Abstract
The association between the triglyceride-glucose (TyG) index and impaired fasting glucose (IFG) in elderly individuals remains uncertain. Our study aimed to explore the association between the TyG index and the risk of future IFG in this population. This retrospective cohort study included 17,746 elderly individuals over 60. In this population, Cox regression models proportional to hazards, along with smooth curve fitting and cubic spline functions, were employed to examine the association between the baseline TyG index and the risk of IFG. Subgroup analyses and sensitivity were also performed to ensure the robustness of the study findings. After adjusting for covariates, a positive association between the TyG index and the risk of IFG was found (HR = 1.43, 95% CI 1.27–1.60, P < 0.0001). The likelihood of IFG rose steadily as the TyG index quartiles (from Q1 to Q4) increased, with Q4 demonstrating a 62% elevated risk compared to Q1 (adjusted HR = 1.62, 95% CI 1.37–1.90). Additionally, we found the association between TyG index and risk of IFG was a linear. Sensitivity and subgroup analyses confirmed the stability of the results. Our study observed a linear association between the TyG index and the development of IFG in elderly Chinese individuals. Recognizing this association can help clinicians identify high-risk individuals and implement targeted interventions to reduce their risk of progressing to diabetes.
Similar content being viewed by others
Introduction
Impaired fasting glucose (IFG) is a glycemic state between normality and diabetes, defined by the American Diabetes Association (ADA) as fasting plasma glucose levels ranging from 5.6 to 6.9 mmol/L1. IFG is prevalent in the elderly globally, more so than diabetes itself in this demographic2,3. IFG not only increases the risk of developing type 2 diabetes but is also closely associated with elevated cardiovascular disease risk4,5,6,7. Additionally, research suggests a potential link between IFG and increased all-cause mortality8. Therefore, early identification and intervention in IFG risk factors are crucial for reducing disease burden.
Metabolic abnormalities, such as hyperuricemia and dyslipidemia, are closely associated with IFG. Uric acid has been identified as a component of metabolic syndrome9 and demonstrates a strong predictive value for hypertension, proteinuria, and peripheral artery disease10,11,12. Furthermore, uric acid is highly correlated with insulin levels, suggesting that it may serve as a potential biomarker for insulin resistance13. High triglycerides (TG) are an important component of dyslipidemia, closely associated with non-alcoholic fatty liver disease, insulin resistance, and atherosclerosis14,15,16. Studies have shown that any type of dyslipidemia is associated with an increased risk of developing diabetes17. High TG may impair glucose metabolism in muscles, reduce insulin sensitivity and metabolic status, and exacerbate insulin resistance18. Therefore, maintaining appropriate triglyceride levels is crucial for preventing insulin resistance, glucose metabolic disorders, and related cardiovascular diseases.
Recently, a blood glucose metabolism index, the triglyceride-glucose (TyG) index, calculated as the product of TG levels and fasting plasma glucose (FPG)19, has emerged as a convenient measure for assessing an individual’s degree of insulin resistance20. The TyG index is closely associated with various diseases. Studies have shown that the TyG index is positively correlated with the incidence of cardiovascular diseases such as carotid atherosclerosis, coronary artery disease, hypertension, and myocardial infarction21. Research has emphasized that the TyG index is one of the most effective markers for diagnosing metabolic syndrome22. Some studies have indicated that the TyG index is closely related to the occurrence and prognosis of diabetes23,24. Overall, the TyG index has important clinical significance in predicting cardiovascular diseases, glucose metabolic disorders, and other related diseases.
However, there is insufficient evidence regarding the association between the TyG index and risk of IFG among the elderly. Our study aims to investigate this association in individuals aged 60 and above, endorsing the TyG index’s clinical utility for early IFG detection, potentially influencing diabetes management and preventive healthcare in the elderly.
