Abstract
The biology of extinct animals is usually reconstructed from external morphological characters and comparison with present-day analogues. Internal soft organs are very rarely preserved in fossils and require high-tech approaches for visualization. Here, we report the internal anatomy of a female and male of the ~ 162 Myr-old lobster Eryma ventrosum from the Jurassic La Voulte-sur-Rhône Konservat-Lagerstätte in France using X-ray synchrotron tomography. The Erymidae is an extinct, species-rich, widespread and ecologically important Mesozoic family of decapod crustaceans. Our investigation revealed the anatomy of the locomotory, respiratory, circulatory, excretory, digestive, nervous and sensory, and reproductive systems at a resolution resembling low-magnification histology. Particularly notable is the detailed preservation of the small brain and the fragile hepatopancreas, the main metabolic organ of decapods that decays rapidly post-mortem. The remarkable preservation shows that the internal anatomy of Eryma ventrosum is closer to that of Nephropidae (clawed lobsters) than Astacidae (freshwater crayfish), their closest living relatives based on skeletal morphology. The microanatomy of the gonads and hepatopancreas indicates that the two specimens investigated were a young, well-nourished female and male prior to sexual maturity. The analysis of the soft anatomy reveals remarkable conservatism over 160 Myr and offers new insights into feeding, reproduction, life history and lifestyle of an important component of the macrozoobenthos of Middle Jurassic seas.
Similar content being viewed by others
Introduction
The remarkable ~ 162 Myr-old (Callovian) La Voulte-sur-Rhône Lagerstätte in southeastern France opens a unique window on the animals inhabiting deep marine settings during the Middle Jurassic1,2. The biota is dominated by arthropods (crustaceans, thylacocephalans, pycnogonids3), cephalopods4 and echinoderms (ophiuroids, asteroids5), all preserving soft-tissues.
The exceptional preservation is the result of early precipitation of fluorapatite, pyrite and other sulfide minerals, accompanied by Mg-calcite cementation which led to the formation of concretions. Different minerals replicated fine anatomical details of different tissues in three dimensions in the arthropods6,7. Recent studies of La Voulte fossils using CT-scan microtomography and X-ray synchrotron tomography have significantly advanced our knowledge of the reproductive strategies in polychelidan lobsters8,9, and the vision and predatory lifestyle of thylacocephalans10.
Here we describe two specimens of the lobster Eryma ventrosum (Decapoda, Erymidae) preserved in carbonate concretions from La Voulte (Fig. 1). Erymid lobsters were previously known only from the subcylindrical carapace, which is characterized by a dorsal intercalated plate in the cephalic region, the massive claws on the first thoracic limb, and an elongate pleon. They are most diverse during the Jurassic and Cretaceous11,12. The only known Palaeozoic representative was originally described as Protoclytiopsis antiqua Birshtein, 1958 from the latest Permian (Changhsingian, 253 Myr) of Russia, reassigned to Eryma (as E. antiquum)13.
Historical specimens of Eryma ventrosum enclosed in carbonate nodules from La Voulte. (a) Dorsal view of male OSUG-ID11543. (b,c) Lateral and dorsal views of female OSUG-IDID11544. (d) Lateral view of carapace MNHN.F.A59527, with large eye and e, close-up view of ommatidia framework. (f–i) 3D reconstruction of female carapace and pleon with line drawings of carapace groove pattern (lateral and dorsal views). (j) Lateral view of large specimen OSUG-ID11906, with first pereiopods. a branchiocardiac groove, b antennal groove, b1 hepatic groove, c postcervical groove, d gastro-orbital groove, e1e cervical groove, i inferior groove, P1 first pereiopod.
The relationships of fossil decapods are difficult to unravel as they rarely preserve the soft-tissue morphology (and, needless to say, genomic data are lacking). A phylogenetic analysis based on morphological characters of the exoskeleton alone, and focused on erymids, placed them as an extinct sister clade to the Infraorder Astacidea, i.e., living lobsters and crayfish, the most economically important group of crustaceans including the familiar Nephrops (the Norway lobster), Homarus (commercial lobsters) and Astacus (freshwater crayfish)14. This phylogeny reveals that erymoids are characterized by a dorsal intercalated plate in the carapace, and the presence of a bulge at the base of the pleura14. The non-erymoid Astacidea are united by features of the carapace: the cervical groove is interrupted in the gastric region; there is a junction between postcervical and branchiocardiac grooves; and the ventral extremity of the branchiocardiac groove is joined to the postcervical groove (characters 2, 9 and 13 listed in14; Fig. 2).
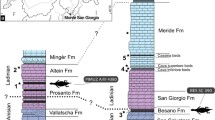
Simplified phylogeny of erymid lobsters and sister groups (after15,16) with a time line showing stratigraphic ranges of taxa. Time-scaled tree generated with the R package strap56. Occurrence data (bold lines) for each taxon were recovered from the literature. Time was scaled using an equal share method57. Line drawings illustrate the main synapomorphies at relevant nodes.
A total evidence phylogenetic analysis (combining morphologic and genomic data) of living lobsters15 placed the origin of Astacidea in the earliest Permian at 297.48 Myr. This implies that they and their sister Erymoidea, which ranges from the Late Permian (253 Myr) to the Paleocene (60 Myr), originated some 44 Myr earlier than the oldest known fossil example12. The extinct clade Erymoidea includes 81 species in 6 genera, which are important components of Mesozoic assemblages because of their abundance and worldwide distribution12,14. Understanding the evolutionary history of Erymoidea is thus critical to interpreting the evolution of modern Astacidea, particularly their adaptive capability and factors that could lead to their extinction.
