictive performances. (1) There were no notable distinctions in clinical features between the LGE+ and LGE− groups, with the exception of a reduced systolic blood pressure in the former (105.60 ± 18.85 mmHg vs. 124.50 ± 20.95 mmHg, P = 0.022). (2) Patients with MM-CA presented with intractable heart failure with preserved ejection fraction (HFpEF). The LVEF in the LGE+ group exhibited a greater reduction (54.27%, IQR 51.59–58.39%) in comparison to the LGE− group (P < 0.05). And MM-CA patients with LGE+ had significantly higher LVMI (90.15 ± 23.69 g/m2), lower MCF (47.39%, IQR 34.28–54.90%), and the LV LAS were more severely damaged (− 9.94 ± 3.42%) than patients with LGE− (all P values < 0.05). (3) The study found that MCF exhibited a significant independent association with LGE, as indicated by an odds ratio of 0.89 (P < 0.05). The cut-off value for MCF was determined to be 64.25% with a 95% confidence interval ranging from 0.758 to 0.983. The sensitivity and specificity of this association were calculated to be 95% and 83%, respectively. MCF is a simple reproducible predict marker of LGE in MM-CA patients. It is a potentially CMR-based method that promise to reduce scan times and costs, and boost the accessibility of CMR.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Multiple myeloma (MM) is the second most common hematologic malignancy, characterized by the abnormal proliferation of clonal plasma cells 1. Pathological, insoluble amyloid produced by monoclonal immunoglobulin light chains in patients with MM can be progressively deposited extracellularly in various organs throughout the body, which can disrupt target organ function and lead to a dysfunction or failure of end organs2. Cardiac involvement carries the worst prognosis and leading causes of death in patients with MM. Without treatment, the median survival time is only six months for MM patients with secondary cardiac amyloidosis (MM-CA) 3.
Between 15 and 30% of individuals diagnosed with MM-CA were frequently subjected to cardiology treatment and erroneously diagnosed with either hypertrophic cardiomyopathy or hypertensive heart disease4. Endomyocardial biopsy (EMB) is considered the gold standard for diagnosing CA due to its high accuracy. However, it is an invasive procedure that carries potential risks such as pericardial perforation and tamponade, arrhythmia, heart block, trauma to the tricuspid valve, puncture of central vessels, arteriovenous fistulae, and pneumothorax5. In clinic, non-invasive techniques including electrocardiogram (ECG), echocardiography, computed tomography (CT) and cardiac magnetic resonance (CMR) are widely used for cardiac disease examination. Among these techniques, CMR is the most stable and provides the best tissue contrast6. With the administration of gadolinium-based contrast agents (GBCAs), the late gadolinium enhancement (LGE) CMR can demonstrate various cardiomyopathies, such as scar, fibrosis, infiltration (e.g., amyloid)7.
Previous studies had reported that LGE CMR is the most accurate predictor of myocardial biopsy confirmed CA, with a diagnostic accuracy of over 90%8. LGE detects patients with CA that other imaging modalities may miss and is highly reproducible in assessing amyloid deposition9. In a large cohort study of patients with CA, Fontana et al.10 showed that LGE on MRI is associated with increased myocardial amyloid infiltration (measured by extracellular volume) and transmural gadolinium enhancement, adding prognostic value to survival. Despite the high accuracy of LGE in detecting CA, there were also studies reported about 20–31% of patients with CA didn’t repent LGE characteristics11. Although LGE is routinely used in clinic, it is generally accepted that patients with severe renal impairment should avoid the use of GBCAs. Diseases associated with gadolinium retention include nephrogenic systemic fibrosis and gadolinium deposition disease. Individuals with allergies may experience allergic reactions, while patients with impaired kidney function may worsen their condition when using contrast agents. About 80% of patients with CA demonstrated severe renal function deficient. Recently, studies of humans and animals have shown gadolinium retention in the brain as well as other organs such as kidney, liver, and bones and have evoked the safety concerns about the application of GBCAs12.Clinicians must carefully weigh the benefits and potential risks of gadolinium-based contrast agents in clinical practice.
