Abstract
This work was inspired by the observation that a majority of MR-electrical properties tomography studies are based on direct comparisons with ex vivo measurements carried out on post-mortem samples in the 90’s. As a result, the in vivo conductivity values obtained from MRI in the megahertz range in different types of tissues (brain, liver, tumors, muscles, etc.) found in the literature may not correspond to their ex vivo equivalent, which still serves as a reference for electromagnetic modelling. This study aims to pave the way for improving current databases since the definition of personalized electromagnetic models (e.g. for Specific Absorption Rate estimation) would benefit from better estimation. Seventeen healthy volunteers underwent MRI of both brain and thorax/abdomen using a three-dimensional ultrashort echo-time (UTE) sequence. We estimated conductivity (S/m) in several classes of macroscopic tissue using a customized reconstruction method from complex UTE images, and give general statistics for each of these regions (mean-median-standard deviation). These values are used to find possible correlations with biological parameters such as age, sex, body mass index and/or fat volume fraction, using linear regression analysis. In short, the collected in vivo values show significant deviations from the ex vivo values in conventional databases, and we show significant relationships with the latter parameters in certain organs for the first time, e.g. a decrease in brain conductivity with age.
Similar content being viewed by others
Introduction
Electrical properties (EPs), namely conductivity \(\upsigma \) and permittivity \(\upepsilon \), are fundamental properties of matter that quantify the degree and nature of its interaction with electromagnetic fields. In human tissues, they are closely related to important biological quantities such as ionic concentration, intra- or extracellular volumes and local architectures. As dispersive parameters, they also vary with the operating frequency, suggesting the possibility of multispectral tissue characterization1. These reasons suggest that they would make useful new biomarkers for the clinical use and that they would provide a better understanding of the interactions between electromagnetic waves and the human body2. Obtaining reliable in vivo EP values, and knowing their variability in the population, is especially important when considering the use of standard electromagnetic models for Specific Absorption Rate (SAR) estimation3. Better modelling might reduce safety margins and, ultimately, examination times. At the same time, the evolution of conductivity in relation to patient characteristics may provide new clues for understanding certain physiological mechanisms, such as aging.
Historically, classical methods for estimating EPs in tissues were performed on excised pieces of tissue4,5. In recent decades, however, theoretical and technical efforts have enabled in vivo measurements, thanks to the combination of stimulation hardware and numerical capabilities for solving challenging problems. As a result, invasive current-injection imaging techniques6 now coexist with induction-based methods7 over a wide frequency range. From this point of view, it was soon realized that MRI, beyond its imaging capabilities, could also be used as a non-invasive induction setup8,9. In the specific MR radiofrequency range, this led to the development of a specific research field now known as MR-Electrical Properties Tomography (MR-EPT)10,11.
One of the main features of MRI is the manipulation of a radiofrequency (RF) field – the transmit field \({\text{B}}_{1}^{+}\) – at the Larmor frequency, to induce resonance. At the same time, these radio waves interact with tissues, forming currents whose imprint can be identified in the measured signal – the receive field \({\text{B}}_{1}^{-}\) – after reception. The aim of MR-EPT is to isolate this signature in the MR signal and infer tissues EPs from an approximate mathematical model. Beyond safety issues, best documented for MRI12 than for current-injection procedures13, the main advantage of MR-EPT is that it can be easily integrated into a standard MRI examination. In addition, it naturally takes advantage of the high-resolution capacity of MR images to better constrain the solving of the inverse problem at hand.
In its simplest form, it is formulated as a homogeneous Helmholtz equation involving one or both RF components of the signal10,14,15,16, \({\text{B}}_{1}^{+}\) and \({\text{B}}_{1}^{-}\) where, more specifically, tissue EPs are derived directly from the spatial second derivatives of the fields. Although very straightforward, this procedure lacks precision as the calculation of second derivatives is very sensitive to the inherent noise of MRI17,18, and because it makes strong assumptions on both the signal and Maxwell equations for sake of simplification19,20,21. In particular, it assumes that spatial variations of EPs are piecewise constant. A common strategy to mitigate noise amplification is to use large voxel sizes, specific differentiation kernels or strong image filters18,22,23,24,25,26 at the cost of amplifying edge effects at tissue boundaries. Meanwhile, numerous attempts have been made to improve the quality of the maps reconstructed in MR-EPT. A first class of advanced methods strives to maintain a direct equation model, closer to physical reality, i.e. not assuming piecewise constant EPs, at the cost of greater sensitivity to noise27,28,29,30,31,32. Another class of advanced methods, which have yet to be validated for in vivo use, employ more general models that may be more faithful to the original Maxwell equations, however they result in a more complex signal equation33,34,35,36, sometimes involving non-measurable quantities, such as the scattered RF electrical field. The last class of advanced methods can be termed data-driven, including methods based on supervised machine learning37,38,39,40 and those using models for signal matching41,42. Importantly, the latter methods make use of a priori information on the data (explicitly or implicitly) or the result to be obtained. While a prior is often needed to better constrain the problem, it can also introduce an undesired bias preventing absolute quantification, e.g. if the solver was trained to match the ex vivo values.
