Abstract
Microplastics (MPs) are defined as plastic particles smaller than 5 mm in size, and nanoplastics (NPs) are those MPs with a particle size of less than 1000 nm or 100 nm. The prevalence of MPs in the environment and human tissues has raised concerns about their potential negative effects on human health. Macrophages are the major defence against foreign substances in the intestine, and can be polarized into two types: the M1 phenotype and the M2 phenotype. However, the effect of NPs on the polarization of macrophages remains unclear. Herein, we selected polystyrene, one of the most plastics in the environment and controlled the particle sizes at 50 nm and 500 nm respectively to study the effects on the polarization of macrophages. We used mouse RAW264.7 cell line models in this macrophage-associated study. Experiments on cell absorption showed that macrophages could quickly ingest polystyrene nanoplastics of both diameters with time-dependent uptake. Compared to the untreated group and 10 μg/mL treatment group, macrophages exposed to 50 μg/mL groups (50 nm and 500 nm) had considerably higher levels of CD86, iNOS, and TNF-α, but decreased levels of aCD206, IL-10, and Arg-1. According to these findings, macrophage M1 and M2 polarization can both be induced and inhibited by 50 μg/mL 50 nm and 500 nm polystyrene nanoplastics. This work provided the first evidence of a possible MPs mode of action with appropriate concentration and size through the production of polarized M1, providing dietary and environmental recommendations for people, particularly those with autoimmune and autoinflammatory illnesses.
Similar content being viewed by others
Introduction
Microplastics (MPs) are described as plastic fragments smaller than 5 mm, and nanoplastics (NPs) asre those smaller than 1000 nm or 100 nm. MPs can be intentionally added to common products and are created when plastic objects deteriorate and weather1,2,3,4. Given their presence in the environment and consumer goods, human exposure to these particles is unavoidable, and concerns about their effects on human health are mounting5,6,7. MPs were formerly assumed to be innocuous particles with no toxicity. Besides inhalation, ingestion is another main exposure scenario for MPs entering the human body: if MPs are ingested, the intestinal barrier protects human beings from MPs, and they are excreted through the gastrointestinal tract8,9. While Yan et al. found that ingesting MPs could lead to inflammatory response, increased permeability, dysfunction of intestinal barrier10. Human beings ingest MPs could come from diverse sources: consuming takeout food in plastic packaging, or drinking water in plastic bottles. People who were more exposed to MPs had more MPs in their stool, and the concentration of fecal MPs was positively correlated with the severity of intestinal inflammation.
Oxidative stress and cytotoxicity may be the major mechanisms of MPs toxicity11,12,13,14,15. Exposure to MPs compounds the histopathological damage of mouse colonic mucosa, leading to increased expression of inflammation factors, oxidative stress and intestinal immune imbalance16,17. Leslie et al. first discovered and measured MPs in human blood theorize that a systemic exposure to MPs and a buildup of MPs in human tissue may have occurred11. It is speculated that MPs also has the ability to transport, exposing distal tissues, especially small-sized nanoplastics. By preventing CD8 + T cell development, T helper cell cytokine production, and T cell activation, circulating MPs may have an impact on the human immune system8,18,19,20,21. Increased exposure to MPs could have adverse effects on autoimmune illnesses and cancer cases.
Macrophages are vital innate immune cells derived from bone marrow-derived monocytes. Macrophages can exist in various tissues, remaining relatively stationary in healthy condition but activated when stimulated22,23,24,25. Macrophages are the immune system’s first line of defense against foreign invaders and are in charge of phagocytosis, antigen presentation, and immunological control26,27,28. Macrophages can become immune-regulating M2 cells or normally inflammatory M1 cells, depending on the activation route, and their fate is contingent upon a range of triggers. M1 polarized macrophages, which are primarily responsible for inducing an inflammatory response by secreting pro-inflammatory mediators like interleukin-6 (IL-6), are induced by foreign substances or pro-inflammatory cytokines29,30,31. Systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and macrophage activation syndrome (MAS) are examples of autoimmune and autoinflammatory disorders that are hypothesized to be pathologically associated to polarization imbalance and hyperactivation of macrophages32,33,34.