Results
Baseline characteristics of participants
The baseline characteristics of the study participants were summarized as showed in Table 1. The mean age was 67.01 ± 6.49 years, with 9751 participants (54.94%) being male. The mean follow-up time was 3.13 years, and 4063 individuals (22.89%) ultimately developed IFG. The study participants were grouped into IFG and Normal groups, with the IFG group displaying significantly higher mean age and BMI compared to the Normal group (P < 0.001). This group also had elevated SBP and DBP, as well as higher FBG levels (P < 0.001). Further differences included higher TG levels, lower HDL-c levels, and lower LDL cholesterol in the IFG group (P < 0.001). Additionally, ALT and Scr levels were significantly higher in the IFG group (P < 0.001). Sex distribution and smoking status also differed significantly between the two groups, while no significant differences were observed in drinking status and family history of diabetes. Notably, the follow-up duration was longer in the IFG group (P < 0.001). The distribution of the TyG index, as shown in Fig. 1, indicated a normal distribution ranging from 6.61 to 11.05, with a mean of 8.57.
Incidence of IFG in participants
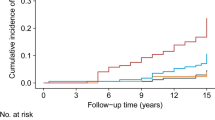
Table 2 and Fig. 2 describe the incidence rates of IFG. Among the participants, 4063 (22.9%) developed IFG. Participants were divided into subgroups based on the quartiles of the TyG index. The incidence rates of IFG per 1000 person-years were 58.89, 64.26, 79.69, and 90.33 for each TyG index quartile. The incidence rates of IFG in each TyG index quartile were as follows: Q1: 18.89%, Q2: 19.93%, Q3: 24.60%, and Q4: 28.08%. Participants with the highest TyG index (Q4) had a higher risk of developing IFG compared to those with the lowest TyG index (Q1) (trend P < 0.001).
The Kaplan–Meier curve depicts the probability of IFG occurrence, stratified by the TyG index. The graph demonstrates that the likelihood of IFG occurrence gradually increases with higher TyG index values. This suggests that patients with the highest TyG index have the highest probability of experiencing IFG.
Multivariable analysis using Cox proportional hazards regression model
In Table 3, the association between the TyG index and the risk of IFG is examined using multivariable analysis with the Cox proportional hazards regression model. The results show that in the crude model, the hazard ratio for the TyG index was statistically significant. When adjusting for age and sex (Model I), the hazard ratio remained significant but slightly decreased. Further adjustments in Model II, which included various additional factors, still showed a significant association between the TyG index and the risk of IFG. Additionally, after adjustments, consistent trends were observed across all quartiles of the TyG index, further highlighting the significant association between the TyG index quartiles and the risk of IFG. These findings suggest that the TyG index is independently associated with an increased risk of IFG, even after accounting for potential confounding factors. This underscores the importance of considering the TyG index in assessing the risk of IFG in clinical and research settings.
Sensitivity analysis
We conducted a series of sensitivity analyses to ensure the validity of our findings. Initially, in Model III using the generalized additive model (GAM) with additional smooth terms for various variables, we observed a HR of 1.48 (95% CI 1.32–1.66, P < 0.0001), indicating a significant association (Table 3). By excluding participants with a BMI ≥ 28 kg/m2 and adjusting for confounding factors, the results consistently showed a positive association between the TyG index and the risk of impaired fasting glucose (IFG) with an HR of 1.42 (95% CI 1.26–1.61, P < 0.0001). Moreover, we conducted sensitivity analyses by excluding individuals aged ≥ 80 years, and the association between the TyG index and IFG risk remained significant (risk ratio = 1.45, 95% CI 1.29–1.64, P < 0.0001) after adjustments. Additionally, when analyzing participant’s SBP < 140 mmHg, the risk ratio was 1.51 (95% CI 1.31–1.74, P < 0.0001) (Table 4). After considering all these sensitivity analyses, we can confidently conclude that our results are dependable and robust, emphasizing the consistent positive association between the TyG index and the risk of IFG.
Association between the TyG index and the risk of IFG
In our study, we found a clear linear association between the TyG index and the risk of IFG. By utilizing a Cox proportional hazards regression model with cubic spline functions, we were able to evaluate this association and confirmed that it is indeed linear (Fig. 3). This finding suggests that as the TyG index increases, so does the risk of developing IFG.