The established prognostic value of LGE in patients with CA is widely recognized in academic literature, it would be beneficial to used GBCAs free CMR techniques to predict LGE characteristics in CA patients. CMR-based cine sequences utilize parameters derived from high spatial resolution cine imaging, without the need for additional acquisition time or complex image post-processing. These parameters accurately reflect both the structure and function of the cardiac. Myocardial strain parameters can be used to evaluate the overall function and synchronization of the cardiac, which can aid in the early diagnosis and management of preclinical lesions, myocardial ischemia, non-ischemic disease, and differences in function between individuals or segments of the myocardium13. The myocardial contrast fraction (MCF), which denotes the ratio of stroke volume to myocardial volume14, exhibits a robust association with global longitudinal strain and has demonstrated prognostic relevance across multiple investigations15. Numerous investigations have exhibited the risk stratification and prognostic significance of LGE in diverse cardiac pathologies16,17. While cine sequence-derived parameters and LGE are both useful for patient assessment, the connection between them is not entirely clear and requires further investigation. If parameters derived from CMR cine sequences can accurately predict LGE outcomes, the use of gadolinium-based contrast agents could be avoided in certain patient cohorts. This approach would not only reduce the associated risks for patients but also decrease the financial and temporal costs of imaging. Consequently, it would extend the benefits of advanced imaging techniques to a broader patient population.
The objective of this study is to investigate the potential of two supplementary LV function markers, namely MCF and LV long axis strain (LAS), obtained through contrast-free CMR, to delineate the regional variability of amyloid deposition and forecast conventional LGE in patients with MM-CA. As far as our knowledge extends, our study is the initial one to exhibit the encouraging potential of CMR through MCF as a convenient diagnostic screening method for assessing the heart involvement in patients with MM.
Patients and methods
Study population
Ethical approval was granted by the Institutional Review Board, and Consistent with the 2013 version of the Declaration of Helsinki. This retrospective study analysed patients with biopsy-proven amyloidosis and overt cardiac involvement or patients with typical clinical and imaging manifestations of myocardial amyloidosis but without biopsy between April 2018 and October 2022. The diagnosis of amyloidosis was established through the utilization of Congo red staining and immunohistochemistry on subendocardial or extracardiac tissue biopsy, which revealed a distinct green–yellow–orange appearance when viewed under polarized light. CA was identified through the use of echocardiographic and CMR techniques, with a specific criterion of interventricular thickness exceeding 12 mm in the absence of any other discernible causes such as increased wall thickness or the presence of a typical LGE pattern, either transmural or subendocardial in nature18,19. MM confirmed by bone marrow biopsy pathology. Most patients had confirmed amyloid deposition by myocardial or extracardiac biopsy (heart [n = 2], kidney [n = 13], tongue [n = 3], abdominal fat [n = 8] and non-pathologically confirmed but with characteristic CMR findings [n = 20]. Following the exclusion of patients with undiagnosed MM via bone marrow aspiration [n = 16], as well as those without cardiac involvement [n = 11], those with poor image quality [n = 1], poorly controlled hypertension [n = 2], myocardial infarction [n = 4], and left ventricular ejection fraction (LVEF) levels below 50% [n = 7], a total of 32 consecutive patients with MM-CA (21 men and 11 women; mean age 62.03 ± 10.06 years [range 46–85]) were ultimately enrolled in the study (Fig. 1). All subjects underwent CMR examination.
CMR protocal
The supine position was utilized to examine all participants via a 3.0 T magnetic resonance imaging scanner (Discovery MR750W; GE Healthcare, Milwaukee, USA), which was equipped with an eight-channel phased-array cardiac coil and incorporated electrocardiogram triggering and respiratory gating technology. Acquisition of data was performed during the end-expiratory breath-hold. Consecutive LV short-axis slices, spanning from the apex to the base, as well as two- and four-chamber long-axis views were obtained for each patient using unenhanced cine steady state free precession (SSFP) sequences. An acquisition of eight to ten consecutive sections in the short-axis view was performed, spanning from the LV apex to the mitral valve. The imaging parameters used were TR 3.9 ms, TE 1.6 ms, matrix 256 × 256, FOV 38 × 38 cm, flip angle 55°, slice thickness 6 mm, and slice spacing 0 mm. The inversion recovery gradient echo sequence was utilized to perform LGE imaging 5–8 min following the intravenous administration of gadobutrol (GadavistⓇ, Bayer Schering Pharma AG, Germany). The imaging parameters utilized were as follows: TR 6.8 ms, TE 3.2 ms, TI 180–300 ms, matrix 256 × 256, FOV 38 × 38 cm, flip angle 20°, slice thickness 6 mm, and slice space 0 mm.