The internal anatomy of fossil decapods is rarely preserved17,18. The unusual taphonomic conditions of the La Voulte-sur-Rhône Lagerstätte, however, have yielded details of the internal anatomy of a number of decapods, including elements of the digestive tract, musculature and ovary of Polychelida lobsters8,9, as well as the muscles, digestive tract, gills, nerve chord and heart of dendrobranchiate shrimps6. Our investigation of two specimens of Eryma ventrosum collected near the end of the nineteenth century and held by the Université Grenoble Alpes in France revealed unprecedented details of the anatomy of erymids using X-ray computed microtomography and propagation phase-contrast synchrotron X-ray tomography. Muscle tissue has been noted previously in E. ventrosum16, without further comment, but the new information includes details of the brain, statocysts, antennal gland, heart and elements of the circulatory system, hepatopancreas and reproductive system.
This new evidence has permitted the first reconstruction of the internal anatomy of this extinct group, including all the major organ systems (Figs. 3, 4, 5; Supplementary Figs. S1–S4; Supplementary Videos S1, S2). The preservation of several features is so detailed that the results resemble those of low-magnification histological sections. Even organs highly prone to decay, such as the hepatopancreas and brain, are evident. Furthermore, we can identify one specimen as female and the other male based on the reproductive system allowing us to confirm the presence of secondary sex characteristics of the exoskeleton.
3D-reconstruction of the exoskeleton and musculature of Eryma ventrosum. (a) Longitudinal view of female with the right half of the exoskeleton removed: cuticle in grey and muscles in red (first pair of chelipeds not preserved). (b) Antero-dorsal view of paired mandible adductors and mandibles. (c) Ventral view of male exoskeleton. (d) View of right second cheliped showing muscle bundles. (e) Dorsal view of left first cheliped showing muscle bundles attached to apodemes. (f) Details of fifth pereiopods with chela in female and terminal dactylus in male. (g) Dorsal view of female endophragmal skeleton. (h) Lateral view of female endophragmal skeleton and overlying gills. (i) Ventral view of male posterior exoskeleton showing gonopores at the base of fifth pereiopods and fused gonopods. (j) Ventral view of female posterior exoskeleton showing gonopores at the base of third pereiopods and spermatheca. P1–P5, pereiopods 1 to 5.
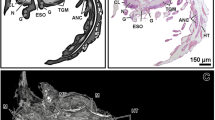
Female specimen of Eryma ventrosum: 3D-reconstruction and X-ray images of internal soft organs. (a) Inner view of stomach above mandibles and oesophagus surrounded by connectives. (b) Lateral view of entire digestive system plus antennal gland in anterior part, and heart and ovaries in posterodorsal part. (c) Dorsal view with exoskeleton removed to show digestive, excretory, respiratory, circulatory and reproductive systems in position. (d) Dorsal view of ovaries full of oocytes. (e) Transverse section of hindgut with folds. (f) Transverse section of basal articles of antennulae with statocysts. (g) Sagittal section of heart. (h) Sagittal section of left ovarian strand and distal extremity of hepatopancreas lobe. (i) Sagittal section of antennal gland. (j) Sagittal section of branchial chamber with gills. (k) Transverse section of cardiac stomach with gastric mill and oesophagus. (l) Transverse section of hepatopancreas lobes. Mco mesocardiac ossicle, Sm sphincter muscles, Uco urocardiac ossicle, Zco zygocardiac ossicle.
Male specimen of Eryma ventrosum: 3D-reconstruction and X-ray images of internal soft organs. (a) Dorsal view with exoskeleton removed to show soft parts. (b) Transverse section of pyloric ampullae. (c) Horizontal section of heart. (d) Transverse section of heart. (e) Reconstruction of heart with muscles (visible through transparency) running in different directions. (f,g) Anterior and lateral views of testis and vas deferens. (h) Transverse section of basal articles of antennulae with their statocysts. (i,j) Dorsal and anterior views of brain. (k) Transverse section of brain. (l) Transverse section of hepatopancreas lobes. (m) Transverse section of hindgut with folds. (n) Sagittal section of hindgut and anus. (o) Transverse section of testis. (p) Horizontal section of testis revealing H-shaped morphology. (q) Transverse section of vas deferens under testis. P1–P5 pereiopods 1 to 5.