Subgroup analysis results
As illustrated in Table 5, a detailed subgroup analysis and interactions were conducted. Firstly, we stratified the analysis by age, gender, BMI, systolic and diastolic blood pressures, smoking and drinking habits, and a family history of diabetes. Through multivariate Cox proportional hazards models, we found statistically significant differences in HR (95% CI) between the 60–70 years old and 70–80 years old groups. However, there were no statistically significant differences in HR (95% CI) between the 80–90 years old and ≥ 90 years old groups. Additionally, in the subgroups of BMI < 18.5 kg/m2 and ≥ 28 kg/m2, there were no statistically significant differences in HR (95% CI). Secondly, we performed an interaction analysis for all subgroups, and we found that no significant interactions were observed between these variables and the TyG index (all interaction P > 0.05).
Discussion
This retrospective cohort study aims to investigate the association between the TyG index and the risk of IFG. We found that the risk of IFG increases with the TyG index, and a linear association between them was observed. The highest quartile of the TyG index has a 1.62 times higher risk of IFG compared to the lowest quartile. These results suggest that the TyG index may be an effective indicator for monitoring IFG.
Globally, the incidence of prediabetes is on the rise. According to estimates by the World Health Organization, in 2021, the global diabetes population was approximately 537 million. The prevalence of diabetes in China rose significantly from 22.5 million individuals in the year 2000 to a staggering 140.9 million by 202125. The number of people with prediabetes exceeds these figures. A study in the United States suggests that about one-third of adults are considered to have prediabetes26. The prevalence of prediabetes in Chinese adults is alarmingly high, with an estimated rate of 50.1%. the prevalence of prediabetes is higher in men, with a rate of 52.1%, compared to women at 48.1%. This suggests that one out of every two Chinese adults may be at risk of developing diabetes if proper measures are not taken27. Studies have indicated that the standardized incidence rate of prediabetes in the overall population of China is 62.6 cases per 1000 person-years (73.8 cases per 1000 person-years among males and 51.2 cases per 1000 person-years among females)28. Additionally, another cohort study in China, which included 4093 Chinese adults with a median follow-up time of 3.25 years, found that 26.2% of participants developed prediabetes29. IFG is a state of prediabetes, and the primary outcome variable studied here was IFG. Our study reveals that over a 5-year period, 22.9% of participants developed impaired fasting glucose (IFG), with an incidence rate of 73.19 per 1000 person-years. The differences in prediabetes incidence rates among various studies could be attributed to variations in participants’ age, follow-up duration, and ethnicity. Notably, less than 11% of individuals are aware of their condition30. Therefore, identifying factors leading to IFG is crucial for preventing diabetes and its complications.
A cohort study in China, involving 201,298 individuals, found that a high TyG index was independently associated with the risk of developing diabetes (HR) = 3.34; 95% CI 3.11–3.60)31. Another cohort study involving 4543 Chinese adults utilized logistic regression analysis adjusted for several confounders, showing that for each standard deviation increase in the TyG index, the risk of prediabetes increased by 1.38 times (95% CI 1.28–1.48)29. Hence, we hypothesize that an increase in the TyG index might be related to an increased risk of IFG in the elderly. Unfortunately, reports on their relationship are scarce. Linhao Zhang et al. found a non-linear association between the TyG index and impaired fasting blood glucose by analyzing data from 25,159 patients32. Xiaoxia Li et al. demonstrated that, based on baseline data, logistic analysis showed that after multivariate adjustment, the TyG index was significantly positively correlated with IFG. However, the association was not significant after further adjustment (HR = 1.06; 95% CI 0.58–1.96; P for trend = 0.784)33. This study may differ from ours because the sample size was not as large as ours. Additionally, the average age of participants in the study by Li et al. (48.2 years) was much younger compared to our study participants (67.01 years). This difference in age may be a significant factor contributing to the discrepancies in study outcomes. However, our study found a positive, linear association between the TyG index and the risk of IFG in the elderly. Our research adds to the existing literature, supporting the hypothesis that an elevated TyG index is associated with an increased IFG risk. Compared to other studies, in our research, the TyG index was utilized in both categorical and continuous forms to examine the association between TyG index and the risk of IFG. Our aim was to minimize loss of information and accurately measure the association between the two variables. Furthermore, the confounding factors adjusted in our study differ from previous ones. We adjusted for a wider variety of variables, such as age, gender, systolic blood pressure, diastolic blood pressure, body mass index, alanine aminotransferase, aspartate aminotransferase, blood urea nitrogen, serum creatinine, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, diabetes family history, alcohol consumption, and tobacco use. Sensitivity analysis confirmed that this association persists even after excluding participants with age ≥ 80 years, BMI ≥ 28 kg/m2, and SBP ≥ 140 mmHg. Additionally, subgroup analyses and interaction tests on age, DBP, BMI, SBP, gender, and family history of diabetes showed no interactions, confirming the stability of the association between the TyG index and IFG risk. This finding provides a reference for clinical interventions to reduce the probability of IFG in the elderly by targeting the TyG index.