Imaging analyses
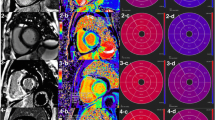
CMR images were initially processed using CVI42 software. The multiplanar long-axis functional module was employed to delineate the endocardial and epicardial boundaries of the left ventricle in both two- and four-chamber cine images. An automated tracking technique was utilized to extract critical variables pertinent to the structural and functional integrity of the left ventricle, as illustrated in Fig. 2A–D. These variables encompassed the end-diastolic volume index (EDVI), end-systolic volume index (ESVI), stroke volume index (SVI), left ventricular ejection fraction (LVEF), cardiac output (CO), cardiac index (CI), left ventricle mass index (LVMI), and LV longitudinal strain (LV LAS). MCF was quantified as the ratio of LV stroke volume to myocardial volume20. LGE images underwent a visual assessment to ascertain the presence or absence of delayed enhancement, along with its pattern. This assessment was performed by a diagnostic imaging resident with four years of experience, supervised by an attending physician with six years of experience in the field. Based on the LGE findings, patients were stratified into two groups: LGE-positive (LGE+) and LGE-negative (LGE−).
LV LAS post-processing diagram and typical CMR and electrocardiogram findings in patients with MM-CA. (A–D) At the end-diastolic and end-systolic phases of the left ventricle, the length of the shortening of the midpoint of the epicardial apical boundary and the beginning of the anteromedial and posterolateral lobes of the mitral valve was measured, and the LAS of the two and four chambers during myocardial contraction was evaluated. (E–G) MM-CA patient with LGE-positive (E: Left ventricular myocardial homogeneous thickening; F: Diffuse subendocardial circumferential and partial myocardial transmural enhancement of the left ventricle at the short-axis level of the LGE sequence; G: electrocardiogram: low voltage in limb leads). (H–J) MM-CA patient with LGE-negative (H: Left ventricular myocardial homogeneous thickening; I: Absence of enhancement in LGE sequences; J: low voltage in limb leads).
Intra- and inter-observer variability
The present study evaluated the intra-observer and inter-observer variability of MCF and LV LAS through independent assessments conducted by two readers (MY. H., YP. S.) across all subjects. The inter-observer variability was determined by comparing the values obtained by two distinct observers, while the intra-observer variability was determined by the same observer repeating the measurement after a one-month interval.
Statistical analyses
The statistical analysis was conducted utilizing SPSS version 25.0 (IBM Corporation, Armonk, New York) and the R programming language (R Foundation for Statistical Computing, Vienna, Austria). The data was subjected to the Kolmogorov–Smirnov normality test to examine its normality. Descriptive statistics were presented as mean ± SD for normally distributed data and median (interquartile range) for non-normally distributed data. Proportions were expressed as percentages. The Student’s t-test analyzed parametric data, while the Mann–Whitney U test examined nonparametric data. Proportional analyses utilized the chi-square or Fisher exact test, contingent on appropriateness. Logistic regression ascertained the predictive power of cardiac parameters for LGE outcomes. CMR parameter efficacy in prognosticating positive LGE was evaluated via receiver operating characteristics (ROC) analysis, computing the area under curve (AUC). The Jorden index identified the optimal threshold, with sensitivity and specificity determined thereafter. Statistical significance was established at P < 0.05.
Ethics approval
The studies involving human participants were reviewed and approved by the Institutional Review Board of the Second Affiliated Hospital of Nanchang University. Since data were evaluated retrospectively, pseudonymously and were solely obtained for treatment purposes, a requirement of informed consent was waived by the Institutional Review Board of the Second Affiliated Hospital of Nanchang University. All methods were performed in accordance with the relevant guidelines and regulations.
Results
Baseline characteristics
Table 1 presents the baseline characteristics of 32 patients diagnosed with MM-CA. The mean age of the patient cohort was 62 years ± 10 (range, 46–85 years), with 21 of the patients being male. Based on the results of LGE, the MM-CA patients were stratified into two subgroups: LGE+ and LGE−. No statistically significant differences were observed in age, sex, and body mass index (BMI) between LGE+ subjects and LGE− patients (all P values > 0.05). With the exception of lower systolic blood pressure in the LGE+ group (105.60 ± 18.85 mmHg), there were no significant differences in clinical characteristics between the LGE+ and LGE− subgroups.