Results
Exoskeleton
The subcylindrical carapace of both specimens displays the fusiform dorsal intercalated plate, groove pattern and densely tuberculate ornamentation diagnostic of Eryma ventrosum (Fig. 1; Supplementary Figs. S3, S4). The rounded stalked eyes display numerous square ommatidia (Fig. 1d, e; Supplementary Fig. S3d). The antennae are wider and longer than the antennulae (Fig. 3c; Supplementary Figs. S3e, S4). The basal articles of the antennulae of both specimens contain a cuticle-lined chamber with a dorsal slit-like opening (Fig. 4f; Supplementary Fig. S1c). These are interpreted as statocysts and reveal statoliths (Fig. 5h). The wide subtriangular mandibles bearing three-segmented palps insert behind the epistome (Fig. 3b; Supplementary Fig. S3d). The first and second maxillae are similar in length, the latter foliate with a lateral scaphognathite (Supplementary Figs. S1e, S3e). A grid-like structure is present in the sternite of the second maxilla (Fig. 3g; Supplementary Fig. S1g). The maxillipeds increase in size from anterior to posterior (Supplementary Figs. S1a, S3e). Pereiopods 1 to 3 are chelate, the first being massive (Fig. 5a; Supplementary Figs. S3, S4). Pereiopod 4 bears a dactylus, whereas pereiopod 5 bears a slightly curved terminal dactylus in the male but a small chela in female (Fig. 3f). The pleopods are biramous except the first pleopods of the male, which are modified to form a uniramous, folded structure corresponding to gonopods (Fig. 3i). The endophragmal skeleton is complex, tapering posteriorly, with well-developed ventral arthrodial cavities, and lateral branchial openings at the base of the pleurites (Fig. 3g, h; Supplementary Fig. S4d). The ventral region of the sternum at pereiopods 4 and 5 (thoracic segments 7 and 8) of the female is modified into an internal, cuticular spermatheca (Fig. 3j; Supplementary Fig. S1a, h). The opening consists of an anterior suture along the longitudinal midline of the sternum and a posterior cup with two oblique lateral notches.
Musculature
Individual muscle strands are preserved, particularly in the appendages and cephalothorax (Fig. 3a). The antagonistic abductor and adductor muscles are evident in the first pair of chelae of the male (attached to the apodemes) and in the pereiopod chelae (Fig. 3c–e). The muscles of the second maxilla arise from the grid-like structure on the corresponding sternite. The most conspicuous muscles preserved in the cephalothorax are the paired mandible adductors, which arise from broad attachment sites on the inner surface of the carapace and run behind the stomach (Figs. 3b, 4l, 5b; Supplementary Fig. S1a). Stomach muscles, which served to move the gastric mill and to form food boluses, are clearly evident dorsally (Fig. 4k; Supplementary Fig. S1d). The most obvious muscles in the pleon are the deep flexors, which are better preserved in the female (Fig. 3a; Supplementary Fig. S1a, f).
Respiratory system
The gills are particularly well preserved in the female, including individual branchiae at the base and lateral filaments at the distal end (Figs. 3h and 4j). The scaphognathite, which generates the respiratory current, can be traced to its attachment at the base of the second maxilla (Supplementary Fig. S1e).
Circulatory system
The heart is located dorsally in the posterior part of the cephalothorax (Figs. 4b, c and 5a; Supplementary Fig. S2b). Individual muscle strands are evident in the myocardium (Figs. 4g and 5c). The pericardium and funnel-shaped ostia (Fig. 5d), as well as parts of the dorsal pleonal artery, are evident in both male and female (Fig. 4b; Supplementary Fig. S1f).
Excretory system
The paired antennal glands are evident in front of the stomach (Figs. 4b, c, 5a). They consist of a dense crescent-shaped nephridial tubule and labyrinth and a relatively large bladder. The duct between bladder and nephropore, and the sphincter muscle, are revealed in sections (Fig. 4i).
Digestive system
Remarkable details of the digestive system are preserved in both specimens (Figs. 4 and 5; Supplementary Figs. S1, S2; Supplementary video S2). The female shows the mouth and esophagus, including musculature (Supplementary Fig. S1d). The cardiac stomach is evident including the zygocardiac, urocardiac, and mesocardiac ossicles (this last particularly substantial) and teeth of the gastric mill (Fig. 4a–c, k; Supplementary Fig. S1a,d). The gland filter (pyloric ampullae) is evident as a paired structure between the cardiac stomach and hepatopancreas in the male (Fig. 5a, b), in addition to the triangular pyloric ossicle. The filter appears to be displaced dorsally due to separation of the stomach and hepatopancreas (Fig. 4b). The dorso-ventral muscles of the cardio-pyloric valve are preserved in the female. The hepatopancreas is evident in both specimens: each half organ consists of an anterior lobe, a medial lobe that extends dorsally, and a long posterior lobe with tubules and large collecting ducts, similar to those of living decapods (Figs. 4a–c, l and 5a, l; Supplementary Figs. S1a, b, g, S2a). The tubules, which are better preserved in the female, are about 100 µm in diameter and 500 µm long (Fig. 4h). The digestive tract is incompletely preserved but the hindgut can be traced to the anus (Figs. 4b and 5a, n). Transverse sections show six indentations typical of the longitudinal hindgut folds in living crayfish (Figs. 4e and 5m).
Nervous and sensory systems
The brain (supraesophageal ganglion) and the connectives linking it to the commissural ganglion are well preserved in the male (Fig. 5a, i, j; Supplementary Figs. S1d, g, S2a, c, d; Supplementary Video S1). Contrasting areas likely represent the protocerebrum, deutocerebrum and tritocerebrum, and lateral olfactory or accessory lobes (Fig. 5k). The optic and antennal nerves are also evident. The brain is poorly preserved in the female but the connectives are evident running to the region below the endophragmal skeleton, where they form the ventral nerve cord. This cord is evident in the pleon of both male and female where it expands into globular structures interpreted as the ganglia, sometimes showing evidence of the right and left halves (Fig. 4b; Supplementary Fig. S2e).