The study has several strengths. Firstly, we have established a linear association between the TyG index and the risk of IFG among the elderly for the first time. In addition, the research involved a substantial group of 17,746 senior citizens and accounted for variables like BMI, age, TC, SBP, BUN, ALT, AST, DBP, Scr, LDL-C, HDL-C, gender, diabetic family history, alcohol consumption, and tobacco use to reduce possible distortions. To ensure the reliability and robustness of the results, sensitivity analysis was performed. Moreover, subgroup analysis and tests for interactions were conducted. The results indicate that the TyG index has different effects on IFG risk across various subgroups, further validating the experiment’s stability.
Despite these strengths, our study has several limitations. First, the average follow-up duration of the study participants was only 5.0 years, which is relatively short. Second, participants were only followed up once, with information available only at two time points (baseline and follow-up). Third, there were no available serum insulin level data, thus the predictive value of the TyG index could not be compared. Fourth, values for impaired glucose tolerance at 2 h or HbA1c were not collected, hence further analysis of the association between the TyG index and changes in glucose tolerance and HbA1c was not possible. Fifth, the study focused only on individuals aged over 60 years, and may not represent all populations; further research should expand to include various age groups. In addition, the original study did not collect data on uric acid. Therefore, we are unable to include uric acid in the current analysis. Future research should consider collecting and analyzing uric acid and other potentially influential factors on IFG to comprehensively elucidate their roles.
Conclusion
This study revealed a linear association between the TyG index and the IFG in Chinese individuals aged over 60. Understanding this linear association can help clinicians identify high-risk elder individuals and implement focused interventions to reduce the risk of developing diabetes.
Methods
Study design
This study utilized data from a previous retrospective cohort study conducted by Chinese researchers34. The target independent variable was the TyG at baseline. The outcome variable was the development from normoglycemia to IFG at follow-up.
Data source
Access to the original dataset was granted at no cost through the DATADRYAD platform (http://www.datadryad.org), courtesy of Ying Chen et al.34. In accordance with Dryad’s usage policy, the data is available for academic and research purposes, allowing users to share, adapt, alter, and build upon the material, provided it is not for commercial use and proper attribution is given to the original authors and source. The dataset was sourced from a publicly accessible study published in 2018 titled “Association of body mass index and age with diabetes onset in Chinese adults: a population-based cohort study,” which can be found at https://doi.org/10.1136/bmjopen-2018-021768. For those interested, the dataset can be retrieved from the following link: https://doi.org/10.5061/dryad.ft8750v.
Ethics approval and consent to participate
The prior study received ethical approval from the Rich Healthcare Group Review Board. Given that the current study involves a secondary analysis of existing data, there was no need for obtaining informed consent or additional ethical approval. All methods were performed in accordance with the relevant guidelines and regulations.
Research population
The primary research included 685,277 individuals aged 20 years and above, all of whom had undergone a minimum of two health assessments. The study focused on participants who, during follow-up, had FPG levels ranging from 6.1 to 6.9 mmol/L without any prior diagnosis of diabetes. The initial selection excluded participants based on several factors: (1) lack of detailed information regarding weight, height, or gender; (2) BMI values outside the normal range (< 15 kg/m2 or > 55 kg/m2); (3) intervals between visits shorter than 2 years; (4) missing FPG readings; (5) individuals diagnosed with diabetes at the start or with uncertain diabetes status at the time of follow-up. Following these criteria, the study retained 211,833 participants.