Intra-observer and inter-observer variability
The present study found a high level of agreement among both intra- and inter-observer groups for MCF and LV LAS measurements. Specifically, the intra-class correlation coefficient values for MCF and LV LAS in the intra-observer analysis were 0.981 and 0.955, respectively. Similarly, the intra-class correlation coefficient values for MCF and LV LAS in the inter-observer analysis were 0.975 and 0.906, respectively.
Functional and structural metrics derived from CMR
The mean values of LV functional and structural parameters were measured in all MM-CA patients (LVEF 57.26%; LVMI 76.48 g/m2; LAS − 12.20%; MCF 58.95%). Patients diagnosed with MM-CA exhibited symptoms of intractable heart failure with preserved ejection fraction (HFpEF). Among the subgroups of MM-CA patients (as illustrated in Fig. 2E–J), those in the LGE+ group displayed a further reduction in LVEF (54.27%, IQR 51.59–58.39%) and an increase in LVMI (90.15 ± 23.69 g/m2), along with more pronounced impairment of LV LAS (− 9.94 ± 3.42%) and MCF (47.39%, IQR 34.28–54.90%) (all P values < 0.05) (refer to Table 2).
The value of imaging markers to predict LGE
All imaging metrics derived from CMR demonstrated predictability of LGE through simple logistic regression analysis, as presented in Table 3. In the multivariable logistic regression analysis of LVMI, LVEF, and LV LAS, solely MCF (odds ratio [OR]: 0.89; 95% CI 0.80–1.00; P = 0.046) demonstrated independent predictability of LGE in patients with MM-CA.
Diagnostic accuracy of imaging marker
To determine the optimal diagnostic threshold for distinguishing LGE+ from LGE− in patients with MM-CA, ROC curves were utilized for LVMI, LVEF, LAS, and MCF. Among these parameters, MCF exhibited the highest discriminative performance, as evidenced by its area under the receiver operating characteristic curve (AUC) of 0.913, surpassing that of LAS (AUC = 0.858), LVMI (AUC = 0.813), and LVEF (AUC = 0.779) (refer to Fig. 3 and Table 4).
Discussion
This study used LAS, a new post-processing method of CMR cine images, to assess the function of left ventricle. Compared to traditional strain measurement techniques, this approach is simpler and faster. Our results showed the potential of functional parameters derived from CMR cine images such as MCF and LAS of left ventricle were significantly different between LGE+ and LGE− patients and can be used to predict the results of LGE. It would be beneficial for patients who were contradict to gadolinium-based contrast. Furthermore, our results demonstrate that MCF exhibits a significantly superior discriminatory capacity compared to LAS, LVMI, and LVEF. Multivariate analyses revealed that MCF was the only independent marker for predicting LGE. Therefore, MCF is a viable tool for predicting the outcome of LGE sequence.
MM is a malignancy of the hematologic system that affects various systems. The term MM-associated CA pertains to a cluster of infrequent illnesses that exhibit the extracellular deposition of amyloid fibrils composed of immunoglobulin light chain in cardiomyocytes21. Although echocardiography is commonly used as the initial diagnostic tool, CMR is presently considered the benchmark for assessing cardiac anatomy and function due to its exceptional stability and reproducibility, superior spatial resolution, and lack of ionizing radiation, rendering it a comprehensive and non-invasive method for evaluating both cardiac structure and function8. According to experts, CMR is a viable option for patients who exhibit suspected CA secondary to MM22, particularly those who present with diffuse LV hypertrophy and reduced myocardial compliance as observed through cine sequences, as well as transmural or diffuse subendocardial circumferential enhancement as seen in LGE sequences23. However, CA patients have a high incidence of renal amyloidosis. In addition, there has recently been a broader focus on brain deposition following the use of gadolinium contrast agents 24. Recent research indicates that qualitative LGE assessment may fail to detect early cardiac involvement in amyloidosis25. However, certain contrast-free quantitative parameters have the potential to identify possible early cardiac involvement in LGE− patients. Consequently, there is a growing interest in non-contrast alternatives for diagnosis24,26. The findings of the present investigation indicate that certain parameters that don’t require contrast administration can not only discern the existence or non-existence of myocardial involvement in individuals with multiple myeloma, but also function as substantial predictors of LGE. However, only MCF emerged as an autonomous predictor of late gadolinium enhancement. These contrast-free parameters furnish evidence-based guidance that empowers clinicians to promptly diagnose this infrequent ailment and furnish precise staging and prognostic recommendations.