Reproductive system
The ovaries of the female are evident (length ca 5 mm) dorsally in the rear of the cephalothorax extending into the first pleonite. The two parallel strands flank the posterior part of the hepatopancreas and the midgut (Fig. 4a, c). The oocytes, about 300 in total, are 80–100 µm in diameter (Fig. 4d, h). The oviducts are not preserved, but the gonopores are evident on pereiopod 3 and the spermatheca is preserved in the ventral region of the sternum (Fig. 3j). A distinct structure in the male extends dorsally from the posterior cephalothorax into the anterior pleon (Fig. 5a). Its position, preserved form, and density contrast with other organs favor its interpretation as the testis (Fig. 5o, p; Supplementary Fig. S2a,b). Vas deferens (sperm ducts) are partly preserved (Fig. 5f, g, q; Supplementary Fig. S2b), and small gonopores are evident on pereiopod 5 (Fig. 3i). In addition, the first pleopods are fused in gonopods (Fig. 3i; Supplementary Fig. S2e).
Discussion
The morphology of erymid lobsters known to date was limited to the carapace and massive chelate first pair of thoracic legs, which are commonly fossilised12. The endophragmal skeleton described here reveals an anterior grid-like structure in both male and female which is the muscle attachment of the second maxilla with its scaphognathite that circulates water within the branchial chamber. The structure is larger than in the living lobster Nephrops17 and crayfish Astacus18 but may be linked to a gill-cleaning mechanism such as that in those forms19. The spermatheca in the posterior part of the female endophragmal skeleton is the best-preserved sperm storage structure known in a fossil decapod.
Muscles are preserved attached to the exoskeleton in a variety of fossil arthropods, including decapod crustaceans as old as Devonian (~ 360 Myr)20. They have been used for the analysis of function and to determine taxonomic identity and evolutionary relationships16. Details of the musculature of Eryma are preserved from antenna to pleon, including all the appendages, and are very similar to those of extant clawed lobsters and crayfish. The mandible adductors are particularly complete with large areas of attachment to the carapace as are the well-developed antagonistic muscles in the first and second pairs of male chelae (Supplementary Fig. S2f, g).
Nervous and circulatory systems of marine arthropods are delicate and prone to rapid decay: preservation is generally limited to Cambrian Burgess-Shale type deposits21,22, concretions from Carboniferous Konservat-Lagerstätten23 and Cenozoic amber inclusions24. The organization of the brain, ventral nerve cord with ganglia, and connectives preserved in both Eryma specimens is very similar to that in extant lobsters and crayfish25. The same applies to the cardiovascular system26 including the hepatopancreatic and dorsal pleonal arteries, unknown in other fossil crustaceans. These two systems in particular illustrate anatomical conservatism for more than 160 Myr, in spite of the substantial morphological and ecological diversification of lobsters and crayfish that occurred subsequently15,27.
The presence of excretory pores at the base of the antenna has been surmised in diverse fossil arthropods28, but the preservation of the complete excretory system in the female Eryma is unique. It presumably functioned not only for excretion of metabolic waste but also for communication (e.g., agonistic, male/female recognition)29 via a urine plume distributed by the exopodites of maxillipeds 1 and 2.
Statocysts are vital sensing organs for Crustacea involved in maintaining equilibrium during movement in three-dimensional space30. They are rarely preserved in fossils and often only as the statoliths (an amalgam of tiny grains and detritus) within them31. The Eryma specimens reveal the location of the statocysts, the shape of the chamber, the statoliths within it, and the associated muscles. Their location and anatomy closely resemble those of statocysts in living crayfish25. Their presence suggests that decapods have been able to sense gravity, frequency vibrations and possibly angular acceleration since at least the Middle Jurassic.
The mandibles bear a simple incisor process and a molar process with a relatively smooth surface. These features resemble those in the carnivorous lobster Nephrops and differ considerably from the mandible of the omnivorous crayfish Astacus, with its more complex incisor process and prominent molar cusps32. This indicates that Eryma fed on soft tissue but this food may have been accessed by using the toothed chelipeds to crush mollusc shells.
The specimens of Eryma are exceptional in preserving details of the digestive system of a fossil crustacean33,34,35 including evidence of musculature and nerves. The gastric mill is small relative to the mandibles: the dimensions of the wide and long mesocardiac ossicle are intermediate between those of the carnivorous Nephrops and omnivorous parastacid crayfish36 and rather indicate omnivory37,38.
The hepatopancreas is decay-prone and rarely preserved and is otherwise known only in some shrimps and thylacocephalans from La Voulte6,10,39. The size and appearance of the hepatopancreas in decapods changes in response to environmental, nutritional and other physiological conditions40. Moreover, most of the reserves of the hepatopancreas are delivered to the ovary as lipids or proteins during vitellogenesis when the hepatopancreas becomes considerably smaller40. The hepatopancreas of the female Eryma occupies most of the cephalothorax indicating good nutrition. The ovaries of living lobsters tend to reduce in size after deposition of the eggs but the persistence of oocytes in small ovaries indicates that this individual was not yet sexually mature41. The hepatopancreas of the male is not as well preserved but it occupies a similar proportion of the cephalothorax to that in the female even though it contains fewer tubules. The number of tubules in the hepatopancreas of decapods increases with age and does not vary with conditions, indicating that the male is relatively young, probably immature42. Thus the male is probably younger than the female even though they are similar in size. Assuming that the male would ultimately become larger than the female, as in living clawed lobsters and crayfish43, it is likely that there was size dimorphism between sexes in this Eryma species. Moreover, very large specimens of Eryma, but of unknown sex, are fossilized in La Voulte (Fig. 1d, j).