The gut has one of the greatest populations of macrophages in the body, which it uses to maintain and control intestinal homeostasis. Intestinal macrophage dysfunction is closely linked to chronic inflammation in the intestinal tract35,36. This research focuses on the action mode of MPs on macrophages since intestinal macrophages are the guardians of intestinal immunological homeostasis and MPs ingested into humans might could serious structural damage to the intestinal mucosa. Therefore, in this study, we analyzed the regulatory effects of 50 nm and 500 nm polystyrene nanoplastics on M1 or M2 macrophage polarization through in vitro experiments, and initially revealed the main mechanism of action of nanoplastics on intestinal inflammation (Scheme 1). Polystyrene nanoplastics with diameter of 50 nm and 500 nm were successfully prepared by emulsion polymerization, which controlled the content of monomer, initiator and methacrylic acid. The toxicity test of the material showed that the macrophage activity was above 85% when the concentration was below 50 μg/mL. Endocytosis experiments showed that the two sizes of polystyrene nanoplastics could be rapidly internalized into macrophages through receptor-mediated internalization and passive transport mechanisms within the incubation time of 9 h, and the smaller sizes had higher internalization efficiency. Additional toxicity mechanism analysis results revealed that, in comparison to the untreated group and the 10 μg/mL 50 nm and 500 nm polystyrene nanoplastics treatment group, macrophages in the 50 μg/mL 50 nm and 500 nm treatment groups expressed high levels of cytokines associated with inflammation (iNOS, CD86 and TNF-α), and low levels of cytokines that alleviate inflammation (CD206, Arg-1 and IL-10). These results proved that 50 μg/mL 50 nm and 500 nm polystyrene nanoplastics could induce macrophages to polarization into M1 type.
Materials and methods
Materials
Styrene (98%), amine persulfate (98.5%), methacrylic acid (98% MAA), anhydrous ethanol and sodium hydroxide (97%) were purchased from Sinopharm Chemical Reagent Co., Ltd. Rhodamine B n-octyl ester (RhB-O) dye was purchased from Tansoole, Shanghai, China. DAPI nuclear staining solution, CCK-8 kit, RIPA lysate, Calcin-AM, and propidium iodide (PI) were purchased from Shanghai Biyuntian Biotechnology. GAPDH (mouse mAb, #ab8245), iNOS (rabbit mAb, #ab210823) was from Abcam. 10% SDS-PAGE was used to separate cell lysate aliquots (Beyotime, Code No. P0012A). All investigations used deionized water (Millipore Milli-Q grade), which has a resistivity of 18.2 M. Water that has undergone diethypyrocarbonate (DEPC) treatment was used to prepare each solution.
Manufacturing of polystyrene nanoplastics
Polystyrene nanoplastics are made by free radical polymerization (Fig. 1)37. A three-nozzle flask with a teflon stirring paddle and a condensing tube is fixed in a thermostatic bath, and the stirring paddle speed is adjusted to 350 r/min. Weigh 20 g of styrene and 2 g of methacrylic acid and mix them in a 50 mL beaker, add the mixed liquid to the three-mouth flask, rinse the beaker with 100 mL of distilled water three times, pour the liquid into the three-mouth flask, and heat up to 85 °C while stirring. After 5 min of constant temperature, 12 mL ammonium persulfate solution (0.3 g ammonium persulfate dissolved in 25 mL distilled water) was added to the three-mouth flask. 2 h after the polymerization, 8 mL ammonium persulfate aqueous solution was added to the three-mouth flask. After a 4 h reaction, the remaining 5 mL of aqueous solution of (NH4)2S2O8 was added to the three-mouth flask. Continue to react for 3 h, then reduce to room temperature and stop stirring. Remove the three-mouth flask from the water bath. After the emulsion in the flask was filtered by gauze, the bulk particles were removed, centrifuged at a rotating speed of 8000 r/min for 30 min, the supernatant was poured away, and the remaining solids were added to distilled water and dispersed by ultrasound for 10–20 min. After repeated ultrasonic washing for three times, 10 mL of distilled water was added to prepare the emulsion, the solid content was determined and set aside. The emulsion polymerization technique was modified by adding rhodamine B dye to produce fluorescently tagged polystyrene nanospheres. The fluorescence stability of rhodamine-labeled polystyrene was studied, and the fluorescence intensity of Rhodamine-labeled polystyrene was not significantly reduced within 1 week. The reason may be that fluorescent molecules are added in the polymerization process of polystyrene nanoparticles, and its stability is mainly affected by the degradation process of polystyrene nanoparticles, and a short time is not enough to make polystyrene physically decompose (Fig. S1).
Characterization of polystyrene nanoplastics
With the use of a scanning electron microscope, the size and shape of polystyrene nanoplastics were determined (Hitachi, Tokyo, Japan). The dynamic light scattering (DLS) method was used to evaluate the size of polystyrene nanoplastics using a Zetasizer Nano-ZS (Malvern Instruments Ltd.). To get the infrared spectra of polystyrene nanoplastics, Tianjin Gangdong Corporation’s Fourier transform infrared spectrometer (FTIR-650) was employed. The fluorescence spectrum of fluorescent polystyrene was measured by a fluorescence spectrophotometer (Edinburgh FS5). Fluorescence microscopic images were obtained using a Nikon microscope (C2, Nikon, Japan).