The deposition of amyloid fibrils is the primary cause of LV hypertrophy, which is the most common imaging manifestation of CA, despite the absence of hypertrophic cells27. The disease is characterized by a progressive nature, with initial stages exhibiting minimal pathological protein accumulation. The extent of myocardial hypertrophy is directly proportional to the degree of myocardial infiltration28. CMR analysis of LVMI furnishes precise quantitative data on the structural characteristics of LV. Several prior investigations have demonstrated that LVMI serves as a dependable prognostic marker for survival in individuals afflicted with CA, with elevated LVMI levels portending a poorer prognosis29. The findings of our study suggest that patients with MM and myocardial involvement exhibit a higher LVMI. Furthermore, our results indicate that LVMI values exceeding 83.85 g/m2 are more effective in predicting positive LGE. The elevated LVMI observed in patients with CA may be attributed to LV remodelling and interstitial cardiac amyloid deposition, resulting in fibrosis25. LGE− patients with higher LVMI may indicate early amyloid deposition, as LGE demonstrated negative results in cases where amyloid deposition was sparse. With disease progression, the gradual increase in LVMI and the progression to LGE positivity in CA patients is directly proportional to the amount of amyloid deposition.
LVEF is a conventional measure utilized to evaluate LV function. In cases of CA, amyloid deposition results in an augmented extracellular space and cardiac stiffening, which leads to restrictive pathophysiology without compensatory dilation. Patients with CA typically exhibit reduced SV and LVEDV. Despite impaired myocardial shortening and systolic function, the LVEF, which is derived from the ratio of SV to LVEDV, typically remains within normal or near-normal limits. The manifestation of established cardiac decompensation symptoms and indicators frequently precedes amyloidosis, resulting in a significant decline in LVEF. The measurement of LVEF in isolation is an inadequate depiction of intricate myocardial contractility patterns and lacks sensitivity towards subtle alterations in myocardial function that typically arise during the later stages of the disease progression. The LVEF serves as a gauge for the comprehensive left ventricular function, encompassing both systolic and diastolic myocardial function, but lacks the precision to detect specific areas of myocardial damage. In a study conducted by Nisha Arenja et al.15, it was determined that LVEF exhibited superior discriminatory capacity compared to LAS or MCF in patients diagnosed with non-ischemic dilated cardiomyopathy (NIDCM). In contrast, our research indicates that both LAS and MCF exhibit greater efficacy in detecting myocardial involvement in MM patients compared to LVEF. This observation may be attributed, in part, to the fact that diminished LVEF served as the primary inclusion criterion for NIDCM patients. Notably, heart failure with reduced ejection fraction constitutes approximately 50% of all heart failure cases 30, with diastolic dysfunction in CA patients and normal systolic ejection fraction until advanced disease. As such, most CA patients not only exhibit early ejection fraction preservation but also HFpEF even in the advanced stage of heart failure31. Consequently, our findings demonstrate that LVEF exhibits lower efficacy compared to LVMI, LV LAS, and MCF in detecting myocardial involvement among individuals diagnosed with MM.
The identification of impaired systolic function resulting from cardiomyocyte dysfunction can be accomplished through the utilization of measurements of myocardial shortening, such as MCF and strain32. In contemporary times, the utilization of myocardial strain imaging has emerged as a proficient technique for the quantitative evaluation of cardiovascular function and has gained greater prevalence in clinical settings. This technique enables the measurement of global and local myocardial strain during the initial subclinical stages, thereby facilitating the early and quantitative assessment of myocardial damage. Numerous research studies have established that CMR strain analysis can detect incipient systolic dysfunction in diverse cardiac ailments prior to the manifestation of alterations in LVEF33. Over the past few decades, numerous CMR techniques have been developed that rely on specialized image acquisition and post-processing methods to quantify myocardial motion and deformation. A plethora of published reports exist on conventional tissue tracking myocardial strain techniques, which aid in the diagnosis of cardiac involvement and provide prognostic information for patients at risk of CA19,34,35,36,37,38. The benefits of LV LAS primarily pertain to its post-processing characteristics, which obviate the need for supplementary image acquisition time. The inclusion of additional acquisition time is a crucial factor that impacts the standard clinical data acquisition modality. Furthermore, LV LAS does not necessitate specialized scan sequences and can be employed for CMR routine cine sequences. It enables the quantification of strain across ventricular chambers and is facile to operate, swift, and semi-automated, thereby reducing post-processing time39,40. In the early stages of CA infiltration, LV LAS exhibits a greater likelihood of abnormality when compared to LVEF. The myocardial thickening of myocardial fibres is affected by abnormal amyloid aggregation through direct toxicity, ischemic injury, and increased ECV.