Many studies have also attempted to provide reference in vivo EP values, associated with specific organs or pathologies such as brain tissues16,42,43,44, brain tumors45,46, breast lesions47,48,49, musculoskeletal tissues50, pelvis51 or rat brain52 and liver53. These works have focused on conductivity rather than permittivity, because permittivity reconstruction is even more prone to noise54. Given the inherent sensitivity and variability of MR-EPT techniques, large discrepancies in conductivity values are found although the orders of magnitude are close. Interestingly, the simplest formulation of MR-EPT has been used in such studies, both because it is very convenient and because advanced methods are difficult to adapt to in vivo data. Even though voxel-wise estimation is still very challenging with such methods, focusing on an average value for the organ of interest greatly tempers the effect of noise37. Authors have compared the measured in vivo values to ex vivo databases, but such comparison should be done carefully since the conditions are very different. In short, existing in vivo studies show significant deviations from these ex vivo reference values, when these ex vivo values are not used as a prior for the reconstruction43,55,56. A recent review of the literature on variations in brain tissue conductivity highlights the uncertainties in this regard, emphasizing that correlations with individual conditions – such as pathologies – are similar however 57.
In this study, we therefore sought to estimate macroscopic values of conductivity in several organs (brain and torso) in a small adult population (17 volunteers) from complex images of a 3D UTE sequence, and using a homogeneous Helmholtz MR-EPT formulation15,19,32,58,59,60. We then explored the relationship of these quantities with biological parameters such as age, Fat Volume Fraction (FVF), BMI (Body Mass Index) or sex, and identify relevant correlations.
Materials and methods
This study was approved by an ethics committee (Comité de Protection des Personnes Sud Est III) and informed written consent was obtained (ClinicalTrials.gov identifier: NCT04645628, first trial registration 27/11/2020). The study was supported by CIC-IT Nancy, sponsored by CHRU Nancy (Département Méthodologie, Promotion, Investigation), and carried out in accordance with the relevant guidelines and regulations.
Simulation
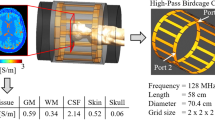
The proof of concept of this study was performed using a simple EM simulation with the commercial software CST Studio Suite 2023 (V6R2023x.FP.CFA.2419, https://software.3ds.com, Computer Simulation Technology, Dassault Systemes, France). A two-port quadrature birdcage coil and a multi-compartment cylindrical conductive volume wereq modeled and used to simulate both RF transmit and receive fields at 123 MHz. The outer diameter of the cylindrical volume is 30 cm, comparable to the dimensions of a human body, and the concentric sections are between 3 and 4 cm. The conductivity range has been chosen to be representative of most tissues. The composite RF field data were then exported to MATLAB, where we added artificial Gaussian noise to obtain an SNR comparable to our experimental images. Reconstructed conductivity values were compared with the reference values in both noiseless and noisy models.
Study population
The average conductivity standard deviation of previous studies16,55 can be approximated at 0.3 S/m for the best results.The sample size required to provide a 95% confidence interval with a 0.16 S/m margin of error is fourteen50. Assuming a dropout rate of 15%, the MR-EPT study was performed on seventeen volunteers (10 males & 7 females) aged between 25 and 73 years old (mean = 43.5, SD = 16.9), with body mass index range from 19.6 to 32.6 kg/m2 (mean = 24.5, SD = 2.6). Besides, this number of volunteers allows us to test a maximum of two predictors.
MRI measurements
Imaging studies were performed on a 3 T MAGNETOM Prisma MRI scanner (Siemens Healthineers, Forchheim, Germany), with standard quadrature birdcage coil for transmission. A 20-channel and 18 + 20-channel phased array coils were respectively used in receive mode for brain and torso mapping.
For the brain study, a reference anatomical volume was acquired using a conventional MPRAGE sequence while complex images from a UTE SpiralVIBE prototype61,62 were used for assessing conductivity. It consists in a stack-of-spirals trajectory with adaptive TE and variable-duration slice encoding to minimize \({\text{T}}_{2}^{*}\) decay63. The relevance of this type of sequence has been demonstrated in the literature for its significant RF weighting15,60 and robustness to off-resonance effect and eddy currents.