Cell culture
From the Shanghai Cell Bank of the Chinese Academy of Sciences, mouse mononuclear macrophage leukemia cells (RAW264.7) were acquired. Gibco was the source of all cell culture additives and medium. RAW264.7 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 2 mM L-glutamine, 0.11 g/L sodium pyruvate, 10% fetal bovine serum (FBS), and 1% penicillin–streptomycin. 37 °C and 5% CO2 were used to culture the cells (CLM-170B-8-NF, ESCO). RAW264.7 cells do not require digestion with pancreatic enzymes prior to passage. Instead, they are dislodged from the culture bottle wall through pipetting, collected, centrifuged, re-suspended, and subsequently inoculated into a new culture vessel to complete the passage process.
Cytotoxicity
The manufacturer’s recommendations were followed while measuring cell proliferation with the cell counting kit-8. Each group’s experiments were carried out in triplicate. RAW264.7 cells were seeded on 96-well plates at a density of 1 × 104 cells per well and allowed to develop for the duration of the night. The cells were then cultivated for an additional two hours at 37 °C after being grown for 24 h in varying concentrations of 50 nm and 500 nm polystyrene nnanospheres with 10 μL of the CCK-8 reagent added to each well. The Varioskan LUX microplate reader was used to measure the absorbance of cells at 450 nm.
Cytoendocytosis of polystyrene nanoplastics
Using flow cytometry (C6, BD Biosciences), polystyrene nanoplastics were found to be taken up by cells. RAW264.7 cell was seeded in 96-well plates at a density of 5 × 104 cells/dish. The culture was continued in the cell incubator after 4 h with the addition of the media containing polystyrene nanospheres. The cells were incubated for 0, 3, 6, and 9 h, respectively, and fixed three times in PBS and preserved at room temperature with 4% paraformaldehyde formaldehyde for 20 min. After fixation, cells were marked with 10 μg/mL Hoechst 33342. With at least 2 × 104 gated cells per sample, the cell absorption of rhodamine B noctyl ester (Rhb-O) modified polystyrene nanoplastics was assessed by flow cytometry. Three times each experiment was carried out.
Real-time quantitative PCR (RTQ-PCR) analysis of the surface marker expression on M1 and M2 macrophages.
RAW264.7 cells were seeded in a plate with six wells at a density of 2 × 104 cells per well and allowed to proliferate throughout the night. For 48 h, RAW264.7 cells were exposed to PBS, 10 μg/mL 50 nm, 10 μg/mL 500 nm, 50 μg/mL 50 nm, and 50 μg/mL 500 nm. The cells were treated with the Trizol reagent (Life Technologies) and the total RNA was isolated following two PBS rinses38. The FastKing RT Kit (With gDNase) was used to reverse-transcribe the mRNA. RTQ-PCR was performed using the ABI7500qPCR system (Life Technologies) and SuperReal premixture SYBRGreen (TIANGEN). The 2−△△Ct method was employed for data analysis. The primer sequences are listed in Table 1.
Western blotting analysis
2 × 105 RAW264.7 cells per well were seeded in 6-well plates, and the cells were then incubated for 24 h. Polystyrene nanoparticles of 10 μg/mL 50 nm, 10 μg/mL 500 nm, 50 μg/mL 50 nm, and 50 μg/mL 500 nm were added to the cell culture medium, respectively, and continued to be cultured for 48 h, with equal amount of PBS as the control group. Using KeyGEN BioTECH’s total extraction sample kit, proteins were recovered from RAW264.7 cells after two rounds of ice-cold PBS washing. The next step was centrifuging the supernatants for 10 min at 12,000 rpm to separate them. The protein sample concentration was determined using the BCA protein assay kit (KeyGEN, China). The 20 μg of proteins from each sample were then electroblotted on PVDF membranes from Merck Millipore (USA) and subjected to sodium dodecyl sulfate- polyacrylamide gel electrophoresis (SDS–PAGE) analysis. The appropriate primary antibodies were incubated on polyvinylidene fluoride (PVDF) membranes at 4 °C for an overnight period. Following this, the membranes were washed in TBS with Tween-20 (TBST), blocked with 5% dried milk (produced from TBST solution), and treated with the peroxidase-coupled secondary antibody for 2 h. An automatic chemiluminescence imaging system (imaging Quant LAS 4000) was used to find several proteins.
Statistical analysis
The data analysis method was to use the statistical analysis software Graphpad to make statistics and analysis on the data of each group, and the findings were presented as the mean and variability, with *P < 0.05; **P < 0.01; ***P < 0.001.