The MCF is defined as the ratio of stroke volume to myocardial volume and can be calculated using imaging techniques such as echocardiography or cardiac magnetic resonance41. MCF represents a volumetric measure of abnormal myocardial shortening, which is independent of chamber size. 20. As shown in our research, MCF is a strong independent predictor of positive LGE. The results of the multivariable ROC curve analysis indicated that the combination of MCF and LVMI had the highest diagnostic value in distinguishing between LGE+ and LGE− cases (AUC = 0.946; 95% CI 0.816–0.997). Which may partly be due to the fact that MCF is a parameter that takes into account both left ventricular structure and function, distinguishing it from several other indicators that only represent one or the other. This approach could improve clinical throughput and improve CMR accessibility by reducing scan time and cost. The concept of MCF bears a strong resemblance to that of myocardial strain. However, whereas myocardial strain gauges the contraction of a specific region of the heart, MCF amalgamates the cumulative contraction data from all directions (namely, longitudinal, radial, and circumferential) into a solitary measurement. The composite measure, MCF, lacks consideration for the timing of myocardial shortening, whereas the strain rate can be computed to determine the timing of strain. Additionally, LVEF, which is the prevailing index of LV systolic function in clinical practice, is reliant on stroke volume. When determining LV ejection volume, the output per beat is divided by LVEDV instead of myocardial volume. In cases where there is an increase in wall thickness or a decrease in LV dimensions, the evaluation of systolic function through LVEF may obscure the presence of significant impairment. Therefore, the assessment of LVEF may be misleading in detecting impaired systolic function in the presence of increased wall thickness or decreased LV size42.
Study limitations
This study has some limitations, such as its single-centre design, relatively small sample size, and inclusion of only 32 patients with biopsy-proven amyloidosis involving both myocardial and extracardiac tissues. It should be noted that MM is a rare disease. In 2018, the age-standardized incidence rate for multiple myeloma was 1.1 per 1000. Moreover, the study did not exclude patients with hypertension and diabetes mellitus, which could have potentially confounded the results due to left ventricular hypertrophy and fibrosis. However, only a small subset of hypertensive and diabetic individuals were included, and no significant differences were observed within the subgroups. Furthermore, the hypertension and diabetes mellitus statuses of these participants were well controlled, reflecting more realistic clinical scenarios. Lastly, our study was limited by the lack of baseline CMR data for our patients, and the possible impact of induction chemotherapy on LV function. Nevertheless, we were able to use baseline echocardiographic data to rule out the possibility of chemotherapy-induced cardiac dysfunction.
Conclusion
This study has shown that LV LAS and MCF have the potential to serve as non-contrast agent alternatives to LGE for tissue characterization in patients with AL-CA, thereby reducing the need for contrast agents in a significant proportion of CMR scans. While these novel contrast-free parameters cannot replace the LGE sequence of CMR or the gold standard EMB, they can have clinical relevance by aiding in further confirmatory examinations or even EMB, particularly in patients with contraindications to contrast.
Data availability
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
References
Röllig, C., Knop, S. & Bornhäuser, M. Multiple myeloma. Lancet 385(9983), 2197–2208 (2015).
Kirichenko, Y. Y. et al. Case report: AL amyloidosis severe restrictive cardiomyopathy associated with multiple myeloma-diagnostic difficulties. Front. Cardiovasc. Med. 9, 862409 (2022).
Gertz, M. A. & Dispenzieri, A. Systemic amyloidosis recognition, prognosis, and therapy: a systematic review. Jama J. Am. Med. Assoc. 324(1), 79–89 (2020).