Regarding torso imaging, UTE images were acquired during a breath-hold period, optimizing resolution, k-space acquisition scheme and slice direction. Reference Water/Fat images were obtained using a standard VIBE DIXON sequence in coronal view. FVF maps were generated from the ratio F/(W + F) applied to each organ. We should point out that we used a dual-echo acquisition to reduce breath-hold time, and this could also lead to a bias in the exact estimation of fat fraction64. If this bias is consistent across all organs, however, the trends measured should be similar.
In each case, an optimal 2-port transmission technique was used65, and receive coil combination was achieved for UTE imaging using the vendor’s reference-based adaptive approach66 which mimics the data from a complete body coil scan. Phase unwrapping was applied when needed as a simple constant shift since the range of phase variations for our applications lies into [\(-\uppi ,\uppi \)]. Moreover, an acceleration factor of 2 was also used with the SPIRiT 67 method to reduce acquisition time, without compromising the reconstruction accuracy44.
For both tissue classes, the specific sequence parameters are reported in Table 1.
Registration and segmentation
Firstly, in each case, the reference images (i.e., MPRAGE and VIBE DIXON images) were resampled and re-aligned to the UTE magnitude images using spline interpolation and a total-variation-regularized non-rigid registration algorithm68, respectively (Fig. 1). Then for organ-specific region-of-interest (ROI) analysis, we used SPM's segmentation tool (SPM12, London, UK) to post-process the brain MPRAGE registered volume to segment gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF). For torso organ segmentation, we used a semantic nnU-Net pipeline69 applied to the AMOS2270 dataset. This specific database contains images labelled with fifteen different tissue classes and was the basis of the recent Multi-Modality Abdominal Multi-Organ Segmentation Challenge (MICCAI 2022). The network was implemented with PyTorch71 and trained in about 8 h on a GPU (NVIDIA Quadro RTX 5000). After training, we applied it to our registered VIBE DIXON data and segmentations took about one minute per 3D volume. In addition to the tissues labeled in the AMOS database, visceral abdominal fat (VAT), heart and lungs were added semi-automatically from 3DSlicer72.
Representation of the reconstruction pipeline for brain (a) and torso (b) images. Native MPRAGE or VIBE DIXON images are first re-aligned to their respective UTE volumes, then segmented using SPM12 or a combination of nnU-Net and manual delineation.The registered segmentation masks provide both a contrast prior (for constrained estimation of the Laplacian), and the ROIs for subsequent statistical analysis.
Conductivity reconstruction
After acquisition, the images were transferred to a workstation and processed using MATLAB (The MathWorks, Natick, MA, USA). The choice of a UTE sequence makes it possible to reconstruct conductivity maps directly from complex images, i.e. the combination of magnitude and phase, according to the following relationship 15,19:
where \(\kappa \underline{\underline{{{\text{def}}}}} \sigma + {\text{i}}\omega \in\) is known as complex admittivity, \(\upmu \) is the local magnetic permeability, assumed to be equal to that of the vacuum \({\upmu }_{0}\) in the RF range, \(\omega =\gamma {\text{B}}_{0}\) is the Larmor frequency, \(\Delta \) is the Laplacian operator and \({\text{B}}_{1}^{+}{\text{B}}_{1}^{-}\) is the RF fields product. From a theoretical point of view, this relationship is obtained from a combination of two homogeneous Helmholtz equations applied to \({\text{B}}_{1}^{+}\) and \({\text{B}}_{1}^{-}\), multiplied by \({\text{B}}_{1}^{-}\) and \({\text{B}}_{1}^{+}\) respectively, to yield the Laplacian of the product \({\text{B}}_{1}^{+}{\text{B}}_{1}^{-}\). The square root appears after the neglected terms have been omitted. The magnitude and phase of \(\sqrt{{\text{B}}_{1}^{+}{\text{B}}_{1}^{-}}\) are defined straightforwardly as the magnitude of the product root and half of its phase, assuming proper unwrapping beforehand. Suitability of the UTE sequence here depends on the use of a small flip angle, allowing the product \({\text{B}}_{1}^{+}{\text{B}}_{1}^{-}\) to appear directly in the signal73 as \({\text{S}}_{\text{UTE}}={\text{I}}_{0}{\text{B}}_{1}^{+}{\text{B}}_{1}^{-}\) (\({\text{I}}_{0}\) = density and relaxation contrasts). This formulation is more general than those involving only the RF phase component30 and more accurate when the correct assumptions are satisfied15, essentially that EPs and \({\text{I}}_{0}\) contrasts are piecewise constant i.e. \(\nabla\upkappa =0\) and \(\nabla {\text{I}}_{0}=0\). In this study, the Laplacian was calculated for each target voxel using a bi-adaptive strategy with a constrained Savitzky-Golay kernel. First, a large selection kernel size31 was used to identify voxels with signal intensity close to the central target voxel. This was done by selecting voxels that belonged to the same segmentation class as the central target voxel, using the segmentation masks obtained from the reference MPRAGE/DIXON images. Once this first mask was obtained, we kept the n nearest neighbors in the sense of the Euclidean distance74 for the actual fitting process. This bi-adaptive strategy allowed to: (i) limit the bias at the boundaries between tissues; (ii) keep the number of voxels sufficiently large for the Savitzky-Golay fit, thereby preventing noise amplification (Fig. 2). The size of the calculation kernel was adapted for each component of this study (brain: N = 153 and torso: N = 233) as the image resolutions were slightly different. This appeared to be the best compromise for preserving final resolution (brain: 1.2 × 1.2x1.2 mm3, torso: 1.95 × 1.95x2.5 mm3) and mitigating the effect of noise. Note that no pre- or post-filtering was used to avoid undesired smoothing effects. Conductivity values were finally estimated within the organ-specific ROIs as mean, median and standard deviation. These ROIs are built from individual segmentations where each organ is uniquely labeled, and on which we perform a single-voxel erosion to reduce edge effects.