Further analysis led to the exclusion of an additional 194,087 participants for reasons including: (1) absence of follow-up FPG readings, (2) baseline FPG levels ≥ 5.6 mmol/L, (3) FPG levels > 6.9 mmol/L during follow-up, (4) unclear diabetes diagnosis at follow-up, and (5) lack of triglyceride (TG) values or being less than 60 years of age. Elder people are defined as those aged over 60 years old or older25. Ultimately, the study included 17,746 participants. The process of selecting participants for this study is depicted in Fig. 4.
Data collection
In this study, data collection included demographic information such as age, diastolic blood pressure (DBP), systolic blood pressure (SBP), height, and weight, from which body mass index (BMI) was calculated. To ensure consistency in data collection, staff received specialized training focusing on demographic data and key measurements, including blood pressure. Tests were uniformly conducted in a standardized laboratory environment for triglycerides (TG), high-density lipoprotein cholesterol (HDL-c), total cholesterol (TC), blood urea nitrogen (BUN), serum creatinine (Scr), alanine aminotransferase (ALT), low-density lipoprotein cholesterol (LDL-c), and FPG. Additionally, the study collected information on the patients’ smoking and drinking histories, defining current drinking as 1, former drinking as 2, never drinking as 3, and unknown drinking status as 4. Similarly, current smoking was coded as 1, former smoking as 2, never smoking as 3, and unknown smoking status as 4. A family history of diabetes mellitus is obtained by inquiring whether the patient’s first-degree relatives (parents, grandparents, siblings, etc.) have diabetes.
Outcome and definitions
At follow-up, our focus was on identifying individuals who had an impaired fasting glucose (IFG) condition. This was determined by having fasting plasma glucose (FPG) levels fall within the range of 5.6–6.9 mmol/L, with no reported cases of new-onset diabetes35. We defined “elderly patients” as aged over 6036.
Missing data processing
In this study, the number of participants with missing data was as follows: 7 (0.00%) each for DBP and SBP, 1 person (0.00%) for TC, 869 (0.69%) for ALT, 72640 (58%) for AST, 60634 (48.39%) for LDL-c, 6192 (4.9%) for Scr, 60579 (48.34%) for HDL-c, and 11452 (9.1%) for BUN. To mitigate the uncertainty caused by missing data, this study utilized multiple imputation techniques. The imputation model included ALT, LDL-c, AST, Scr, HDL-c, and BUN, with 5 iterations using linear regression. Data analysis assumed that missingness was random (MAR)37,38.
Statistical analysis
The main variable examined in this research is the TyG index, which is defined by the equation: TyG index = ln [FPG (mg/dL) × TG (mg/dL)/2]39,40,41. We divided it into four quartiles and considered it as a continuous variable. We presented the mean and standard deviation for continuous variables that followed a normal distribution, while the median was reported for data that did not follow a normal distribution. For categorical variables, we presented the frequency and proportion of the data in our study. To analyze the differences between different TyG index groups, we utilized the Kruskal–Wallis H test for data that was not normally distributed, one-way analysis of variance for normally distributed data, and the chi-square test for categorical variables.
We developed numerous models to evaluate the association between the TyG index and the risk of IFG: a baseline model without any adjustments, a simplified model adjusting for gender and age only (Model I), and a comprehensive model adjusting for multiple covariates (Model II: recording a variety of demographic and health-related variables, such as age, gender, BMI, blood pressure, liver enzymes, cholesterol levels, serum creatinine, family history of diabetes, alcohol consumption, and smoking status, in our study. From each model, we noted the effect size (hazard ratio HR) along with its 95% confidence interval (CI).
To validate our findings, we conducted a series of sensitivity analyses. By incorporating continuous variables into a generalized additive model (GAM) in curve form, we further confirmed the robustness of the results. Additionally, we conducted analyses using stratified Cox proportional hazards models in different subgroups (such as age, gender, blood pressure, smoking, and drinking status). Finally, we used likelihood ratio tests to examine whether there were interactions in the model, both in models including interaction terms and those without. All analyses were performed using Empower Stats (X&Y Solutions, Inc., Boston, MA, http://www.empowerstats.com), with a statistical significance level set at a two-sided P value less than 0.05.