Fossil evidence of reproductive systems in marine arthropods are rare with some exceptions among eurypterids44, ostracods45 and malacostracans8,34. Mature ovaries and eggs attached to the pleon of polychelidan lobsters8,9 from the La Voulte Lagerstätte reveal details of brood care and reproductive strategy. Preserved features of the reproductive organs in Eryma include oocyte-filled ovaries, gonopores, a spermatheca in the female, and a lobulate testis, gonopores and gonopods in the male. The oocytes are reminiscent of pyrite framboids, which occur as iron oxide in some La Voulte fossils7, but are normally much smaller. The size46, number and location of the features in the ovaries is consistent with their interpretation as mineralized oocytes. The spermatheca of Eryma shows greater similarity to that in nephropid lobsters (Nephropidae) than in cambarid crayfish (Cambaridae); other freshwater crayfish (Astacidae and Parastacidae) lack such a sperm storage organ47. The gross morphology of the ovary, with two separate ovarian strands, is also similar to that in clawed lobsters25. The small size of the oocytes (80–100 µm) corresponds to that at the beginning of vitellogenesis in living clawed lobsters and crayfish, where mature eggs are usually 1–2 mm in diameter, depending on species25. The presence of about 300 oocytes indicates the likely reproductive strategy of Eryma. Living crayfish of average size usually carry several hundred eggs, depending on species, rarely as many as one thousand47. Eggs in smaller species are numbered in the tens. Adults of the living crayfish Gramastacus, for example, are similar in size to Eryma and carry about 80 eggs48. The lobsters Nephrops and Homarus, in contrast, produce eggs numbering in the thousands and ten thousands, respectively49. The number of eggs in Eryma suggests a tendency toward a K-strategy as in crayfish, whereas nephropid lobsters are r-strategists. Thus the reproductive anatomy of Eryma resembles that of clawed lobsters whereas the number of eggs and presumed K-strategy are closer to those in crayfish. Secondary sexual characters on the pereiopods of erymids have been noted previously50,51. A terminal dactylus is present on pereiopod 4 in both male and female. Pereiopod 5 also bears a terminal dactylus in the male, but is chelate in the female, an arrangement similar to that in many living clawed lobsters. The soft parts described here provide a definitive identification of the Eryma specimens as female and male.
Eryma ventrosum is the most common species of clawed lobster in the Jurassic of Western Europe52. Although the species was first described in 183551 (as Glyphea ventrosa) the internal anatomy was previously unknown. The two specimens described here allow an almost complete 3D reconstruction of an extinct decapod crustacean. The remarkable preservation of respiratory, circulatory, excretory, digestive, nervous and sensory, and reproductive systems provides details to rival low-magnification histology, and allows direct comparison with living lobsters. The internal organs of Eryma show a close similarity to those of living clawed lobsters consistent with the position of erymids as the stem-group of Astacidea14(Fig. 2). Their feeding (mandibles and gastric ossicles) and reproductive (spermatheca) systems more closely resemble those of Nephropidae (clawed lobsters) than Astacidae (crayfish). The additional details revealed by our study point a way forward to incorporating internal anatomy into phylogenetic analyses of fossil decapods but more examples need to be investigated in the same way to provide comparable data for other higher taxa in order to realize this goal. In the meantime, the stem-group status of erymid lobsters suggests that the character states they share with clawed lobsters are ancestral for Astacidea, whereas the internal anatomy of crayfish (Astacidae) may be more derived. The preservation of details of the reproductive organs allows the female and male to be distinguished. Moreover, evidence of the gonads and hepatopancreas indicate that these were young well-fed lobsters, not yet of reproductive age.
Material and methods
Fossil specimens
This study is based on two specimens of Eryma ventrosum (Meyer, 1835) (UJF-ID11543 and ID11544; Fig. 1a–c) housed at the Observatoire des Sciences de l’Univers de Grenoble, Université Grenoble Alpes, France. They were collected from the Middle Jurassic (early Callovian) marls at La Voulte-sur-Rhône at the end of the nineteenth century by Alfred Gevrey, Adviser to the Court of Appeal of Grenoble and fossil collector1. The fossils occur in carbonate concretions which were mechanically opened to reveal dorsal (UJF-ID11543) and left lateral UJF-ID11544 views respectively. The matrix surrounding the fossil was covered with Indian ink for the figures in Van Straelen (1925, Pl. 8, figs. 5 and 6 as Eryma Mandelslohi).
We analysed two additional larger specimens of Eryma ventrosum from La Voulte-sur-Rhône, likewise three-dimensionally preserved in carbonate concretions (Fig. 1d, j): MNHN.F.A59527 is an isolated carapace and well-preserved eye with ommatidia, and UJF-ID11906 is a very large but incomplete adult.