Results
Preparation and characterization of polystyrene nanoplastics
Polystyrene was prepared by addition polymerization with styrene as monomer and ammonium persulfate as initiator. The experimental results indicated that, as the dose of methacrylic acid (MAA) was raised, the polymerization rate and the final conversion rate first improved and then decreased. Since MAA is a hydrophilic monomer, it acts as an emulsifier in soap-free emulsion polymerization. The reaction system is less stable when MAA is not utilized, and latex particles are more likely to clump together. With an increase in MAA content, the produced polystyrene nanospheres’ particle size shrank. It can be seen from Fig. 2a–d that 50 nm and 500 nm diameter polystyrene nanospheres were successfully prepared by controlling monomer, initiator and MAA content. The particle size and diameter of nanospheres were determined by SEM (scanning electron microscope). The produced nanospheres exhibit uniform particle size and good sphericity, as can be shown in Fig. 2a, b. Figure 2e shows the infrared spectrum of the prepared polystyrene nanospheres. The peak that appears at 3435.3 cm−1 in the figure may be shown to be the O–H bond stretching vibration peak of airborne H2O molecules. Unsaturated C–H bonds on the benzene ring have stretching vibration maxima at 3058.4 and 3023.3 cm−1. On saturated –CH– and –CH2–, respectively, the stretching vibration maximum of the C–H bond can be seen at 2916.8 and 2839.6 cm−1. Rigid vibrations of the phenyl ring caused a peak of 1609.2 cm−1, which is what causes the skeleton to vibrate. The in-plane flexural vibration peak of the C–H bond on –CH is located at 1494.5 cm−1. The C–H bond on –CH2 has an out-of-plane bending vibration peak at 1443.7 cm−1. The C–H bond on the single substituted benzene ring exhibits in-plane bending vibration at 754.8 cm−1 and out-of-plane bending vibration at 698.3 cm−1, respectively. The above results indicate that styrene is in polymerization state.
To evaluate the stability of the synthesized polystyrene plastic, we measured the particle size change of 50 nm polystyrene plastic in phosphate buffer solution (PBS) to evaluate its stability. As can be seen from Fig. 2f, the particle size of 50 nm polystyrene nanoplastics in PBS barely changed in a week, indicating that synthetic polystyrene nanoplastics have good stability in solution and can be stored for subsequent cell stimulation experiments.
Toxicity of polystyrene nanoplastics
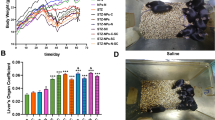
Cell stimulation experiments of polystyrene nanoplastics are achieved by incubating these nanomaterials with macrophages for about 4–24 h. As shown in Fig. 3a, b, both 50 nm and 500 nm polystyrene nanoplastics exhibit good cytocompatibility at low concentrations. Figure 3c shows the cell counting kit-8 (CCK-8) detection results of macrophages treated with 50 nm and 500 nm polystyrene nanoplastics at different concentrations for 24 h. When the concentration of polystyrene nanoplastics was in the range of 0–50 μg/mL, with the increase of the concentration of polystyrene nanoplastics, the activity of macrophages did not change significantly with the increase of time, and the cell activity was basically above 85%. However, when the concentration of polystyrene nanoplastics continued to increase, the activity of macrophages significantly decreased with the increase of the concentration, and 50 μg/mL could be used as the critical point concentration of macrophage activity. It is essential to realize that at high concentrations, the cell survival of 500 nm is slightly higher compared with that of 50 nm, probably because of the less effective cell endocytosis caused by 500 nm’s bigger size.
Cellular uptake of polystyrene nanoplastics in vitro
To create fluorescently modified polystyrene nanoplastics for use in later endocytosis studies, Rhodamine dye was first added to the polymerization of polystyrene nanoplastics. The excitation and emission spectra of Rhodamine B n-octyl ester (Rhb-O) modified polystyrene nanoplastics are shown in Fig. 4a. To study the stability of fluorescent nanoplastics prepared by embedding method, fluorescent nanoplastics were dispersed in different pH solutions to detect their fluorescence changes. As observed in Fig. 4b, Rhb-O’s fluorescence intensity varies significantly in aqueous solutions with various pH values, whereas Rhb-O fluorescent nanospheres made using the embedding method exhibit essentially little variation in fluorescence intensity. This is because with the decrease of pH, the two tertiary amine groups in RhB-O gradually become quaternary ammonium salts, which are strong electron absorbing groups, which will cause the fluorescence of the parent dye to weaken. In the nanospheres prepared by embedding method, RhB-O is protected by polystyrene long chain, isolating the dye from the external environment, so the fluorescence intensity of RhB-O fluorescent nanospheres prepared by embedding method is almost unchanged in aqueous solutions of different pH. Figure 4c–f depicts the appearance of fluorescence changed by polystyrene nanoplastics after 3 h of incubation between polystyrene nanoplastics and RAW264.7 cells. With the extension of co-incubation time, the fluorescence intensity also increased, and reached the highest fluorescence intensity at 9 h. This indicated that macrophages were time-dependent in endocytosis of polystyrene nanoplastics. Therefore, in order to ensure adequate endocytosis, incubation time of 12 h was used as a condition for subsequent experiments to ensure a large amount of nanocomplex enrichment in the cells.