Ethics statement
The studies involving human participants were reviewed and approved by Rich Healthcare Group Review Board. The ethics committee waived the requirement of written informed consent for participation.
Data availability
The dataset was sourced from a publicly accessible study published in 2018 titled “Association of body mass index and age with diabetes onset in Chinese adults: a population-based cohort study,” which can be found at https://doi.org/10.1136/bmjopen-2018-021768. For those interested, the dataset can be retrieved from the following link: https://doi.org/10.5061/dryad.ft8750v.
References
American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care 33(Suppl 1), S11–S61 (2010).
Jorge-Galarza, E. et al. Adipose tissue dysfunction increases fatty liver association with prediabetes and newly diagnosed type 2 diabetes mellitus. Diabetol. Metab. Syndr. 8, 73 (2016).
Ek, A. E., Rössner, S. M., Hagman, E. & Marcus, C. High prevalence of prediabetes in a Swedish cohort of severely obese children. Pediatr. Diabetes 16, 117–128 (2015).
Kim, M. K. et al. Cumulative exposure to impaired fasting glucose and future risk of type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 175, 108799 (2021).
Kim, J. H. & Lim, J. S. Trends of diabetes and prediabetes prevalence among Korean adolescents from 2007 to 2018. J. Korean Med. Sci. 36, e112 (2021).
Zuo, Y. et al. Association of impaired fasting glucose with cardiovascular disease in the absence of risk factor. J. Clin. Endocrinol. Metab. 107, e1710–e1718 (2022).
Lind, V. et al. Impaired fasting glucose: A risk factor for atrial fibrillation and heart failure. Cardiovasc. Diabetol. 20, 227 (2021).
Lee, S. H., Han, K., Kwon, H. S. & Kim, M. K. Frequency of exposure to impaired fasting glucose and risk of mortality and cardiovascular outcomes. Endocrinol. Metab. 36, 1007–1015 (2021).
Yang, T. et al. Uric acid level as a risk marker for metabolic syndrome: A Chinese cohort study. Atherosclerosis 220, 525–531 (2012).
Yang, T. et al. Uric acid concentration as a risk marker for blood pressure progression and incident hypertension: A Chinese cohort study. Metabolism 61, 1747–1755 (2012).
Tseng, C. H. Correlation of uric acid and urinary albumin excretion rate in patients with type 2 diabetes mellitus in Taiwan. Kidney Int. 68, 796–801 (2005).
Tseng, C. H. Independent association of uric acid levels with peripheral arterial disease in Taiwanese patients with Type 2 diabetes. Diabetic Med. 21, 724–729 (2004).
Tseng, C. H. & Tai, T. Y. Risk factors for hyperinsulinemia in chlorpropamide-treated diabetic patients: A three-year follow-up. J. Formos. Med. Assoc. 91, 770–774 (1992).
Farrell, G. C. & Larter, C. Z. Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology 43, S99–S112 (2006).
Jin, R. et al. Dietary fructose reduction improves markers of cardiovascular disease risk in Hispanic-American adolescents with NAFLD. Nutrients 6, 3187–3201 (2014).
Hsieh, M. H. et al. Waist circumference, body mass index, serum uric acid, blood sugar, and triglyceride levels are important risk factors for abnormal liver function tests in the Taiwanese population. Kaohsiung J. Med. Sci. 28(9), 470–476 (2012).
Mooradian, A. D. Dyslipidemia in type 2 diabetes mellitus. Nat. Clin. Pract. Endocrinol. Metab. 5, 150–159 (2009).
Guerrero-Romero, F. et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 95, 3347–3351 (2010).
Unger, G., Benozzi, S. F., Perruzza, F. & Pennacchiotti, G. L. Triglycerides and glucose index: A useful indicator of insulin resistance. Endocrinol. Nutr. 61, 533–540 (2014).
Fritz, J. et al. The triglyceride-glucose index as a measure of insulin resistance and risk of obesity-related cancers. Int. J. Epidemiol. 49, 193–204 (2020).