Computed microtomography scanning
The specimens were first imaged at the AST-RX technical platform of the MNHN, Paris, with a GE Phoenix v│tome│ × 240 L tomograph equipped with a microfocus 240 kV/320 W tube delivering a current/voltage of 240 µA/130 kV for UJF-ID11543 and 240 µA/140 kV for UJF-ID11544. A voxel size of 31.253 µm for UJF-ID11543 and 32.778 µm for UJF-ID11544 was obtained.
In order to get better contrast and resolution, phase-contrast synchrotron X-ray microtomography was performed at the PSICHÉ beamline of the SOLEIL synchrotron (Saint-Aubin, France). Each specimen was scanned with a voxel size of 11.139 µm and a single propagation distance of 1150 mm. A pink beam of 120 keV was selected from the spectrum of the wiggler insertion device by filtering with 20 mm of copper. The detector camera was a Hamamatsu ORCA Flash 4.0 sCMOS, and the scintillator a 0.25 mm LuAG, coupled with a Hasselblad objectives in a tandem configuration. Due to the limited field of view and the size of the samples, a series of acquisitions with vertical movement of the sample was recorded. We performed scans of 6200 projections of 0.017 s exposure time over 360 degrees with the rotation axis offset from the centre of the projection. The scans were then assembled in a mosaic in order to reconstruct the whole volume. Volumes were reconstructed using filtered back projections with ESRF PyHST2 software53, using Paganin phase retrival.
Segmentation and surface model visualization
The original image stacks were optimized using ImageJ254: the images were cropped, rotated, converted to 8-bit grayscale and contrasted. Segmentation and 3D reconstruction were performed using Materialise Mimics innovation suite 24.0 (research edition) and 3DSlicer 4.11 (BSD-license55). 3D rendering was performed with Maxon Cinema 4D R25.010.
Ethics
This research complies with all relevant ethical regulations.
Data availability
Selection of X-ray sections and 3D models of the male (UJF-ID11543) and female (UJF-ID11544) of Eryma ventrosum are respectively available at: https://doi.org/10.48579/PRO/YJHTEY, https://doi.org/10.48579/PRO/O1TPJ6. Supplementary video S1 of 3D model of the male (UJF-ID11543) of Eryma ventrosum is available at: https://doi.org/10.48579/PRO/X1CBMX. Supplementary video S2 of 3D model of the female (UJF-ID11544) of Eryma ventrosum is available at: https://doi.org/10.48579/PRO/OE6XPK.
References
Charbonnier, S. L. Lagerstätte de La Voulte: Un Environnement Bathyal au Jurassique (Publications Scientifiques du Muséum, 2009).
Charbonnier, S., Vannier, J., Hantzpergue, P. & Gaillard, C. Ecological significance of the arthropod fauna from the Jurassic (Callovian) La Voulte Lagerstätte. Acta Palaeontol. Pol. 55, 111–132 (2010).
Charbonnier, S., Vannier, J. & Riou, B. New sea spiders from the Jurassic La Voulte-sur-Rhône Lagerstätte. Proc. R. Soc. B Biol. Sci. 274, 2555–2561 (2007).
Rowe, A. et al. Exceptional soft-tissue preservation of Jurassic Vampyronassa rhodanica provides new insights on the evolution and palaeoecology of vampyroteuthids. Sci. Rep. https://doi.org/10.1038/s41598-022-12269-3 (2022).
Villier, L., Charbonnier, S. & Riou, B. Sea stars from Middle Jurassic lagerstätte of La Voulte-sur-Rhône (Ardèche, France). J. Paleontol. 83, 389–398 (2009).
Wilby, P. R., Briggs, D. E. G. & Riou, B. Mineralization of soft-bodied invertebrates in a Jurassic metalliferous deposit. Geology 24, 847–850 (1996).
Jauvion, C. et al. Exceptional preservation requires fast biodegradation: thylacocephalan specimens from La Voulte‐sur‐Rhône (Callovian, Jurassic, France). Palaeontology pala.12456. https://doi.org/10.1111/pala.12456 (2019).
Jauvion, C., Audo, D., Charbonnier, S. & Vannier, J. Virtual dissection and lifestyle of a 165-million-year-old female polychelidan lobster. Arthropod Struct. Dev. 45, 122–132 (2016).
Jauvion, C. et al. A new polychelidan lobster preserved with its eggs in a 165 Ma nodule. Sci. Rep. 10, 3574 (2020).
Vannier, J., Schoenemann, B., Gillot, T., Charbonnier, S. & Clarkson, E. Exceptional preservation of eye structure in arthropod visual predators from the Middle Jurassic. Nat. Commun. 7, 10320 (2016).
Feldmann, R. M. & Schweitzer, C. E. Lobster (Decapoda) diversity and evolutionary patterns through time. J. Crustac. Biol. 34, 820–847 (2014).
Devillez, J. & Charbonnier, S. A synthesis of the evolutionary history of erymoid lobsters (Crustacea, Decapoda, Erymoidea). Geodiversitas 44, 17 (2022).
Devillez, J. & Charbonnier, S. The genus Eryma Meyer, 1840 (Crustacea: Decapoda: Erymidae): New synonyms, systematic and stratigraphic implications. Bull. Soc. Géol. Fr. 188, 15 (2017).
Devillez, J., Charbonnier, S. & Barriel, V. An attempt to clarify phylogenetic affinities of erymid lobsters (Decapoda) using morphological characters. Arthropod Syst. Phylogeny 77, 365–395 (2019).