Cellular uptake of polystyrene nanoplastics. (a) Fluorescene excition and emission spectrum of RhB-O labeled fluorescent polystyrene nanoplastics. (b) Fluorescene intensity of RhB-O and RhB-O fluorescent polystyrene nanoplastics in different pH. (c,d) Flow cytometry analysis of 50 nm and 500 nm polystyrene nanoplastics uptake by RAW264.7 cells at 0 h, 3 h, 6 h, and 9 h. (e,f) Fluorescence intensity analysis of 50 nm and 500 nm polystyrene nanoplastics uptake by flow cytometry.
RTQ-PCR and fluorescence imaging analysis of the surface marker expression on M1 and M2 macrophages
The degree of macrophage polarization was next assessed by staining for the M1 and M2 cellular markers, iNOS and Arg-1 (Fig. 5a–e). The red fluorescence of Arg-1 in the control, 50 nm (10 μg/mL), and 500 nm (10 μg/mL) experimental groups was greater than the green fluorescence of iNOS. As a result, nearly all of the macrophages in the experimental group stained positively for iNOS at 50 nm (50 μg/mL) and 500 nm (50 μg/mL).
Polystyrene nanoplastics enhance M1 polarization of macrophages. (a–e) After 3 days of culture, photos of the M1 and M2 markers Arg-1 and iNOS were labeled with immunofluorescence. (f) Expression levels of M1 and M2 related genes in macrophages treated with 50 nm and 500 nm polystyrene nanoplastics for 24 h.
To further determine whether polystyrene nanoplastics can induce macrophages to enter the M1 stage, RTQ-PCR was used in this experiment to detect control, 50 nm (10 μg/mL), mRNA expression difference of M1 (CD86, TNF-α, iNOS) and M2 (CD206, IL-10, Arg-1) phenotypes in 500 nm (10 μg/mL), 50 nm (50 μg/mL) and 500 nm (50 μg/mL) transcriptomes. Figure 5f shows that, in comparison to the untreated group, all of the treatment groups exhibited greater levels of M1 macrophage cytokine expression, with the macrophage gene expression levels in the 50 μg/mL 50 nm and 500 nm groups being the highest. Accordingly, M2-type cytokine expression in the 10 μg/mL 50 nm and 500 nm treatment groups did not differ substantially from that of the control group, however it dramatically decreased in the 50 μg/mL 50 nm and 500 nm treatment groups.
Western blotting analysis
Figure 6 and Figs. S2, S3 shows the blotting of macrophages treated with control, 50 nm (10 μg/mL), 500 nm (10 μg/mL), 50 nm (50 μg/mL) and 500 nm (50 μg/mL) for 24 h. In accordance with the RTQ-PCR results above, the iNOS blot in the 50 nm (50 μg/mL) and 500 nm (50 μg/mL) treatment groups got deeper than the control group when glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal reference. It was confirmed that 50 μg/mL polystyrene nanoplastics enhanced M1 polarization of macrophages at the intracellular protein level. Therefore, the above data supported the view that polystyrene nanoplastics enhanced M1 polarization and inhibited M2 polarization of macrophages.
Discussion
Plastic is a major component of human production and life waste, the world’s annual production of more than 300 million tons of plastic, of which about 20% of the plastic will enter the ocean, air and other natural environment39. Packaging is the largest market for plastics, and polystyrene is one of the “five general plastics” with the largest usage in the world, the fourth-largest variety after polyethylene, polypropylene and polyvinyl chloride. Polystyrene is a thermoplastic resin produced by free radical polymerization of styrene monomers. Due to its hard, transparent, rigid, electrical insulation, low moisture absorption and excellent processing properties, polystyrene is widely used in daily necessities, such as bowls, dishes, cups, disposable lunch boxes, which are fast consumables, easy to bring “white pollution” problems40. Eliminating these plastics poses a significant challenge, necessitating destructive thermal methods like combustion or pyrolysis. These methods can weaken plastics and cause the materials to break down into particles known to be millimeters (MPs) or nanometres (NPs) in size2. Once broken down, plastics can infiltrate the human body by inhaling or ingesting them. Prior investigations have verified the discovery of nanoplastic particles in both human feces, blood and even tissue10,11,41.