Bahlis, N. J. & Lazarus, H. M. Multiple myeloma-associated AL amyloidosis: Is a distinctive therapeutic approach warranted?. Bone Marrow Transpl. 38(1), 7–15 (2006).
Elliott, P. & Arbustini, E. The role of endomyocardial biopsy in the management of cardiovascular disease: A commentary on joint AHA/ACC/ESC guidelines. Heart 95(9), 759–760 (2009).
Chacko, L. et al. Cardiac amyloidosis: Updates in imaging. Curr. Cardiol. Rep. 21(9), 108 (2019).
Carvalho, F. P., Erthal, F. & Azevedo, C. F. The role of cardiac MR imaging in the assessment of patients with cardiac amyloidosis. Magn. Reson. Imaging Clin. N. Am. 27(3), 453–463 (2019).
Chatzantonis, G. et al. Diagnostic value of cardiovascular magnetic resonance in comparison to endomyocardial biopsy in cardiac amyloidosis: A multi-centre study. Clin. Res. Cardiol. 110, 555–568 (2020).
Chatzantonis, G. et al. Diagnostic value of cardiovascular magnetic resonance in comparison to endomyocardial biopsy in cardiac amyloidosis: A multi-centre study. Clin. Res. Cardiol. 110(4), 555–568 (2021).
Fontana, M. et al. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 132(16), 1570–1579 (2015).
Perugini, E. et al. Non-invasive evaluation of the myocardial substrate of cardiac amyloidosis by gadolinium cardiac magnetic resonance. Heart 92(3), 343–349 (2006).
Gulani, V. et al. Gadolinium deposition in the brain: Summary of evidence and recommendations. Lancet Neurol. 16(7), 564–570 (2017).
Pedrizzetti, G. et al. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J. Cardiovasc. Magn. Reson. 18(1), 51 (2016).
Tendler, A. et al. The myocardial contraction fraction is superior to ejection fraction in predicting survival in patients with AL cardiac amyloidosis. Amyloid 22(1), 61–66 (2015).
Arenja, N. et al. Diagnostic and prognostic value of long-axis strain and myocardial contraction fraction using standard cardiovascular MR imaging in patients with nonischemic dilated cardiomyopathies. Radiology 283(3), 681–691 (2017).
Stevenson, A. et al. Prognostic value of late gadolinium enhancement detected on cardiac magnetic resonance in cardiac sarcoidosis. JACC Cardiovasc. Imaging 16, 345–357 (2022).
Georgiopoulos, G. et al. Comparison of demographic, clinical, biochemical, and imaging findings in hypertrophic cardiomyopathy prognosis: A network meta-analysis. JACC Heart Fail. 11(1), 30–41 (2023).
Falk, R. H. Diagnosis and management of the cardiac amyloidoses. Circulation 112(13), 2047–2060 (2005).
Wan, K. et al. Left ventricular myocardial deformation on cine MR images: Relationship to severity of disease and prognosis in light-chain amyloidosis. Radiology 288(1), 73–80 (2018).
Matthews, S. D. et al. Myocardial contraction fraction: A volumetric measure of myocardial shortening analogous to strain. J. Am. Coll. Cardiol. 71(2), 255–256 (2018).
Biliński, J., Biernacka, E. K. & Januszewicz, A. Cardiac amyloidosis secondary to multiple myeloma: Successful sequential cardiac and autologous stem cell transplantations with excellent outcome. Eur. Heart J. 42(36), 3803 (2021).
van de Donk, N., Pawlyn, C. & Yong, K. L. Multiple myeloma. Lancet 397(10272), 410–427 (2021).
Giusca, S. et al. Multi-parametric assessment of left ventricular hypertrophy using late gadolinium enhancement, T1 mapping and strain-encoded cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 23(1), 92 (2021).
Baggiano, A. et al. Noncontrast magnetic resonance for the diagnosis of cardiac amyloidosis. JACC Cardiovasc. Imaging 13(1 Pt 1), 69–80 (2020).
Kuetting, D. L. et al. Quantitative assessment of systolic and diastolic function in patients with LGE negative systemic amyloidosis using CMR. Int. J. Cardiol. 232, 336–341 (2017).
Li, X. et al. Nonenhanced chemical exchange saturation transfer cardiac magnetic resonance imaging in patients with amyloid light-chain amyloidosis. J. Magn. Reson. Imaging 55(2), 567–576 (2022).