Bracken-Grissom, H. D. et al. The emergence of lobsters: Phylogenetic relationships, morphological evolution and divergence time comparisons of an ancient group (Decapoda: Achelata, Astacidea, Glypheidea, Polychelida). Syst. Biol. 63, 457–479 (2014).
Klompmaker, A. A. et al. Muscles and muscle scars in fossil malacostracan crustaceans. Earth-Sci. Rev. 194, 306–326 (2019).
Secretan-Rey, S. Monographie du Squelette Axial de Nephrops norvegicus (Linné, 1758). https://doi.org/10.5281/ZENODO.5756017 (2002).
Biology of Freshwater Crayfish. (Blackwell Science ; Iowa State University Press [distributor], 2002).
Wilkens, J. L. & Young, R. E. Patterns and bilateral coordination of scaphognathite rhythms in the lobster Homarus americanus. J. Exp. Biol. 63, 219–235 (1975).
Feldmann, R. M. & Schweitzer, C. E. The oldest shrimp (Devonian: Famennian) and remarkable preservation of soft tissue. J. Crustac. Biol. 30, 629–635 (2010).
Strausfeld, N. J., Ma, X. & Edgecombe, G. D. Fossils and the evolution of the arthropod brain. Curr. Biol. 26, R989–R1000 (2016).
Ma, X., Cong, P., Hou, X., Edgecombe, G. D. & Strausfeld, N. J. An exceptionally preserved arthropod cardiovascular system from the early Cambrian. Nat. Commun. 5, 3560 (2014).
Bicknell, R. D. C., Ortega-Hernández, J., Edgecombe, G. D., Gaines, R. R. & Paterson, J. R. Central nervous system of a 310-m.y.-old horseshoe crab: Expanding the taphonomic window for nervous system preservation. Geology 49, 1381–1385 (2021).
Pohl, H., Wipfler, B., Grimaldi, D., Beckmann, F. & Beutel, R. G. Reconstructing the anatomy of the 42-million-year-old fossil †Mengea tertiaria (Insecta, Strepsiptera). Naturwissenschaften 97, 855–859 (2010).
Vogt, G. Functional anatomy. In Biology of freshwater crayfish (ed. Holdich, D. M.) 53–151 (Blackwell Science ; Iowa State University Press [distributor], 2002).
Scholz, S., Richter, S. & Wirkner, C. S. Constant morphological patterns in the hemolymph vascular system of crayfish (Crustacea, Decapoda). Arthropod Struct. Dev. 47, 248–267 (2018).
Karasawa, H., Schweitzer, C. E. & Feldmann, R. M. Phylogeny and systematics of extant and extinct lobsters. J. Crustac. Biol. 33, 78–123 (2013).
Waloszek, D., Maas, A., Olesen, J., Haug, C. & Haug, J. T. A eucrustacean from the Cambrian ‘Orsten’ of Sweden with epipods and a maxillary excretory opening. Palaeontology 57, 909–930 (2014).
Aggio, J. & Derby, C. D. Chemical communication in lobsters. In Chemical Communication in Crustaceans (eds. Breithaupt, T. & Thiel, M.). 239–256. https://doi.org/10.1007/978-0-387-77101-4_12 (Springer, 2010).
Budelmann, B.-U. Morphological diversity of equilibrium receptor systems in aquatic invertebrates. In Sensory Biology of Aquatic Animals (eds. Atema, J., Fay, R. R., Popper, A. N. & Tavolga, W. N.). 757–782 https://doi.org/10.1007/978-1-4612-3714-3_30 (Springer, 1988).
Wittmann, K. J., Schlacher, T. A. & Ariani, A. P. Structure of recent and fossil mysid statoliths (Crustacea, Mysidacea). J. Morphol. 215, 31–49 (1993).
Vojkovská, R., Horká, I. & Ďuriš, Z. Comparative morphology of crayfish mandibles, with insight into their evolution. J. Morphol. 281, 365–376 (2020).
Klompmaker, A., Kloess, P., Jauvion, C., Brezina, J. & Landman, N. Internal anatomy of a brachyuran crab from a Late Cretaceous methane seep and an overview of internal soft tissues in fossil decapod crustaceans. Palaeontol. Electron. https://doi.org/10.26879/1277 (2023).
De Mazancourt, V., Wappler, T. & Wedmann, S. Exceptional preservation of internal organs in a new fossil species of freshwater shrimp (Caridea: Palaemonoidea) from the Eocene of Messel (Germany). Sci. Rep. 12, 18114 (2022).
Xing, L. et al. The first shrimp preserved in mid-cretaceous Kachin amber: Systematics, palaeoecology, and taphonomy. Sci. Bull. 66, 1723–1726 (2021).
Becker, C., Dick, J. T. A., Cunningham, E. M., Schmitt, C. & Sigwart, J. D. The crustacean cuticle does not record chronological age: New evidence from the gastric mill ossicles. Arthropod Struct. Dev. 47, 498–512 (2018).
Vogt, G. Synthesis of digestive enzymes, food processing, and nutrient absorption in decapod crustaceans: A comparison to the mammalian model of digestion. Zoology 147, 125945 (2021).
Kawai, T. & Patoka, J. Morphology of gastric mills and mandibles of New Guinean parastacid crayfishes, with comparisons with other Astacidea (Decapoda). J. Crustac. Biol. 40, 692–703 (2020).