Intestine is one of the main entries for MPs into the body. Macrophages, as “goalkeepers” of the intestine, play an important role in maintaining homeostasis of the local intestinal microenvironment. They can phagocytose foreign invaders and polarize in response to stimulation, developing into an anti-inflammatory M1 or anti-inflammatory M2 phenotype. The phenotype of M1 with CD86 expression may be driven by microbial products or pro-inflammatory cytokines, and can lead to tissue damage by secretion of pro-inflammatory mediators, such as IL-6, and iNOS; while M2 phenotype with CD206 expression can alleviate inflammation and promote tissue healing and repair by secretion of anti-inflammatory mediators, such as transforming growth factor-β (TGF-β), and IL-1042. Overactivation and polarization imbalance of macrophages play a key role in the pathogenesis of autoimmune and autoinflammatory diseases, such as RA43,44. While plastics have long been believed to be inert chemicals, recent studies indicate their potential to inflict damage on local intestinal immune microenvironment.
Do MP ingested into the intestine lead to local inflammation by acting on macrophages, and what is the mechanism? Studies have demonstrated that MPs can be engulfed into macrophages through endocytosis, by binding to the receptors on the cell surface. It is reported that the ideal particle size for cell internalization is 40–50 nm because if the particle size is too small, it won’t bind to enough membrane surface receptors, which will limit cell internalization; if the particle size is too large, cells find it challenging to internalize it45,46,47. Different sized plastic particles can be found in the environment. Is there a relationship between the concentration of various plastic particle sizes that macrophages ingest and the dysfunction that macrophages cause? No research has been published in this field. For this reason, we chose to investigate the effects of 50 nm and 500 nm polystyrene particles on endocytosis and macrophage polarization. The findings demonstrated that macrophages demonstrated fast phagocytosis at both 50 and 500 nm, in contrast to other cells’ challenging phagocytosis behavior of large particles48. This observation may be connected to the interaction between macrophages and microplastics that is facilitated by Tim4, a receptor on macrophages that identifies apoptotic cells.
To study the effects of plastic particles on macrophage activity, we concentrated on macrophage polarization and investigated how polystyrene nanoplastics varying in size and concentration impact the polarization. Latest findings about the impact of plastic particles on macrophage polarization were not uniform: Hu et al. discovered that polystyrene nanoplastics could disturb maternal–fetal immune balance by converting macrophages into a dominant M2-subtype in pregnant mice49; Stock et al. found no interference of polystyrene nanoplastics with the activation and polarization of human macrophage model50; Li et al. discovered that polystyrene nanoplastics have the potential to initiate the M1 polarization phenotype in testicular tissues51. In our study, we confirmed the pro-inflammatory feature of polystyrene nanoplastics by promoting M1 polarization without being impacted by size, and discovered that 50 μg/mL was the breaking point of polystyrene nanoplastics acting on macrophages. When the concentration of polystyrene nanoplastics was above the threshold, the activity of macrophages was obviously impacted and a polarization imbalance of macrophages manifested itself: M1 polarization was stimulated, whilst M2 polarization was repressed. This may be a factor in the inconsistency of previous results, as these studies did not take concentration into account. The impact of the surrounding microenvironment, particularly with regard to the electric potential, on the plastic particles could be another element. The electric potential of the microplastics will alter dramatically after being exposed to various environments, which will have an impact on the strength of the particles' adherence to cells as well as their internalization once they have entered them52.
Macrophage polarization imbalance in the local intestinal immune microenvironment is closely linked to the development of autoimmune and autoinflammatory diseases, such as RA and SLE53,54,55. We confirmed the critical point concentration of polystyrene nanoplastics that may promote M1 polarization, serving as a vital dietary reference for these patients. Once these patients have long been consuming MPs-rich diet (plastic takeaways or bottled water), the local concentration of MPs in the intestine may reach a certain limit, and this may promote M1 polarization and aggravate the disease progress. Minimize the use of pre-packaged foods, especially those with a high degree of processing. And avoid decomposing operations such as heating plastic packaging. Future research will focus on verifying the effects of MPs-rich diet on macrophage funtion, and figuring out the mechanisms by which MPs may cause or exacerbate disease.