Ardehali, H. et al. Endomyocardial biopsy plays a role in diagnosing patients with unexplained cardiomyopathy. Am. Heart J. 147(5), 919–923 (2004).
Martini, N. et al. Deep learning to diagnose cardiac amyloidosis from cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 22(1), 84 (2020).
Huang, H. et al. Independent prognostic value of left ventricular mass index in patients with light-chain amyloidosis. Acta Cardiol. 77(9), 815–821 (2022).
Hahn, V. S. et al. Myocardial gene expression signatures in human heart failure with preserved ejection fraction. Circulation 143(2), 120–134 (2021).
Ritts, A. J. et al. Current concepts of cardiac amyloidosis: Diagnosis, clinical management, and the need for collaboration. Heart Fail Clin. 13(2), 409–416 (2017).
Arenja, N. et al. Myocardial contraction fraction derived from cardiovascular magnetic resonance cine images-reference values and performance in patients with heart failure and left ventricular hypertrophy. Eur. Heart J. Cardiovasc. Imaging 18(12), 1414–1422 (2017).
Hor, K. N. et al. Circumferential strain analysis identifies strata of cardiomyopathy in Duchenne muscular dystrophy: A cardiac magnetic resonance tagging study. J. Am. Coll. Cardiol. 53(14), 1204–1210 (2009).
Bhatti, S. et al. Myocardial strain pattern in patients with cardiac amyloidosis secondary to multiple myeloma: A cardiac MRI feature tracking study. Int. J. Cardiovasc. Imaging 34(1), 27–33 (2018).
Wu, Y. et al. A risk score to diagnose cardiac involvement and provide prognosis information in patients at risk of cardiac light-chain amyloidosis. Front. Cardiovasc. Med. 9, 817456 (2022).
Williams, L. K. et al. Patterns of CMR measured longitudinal strain and its association with late gadolinium enhancement in patients with cardiac amyloidosis and its mimics. J. Cardiovasc. Magn. Reson. 19(1), 61 (2017).
Liu, H. et al. The prognostic value of right ventricular deformation derived from cardiac magnetic resonance tissue tracking for all-cause mortality in light-chain amyloidosis patients. Cardiovasc. Diagn. Ther. 10(2), 161–172 (2020).
Li, X. et al. Left and right ventricular myocardial deformation and late gadolinium enhancement: Incremental prognostic value in amyloid light-chain amyloidosis. Cardiovasc. Diagn. Ther. 10(3), 470–480 (2020).
Leng, S. et al. Fast long-axis strain: A simple, automatic approach for assessing left ventricular longitudinal function with cine cardiovascular magnetic resonance. Eur. Radiol. 30(7), 3672–3683 (2020).
Riffel, J. H. et al. Fast assessment of long axis strain with standard cardiovascular magnetic resonance: A validation study of a novel parameter with reference values. J. Cardiovasc. Magn. Reson. 17(1), 69 (2015).
Maurer, M. S. & Packer, M. How should physicians assess myocardial contraction? Redefining heart failure with a preserved ejection fraction. JACC Cardiovasc. Imaging 13(3), 873–878 (2020).
Knight, D. S. et al. Cardiac structural and functional consequences of amyloid deposition by cardiac magnetic resonance and echocardiography and their prognostic roles. JACC Cardiovasc. Imaging 12(5), 823–833 (2019).
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 82260342, 81860316); Postgraduate Innovation Fund of Jiangxi Province (YC2023-B084).
Author information
Authors and Affiliations
Contributions
(1) Conception and design: H.Y., L.G.; (2) Administrative support: L.G.; (3) Provision of study materials or patients: C.Y., J.W., W.Z.; (4) Collection and assembly of data: A.K., P.Y.; (5) Data analysis and interpretation: L.X., J.D.; (6) Manuscript writing: All authors; (7) Final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Competing interests
Jiankun Dai reports a relationship with Clinical and Technical Support, GE Healthcare, Beijing, China that includes: employment. Other remaining authors has no conflict of interest to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, M., Song, Y., Yang, C. et al. The value of myocardial contraction fraction and long-axis strain to predict late gadolinium enhancement in multiple myeloma patients with secondary cardiac amyloidosis. Sci Rep 14, 16832 (2024). https://doi.org/10.1038/s41598-024-67544-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67544-2
- Springer Nature Limited