Huang, R. et al. Prognostic value of triglyceride glucose (TyG) index in patients with acute decompensated heart failure. Cardiovasc. Diabetol. 21, 88 (2022).
Khan, S. H. et al. Metabolic clustering of risk factors: Evaluation of Triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol. Metab. Syndr. 10, 74 (2018).
Li, X. et al. Association between triglyceride-glucose index and risk of incident diabetes: A secondary analysis based on a Chinese cohort study: TyG index and incident diabetes. Lipids Health Dis. 19, 236 (2020).
Luo, E. et al. High triglyceride-glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc. Diabetol. 18, 150 (2019).
Sun, H. et al. Erratum to “Idf Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045” [Diabetes Res. Clin. Pract. 183 (2022) 109119]. Diabetes Res. Clin. Pract. 204, 110945 (2023).
Ogurtsova, K. et al. Idf Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 128, 40–50 (2017).
Xu, Y. et al. Prevalence and control of diabetes in Chinese adults. JAMA 310, 948–959 (2013).
Cao, C. et al. Nonlinear relationship between aspartate aminotransferase to alanine aminotransferase ratio and the risk of prediabetes: A retrospective study based on Chinese adults. Front. Endocrinol. 13, 1041616 (2022).
Wen, J. et al. Elevated triglyceride-glucose (Tyg) index predicts incidence of prediabetes: A prospective cohort study in China. Lipids Health Dis. 19, 226 (2020).
Horstman, C., Aronne, L., Wing, R., Ryan, D. H. & Johnson, W. D. Implementing an online weight-management intervention to an employee population: Initial experience with real appeal. Obesity 26, 1704–1708 (2018).
Li, X. et al. Association between triglyceride-glucose index and risk of incident diabetes: A secondary analysis based on a Chinese cohort study: Tyg index and incident diabetes. Lipids Health Dis. 19, 236 (2020).
Zhang, L. & Zeng, L. Non-linear association of triglyceride-glucose index with prevalence of prediabetes and diabetes: A cross-sectional study. Front. Endocrinol. 14, 1295641 (2023).
Li, X. et al. Association of non-insulin-based insulin resistance indices with risk of incident prediabetes and diabetes in a Chinese rural population: A 12-year prospective study. Diabetes Metab. Syndr. Obes. 15, 3809–3819 (2022).
Chen, Y. et al. Association of body mass index and age with incident diabetes in Chinese adults: A population-based cohort study. BMJ Open 8, e21768 (2018).
American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care 44, S15–S33 (2021).
Torreggiani, M. et al. Spontaneously low protein intake in elderly CKD patients: Myth or reality? Analysis of baseline protein intake in a large cohort of patients with advanced CKD. Nutrients 13, 4371 (2021).
White, I. R., Royston, P. & Wood, A. M. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 30, 377–399 (2011).
Groenwold, R. H. et al. Missing covariate data in clinical research: When and when not to use the missing-indicator method for analysis. Can. Med. Assoc. J. 184, 1265–1269 (2012).
Qin, Y. et al. A high triglyceride-glucose index is associated with contrast-induced acute kidney injury in Chinese patients with type 2 diabetes mellitus. Front. Endocrinol. 11, 522883 (2020).
Chen, X., Liu, D., He, W., Hu, H. & Wang, W. Predictive performance of triglyceride glucose index (TyG index) to identify glucose status conversion: A 5-year longitudinal cohort study in Chinese pre-diabetes people. J. Transl. Med. 21, 624 (2023).
Zhang, Q., Xiao, S., Jiao, X. & Shen, Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: Evidence from NHANES 2001–2018. Cardiovasc. Diabetol. 22, 279 (2023).
Author information
Authors and Affiliations
Contributions
Jie Liu, Feng Yi, and Kai Duan contributed to the study concept and design, researched, and interpreted the data, and drafted the manuscript. Haibo Liu analyzed the data and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, J., Yi, F., Duan, K. et al. Triglyceride-glucose index is associated with the risk of impaired fasting glucose in Chinese elderly individuals. Sci Rep 14, 16033 (2024). https://doi.org/10.1038/s41598-024-67081-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67081-y
- Springer Nature Limited