Conclusion
Polystyrene nanoplastics with diameters of 50 nm and 500 nm were successfully prepared in our study. Both sizes of polystyrene nanoplastics could be rapidly internalized into macrophages and were toxic to macrophage activity. Only at a concentration above of 50 μg/ml, polystyrene nanoplastics might cause macrophages to polarize to inflammatory M1 but not pro-inflammatory M2. Since the imbalance of macrophage polarization is thought to be one of the pathological bases in the progression of autoimmune and autoimmune diseases, and people are exposed to more and more plastics with the aggravation of “white pollution”, this study provides dietary advice for these patients, and provides research direction to investigate the mechanism of MPs participating in the progression of these diseases.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Law, K. L. & Thompson, R. C. Oceans. Microplastics in the seas. Science 345, 144–145 (2014).
Geyer, B., Jambeck, J. R. & Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782 (2017).
Andrady, A. L. Microplastics in the marine environment. Mar. Pollut. Bull. 62, 1596–1605 (2011).
Wright, S. L. & Kelly, F. J. Plastic and human health: A micro issue?. Environ. Sci. Technol. 51, 6634–6647 (2017).
Andrady, A. L. The plastic in microplastics: A review. Mar. Pollut. Bull. 119, 12–22 (2017).
Koelmans, A. A. et al. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 155, 410–422 (2019).
Van Cauwenberghe, L. & Janssen, C. R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 193, 65–70 (2014).
Prata, J. C. et al. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 702, 134455 (2020).
Mamun, A. A. et al. Microplastics in human food chains: Food becoming a threat to health safety. Sci. Total Environ. 858, 159834 (2023).
Yan, Z. et al. Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ. Sci. Technol. 56, 414–421 (2022).
Leslie, H. A. et al. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 163, 107199 (2022).
Dong, C.-D. et al. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J. Hazard. Mater. 385, 121575 (2020).
Luo, H. et al. Environmental behaviors of microplastics in aquatic systems: A systematic review on degradation, adsorption, toxicity and biofilm under aging conditions. J. Hazard. Mater. 423, 126915 (2022).
Yuan, Z., Nag, R. & Cummins, E. Human health concerns regarding microplastics in the aquatic environment—From marine to food systems. Sci. Total Environ. 823, 153730 (2022).
Cao, J. et al. Polyethylene microplastics trigger cell apoptosis and inflammation via inducing oxidative stress and activation of the NLRP3 inflammasome in carp gills. Fish Shellfish Immunol. 132, 108470 (2023).
Liu, S. et al. Polystyrene microplastics aggravate inflammatory damage in mice with intestinal immune imbalance. Sci. Total Environ. 833, 155198 (2022).
Deng, Y. et al. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 7, 46687 (2017).
Li, Y. et al. In vitro study on the toxicity of nanoplastics with different charges to murine splenic lymphocytes. J. Hazard. Mater. 424, 127508 (2022).
Kögel, T. et al. Micro- and nanoplastic toxicity on aquatic life: Determining factors. Sci. Total Environ. 709, 136050 (2020).
Yan, Z. et al. Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ. Sci. Technol. 56, 414–421 (2021).
Rubio, L. et al. Biological effects, including oxidative stress and genotoxic damage, of polystyrene nanoparticles in different human hematopoietic cell lines. J. Hazard. Mater. 398, 122900 (2020).
Louiselle, A. E. et al. Macrophage polarization and diabetic wound healing. Transl. Res. 236, 109–116 (2021).
Mantovani, A. et al. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 21, 799–820 (2022).
Chen, W. et al. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat. Rev. Cardiol. 19, 228–249 (2021).
Christofides, A. et al. The complex role of tumor-infiltrating macrophages. Nat. Immunol. 23, 1148–1156 (2022).
Locati, M., Curtale, G. & Mantovani, A. Diversity, mechanisms, and significance of macrophage plasticity. Annu. Rev. Pathol. 15, 123–147 (2020).
Lazarov, T. et al. Physiology and diseases of tissue-resident macrophages. Nature 618, 698–707 (2023).
Watanabe, S. et al. The role of macrophages in the resolution of inflammation. J. Clin. Investig. 129, 2619–2628 (2019).
Kadomoto, S., Izumi, K. & Mizokami, A. Macrophage polarity and disease control. Int. J. Mol. Sci. 23, 144 (2021).
Ahamada, M. M., Jia, Y. & Wu, X. Macrophage polarization and plasticity in systemic lupus erythematosus. Front. Immunol. 12, 734008 (2021).
Cutolo, M. et al. The Role of M1/M2 macrophage polarization in rheumatoid arthritis synovitis. Front. Immunol. 13, 867260 (2022).
Ross, E. A., Devitt, A. & Johnson, J. R. Macrophages: The good, the bad, and the gluttony. Front. Immunol. 12, 708186 (2021).
Yang, S., Zhao, M. & Jia, S. Macrophage: Key player in the pathogenesis of autoimmune diseases. Front. Immunol. 14, 1080310 (2023).