Secretan, S. Conchyliocarida, a class of fossil crustaceans: relationships to Malacostraca and postulated behaviour. Earth Environ. Sci. Trans. R. Soc. Edinb. 76, 381–389 (1985).
Vogt, G. Functional cytology of the hepatopancreas of decapod crustaceans. J. Morphol. 280, 1405–1444 (2019).
Cabiddu, S., Follesa, M. C., Gastoni, A., Porcu, C. & Cau, A. Gonad development of the deep-sea lobster Polycheles typhlops (Decapoda: Polichelidae) from the Central Western Mediterranean. J. Crust. Biol. 28, 494–501 (2008).
Vogt, G. Ageing and longevity in the Decapoda (Crustacea): A review. Zool. Anz.-J. Comp. Zool. 251, 1–25 (2012).
Waddy, S. L., Aiken, D. E. & Kleijn, D. P. V. D. Chapter 10—Control of growth and reproduction. In Biology of the Lobster (ed. Factor, J. R.). 217–266 https://doi.org/10.1016/B978-012247570-2/50032-3 (Academic Press, 1995).
Kamenz, C., Staude, A. & Dunlop, J. A. Sperm carriers in Silurian sea scorpions. Naturwissenschaften 98, 889–896 (2011).
Matzke-Karasz, R. & Smith, R. J. A review of exceptional preservation in fossil ostracods (Ostracoda, Crustacea). Mar. Micropaleontol. 174, 101940 (2022).
Craveiro, C. et al. Ovarian maturation of Penaeus subtilis (Decapoda: Penaeidae): A new insight to describe oocyte development and somatic structures. Act. Zool. 104, 506–515 (2023).
Mclay, C. L. & van den Brink, A. Crayfish growth and reproduction. In Biology and Ecology of Crayfish (eds. Longshaw, M. & Stebbing, P.). 62–116 https://doi.org/10.1201/b20073-4 (CRC Press, 2016).
McCormack, R. The eastern swamp crayfish Gramastacus lacus sp. n. (Decapoda, Parastacidae) a new species of freshwater crayfish from coastal New South Wales, Australia. ZooKeys 398, 53–67 (2014).
Pollock, D. E. Egg production and life-history strategies in some clawed and spiny lobster populations. Bull. Mar. Sci. 61, 97–109 (1997).
Schram, F. R. & Dixon, C. J. Decapod phylogeny: Addition of fossil evidence to a robust morphological cladistic data set. Bull. Mizunami Foss. Mus. 31, 1–19 (2023).
Charbonnier, S., Pérès, D. & Letenneur, C. Exceptionally preserved crustaceans from the Oxfordian of eastern France (Terrain à Chailles Formation, Haute-Saône). Geodiversitas 34, 531–568 (2012).
Devillez, J. & Charbonnier, S. Review of the Late Jurassic erymoid lobsters (Crustacea: Decapoda). Geodiversitas 43, 25–73 (2021).
Mirone, A., Brun, E., Gouillart, E., Tafforeau, P. & Kieffer, J. The PyHST2 hybrid distributed code for high speed tomographic reconstruction with iterative reconstruction and a priori knowledge capabilities. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 324, 41–48 (2014).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Fedorov, A. et al. 3D Slicer as an image computing platform for the quantitative imaging network. Magn. Reson. Imaging 30, 1323–1341 (2012).
Bell, M. A. & Lloyd, G. T. strap: An R package for plotting phylogenies against stratigraphy and assessing their stratigraphic congruence. Palaeontology 58, 379–389 (2015).
Brusatte, S. L., Benton, M. J., Ruta, M. & Lloyd, G. T. Superiority, competition, and opportunism in the evolutionary radiation of dinosaurs. Science 321, 1485–1488 (2008).
Acknowledgements
This paper is dedicated to the memory of our dear co-worker Günter Vogt, who passed away on June 23, 2023. Special thanks to U. Bieberstein (Heidelberg University, Germany), who helped us to recover his thoughts, observations and digital files about the Eryma project. The authors thank F. Giraud for access to the palaeontological collections of the Observatoire des Sciences de l’Univers de Grenoble (France). We thank P. Loubry (CR2P, MNHN) and N. Podevigne (Université Lyon 1) for the photographic work of Fig. 1a–e, and j, respectively. Computed tomography scanning of Eryma was performed at the Synchrotron Soleil (PSICHÉ beamline) at Gif-sur-Yvette (France), with the support of Proposal 20210093.
Author information
Authors and Affiliations
Contributions
Conceptualization: S.C., G.V., M.-B.F and D.E.G.B. Methodology: T.L., A.K. and N.P.-C. Investigation: T.L. and A.K. Visualization: N.H. and N.P.-C. Funding acquisition: T.L., M.-B.F. and S.C. Specimen access and geological data: S.C. and J.D. Project administration: S.C. Supervision: S.C. Writing of original draft: S.C., G.V., M.-B.F. and D.E.G.B. Writing (review and editing): S.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Charbonnier, S., Vogt, G., Forel, MB. et al. The La Voulte-sur-Rhône Konservat-Lagerstätte reveals the male and female internal anatomy of the Middle Jurassic clawed lobster Eryma ventrosum. Sci Rep 14, 17744 (2024). https://doi.org/10.1038/s41598-024-67357-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67357-3
- Springer Nature Limited