Gerasimova, E. V. et al. Macrophage dysfunction in autoimmune rheumatic diseases and atherosclerosis. Int. J. Mol. Sci. 23, 4513 (2022).
Na, Y. R. et al. Macrophages in intestinal inflammation and resolution: A potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 16, 531–543 (2019).
Delfini, M. et al. Macrophages in the gut: Masters in multitasking. Immunity 55, 1530–1548 (2022).
Binbin, Y. et al. Preparation of monodisperse polystyrene microspheres with different functional groups using soap-free emulsion polymerization. Colloid Polym. Sci. 299, 1095–1102 (2021).
Wu, T. M. et al. Sarcodia suieae acetyl-xylogalactan regulate RAW 264.7 macrophage NF-kappa B activation and IL-1 beta cytokine production in macrophage polarization. Sci. Rep. 9, 19627 (2019).
Ekanayaka, A. H. et al. A review of the fungi that degrade plastic. J. Fungi 8, 772 (2022).
Ali, S. S. et al. Plastic wastes biodegradation: Mechanisms, challenges and future prospects. Sci. Total Environ. 780, 146590 (2021).
Marfella, R. et al. Microplastics and nanoplastics in atheromas and cardiovascular events. N. Engl. J. Med. 390, 900–910 (2024).
Yunna, C. et al. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 877, 173090 (2020).
Tardito, S. et al. Macrophage M1/M2 polarization and rheumatoid arthritis: A systematic review. Autoimmun. Rev. 18, 102397 (2019).
Peng, Y. et al. Regulatory mechanism of M1/M2 macrophage polarization in the development of autoimmune diseases. Mediat. Inflamm. 2023, 8821610 (2023).
Cabral, H. et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 6, 815–823 (2011).
Jiang, W. et al. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 3, 145–150 (2008).
Lv, S. et al. One-step microfluidic fabrication of multi-responsive liposomes for targeted delivery of doxorubicin synergism with photothermal effect. Int. J. Nanomed. 16, 7759–7772 (2021).
Keller, S., Berghoff, K. & Kress, H. Phagosomal transport depends strongly on phagosome size. Sci. Rep. 7, 17068 (2017).
Hu, J. et al. Polystyrene microplastics disturb maternal–fetal immune balance and cause reproductive toxicity in pregnant mice. Reprod. Toxicol. 106, 42–50 (2021).
Stock, V. et al. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Arch. Toxicol. 93, 1817–1833 (2019).
Li, S. et al. The crosstalk between M1 macrophage polarization and energy metabolism disorder contributes to polystyrene nanoplastics-triggered testicular inflammation. Food Chem. Toxicol. 180, 114002 (2023).
Kuroiwa, M. et al. Tim4, a macrophage receptor for apoptotic cells, binds polystyrene microplastics via aromatic–aromatic interactions. Sci. Total Environ. 875, 162586 (2023).
Jiang, L. et al. A high-fiber diet synergizes with Prevotella copri and exacerbates rheumatoid arthritis. Cell. Mol. Immunol. 19, 1414–1424 (2022).
Jia, X. M. et al. Compositional and functional aberrance of the gut microbiota in treatment naive patients with primary Sjogren’s syndrome. J. Autoimmun. 134, 102958 (2022).
Chen, B. D. et al. An autoimmunogenic and proinflammatory profile defined by the gut microbiota of patients with untreated systemic lupus erythematosus. Arthritis Rheumatol. 73, 232–243 (2021).
Acknowledgements
The research was funded by the National Natural Science Foundation of China (22204009), State Key Laboratory of Analytical Chemistry for Life Science (SKLACLS2315), the Natural Science Foundation of the Jiangsu Higher Education Institution of China (22KJB150019), the Initial Research Funding of Changzhou University (ZMF19020379, KYH21020405), Science and Technology Project of Changzhou Health Committee for Young Talents (QN202204), Leading Talent of Changzhou “The 14th Five-Year Plan” High-Level Health Talents Training Project (2022260).
Author information
Authors and Affiliations
Contributions
Conceptualization, W.J., Y.L., validation, Y.L. and Y.W.; formal analysis, Y.W.; and L.Z.; investigation, B.Z., and S.Z.; resources, M.W. and S.L.; data curation, W.J., and Y.L., writing—original draft preparation, W.J. and Y.L.; writing—review and editing, P.Z.; M.W. and T.X.; funding acquisition, S.L.; All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, W., Liu, Y., Wu, Y. et al. Polystyrene nanoplastics of different particle sizes regulate the polarization of pro-inflammatory macrophages. Sci Rep 14, 16329 (2024). https://doi.org/10.1038/s41598-024-67289-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67289-y
- Springer Nature Limited