Simulation study as proof of concept. The bi-adaptive kernel (a) optimizes the fitting area and constrains the homogeneity of the Laplacian estimate, based on both contrast proximity and a fixed number of voxels. Both reconstruction pipelines are simulated (b) and the reconstruction results compared with the conductivity model in each case, with and without noise. The respective SNRs are defined according to the values obtained in our in vivo images. The associated tables show the results for each ROI, noting the closest proximity of the median to the reference values.
Statistical analysis
For this analysis, we included the three types of brain tissue (WM/GM/CSF), as well as fourteen classes of torso tissues from the segmentation step: [Spleen, Right and Left Kidneys, Gallbladder, Esophagus, Liver, Stomach, Aorta, Postcava, Pancreas, Duodenum] from nnU-Net69 and [Heart, Lungs, Visceral Fat (VAT)] from manual segmentation.
Data were pooled and grouped according to tissue types, in order to determine (i) an estimate of mean/median/SD conductivity for each tissue, (ii) which significant independent variables account for differences in conductivity, and (iii) any extended relationship between conductivity and reported significant variables.
Boxplot diagrams, presenting the ROIs medians, means and standard deviations of conductivity measurements in our cohort were generated to display variations in conductivity within different tissue classes. We also provide metrics on the number of voxels associated with each ROI, as well as Fat Volume Fraction (FVF) statistics for torso organs only. Note that these numbers of voxels are an important reliability criterion: the higher they are, the more effective the compensation for reconstruction errors.
We aimed to test the independent influence of sex, age, BMI or FVF on tissues conductivity (Two-tailed Pearson’s correlation) with a linear model using the MATLAB fit linear model function. Pearson correlation coefficient r and p values for all estimators were given, and p values below 0.05 were considered to indicate statistical significance. Once the univariate regressions have been obtained, and if two predictors are significant, we use multivariate models (glmfit) to test for weighted dependence. The correlation between predictors is also estimated using regression. Linear regression curves were generated for univariate models.
Results
Brain data from all volunteers (n = 17) were used for the analyses whereas only fifteen torso data were usable. One of the volunteers had a known intestinal pathology, and an incidental finding was made in another one. They were excluded from the subsequent statistical analyses, but we do provide reconstruction data for illustrative purposes in the Supplementary Material (Fig. S1).
Simulation study
The use of a bi-adaptive computation kernel optimizes the role of the prior in the reconstruction process as it allows a voxel selection that matches the piecewise constant model equation. A simple illustration is provided in Fig. 2. As the typical SNR of our brain images was ~ 30 dB, and that of our torso images was ~ 15 dB, we mimicked both reconstruction pipelines and adapted the size of the computation kernel accordingly. While it maintains a very good accuracy in each ROI without excessive smoothing, it is also robust to noise (Fig. 2). There are however some visible deviations due to the lack of signal at the edge of the calculation area, as well as visible inconsistencies due to inherent noise amplification. SD values reflect both the effect of smoothing and noise. It is also worth noting here that the median is a better absolute estimator than the mean for central compartments.
Conductivity maps
Examples of reconstructed maps are shown in Fig. 3 with associated ROIs. The visible segmentations were subsequently eroded for statistical analysis, as the decrease in quality of conductivity maps at the periphery or in certain transition zones, such as the CSF or the heart, quickly became apparent. We also note that the proposed technique resulted in non-physical (negative) conductivity values in the lung. Reconstructed images of subjects with known or incidentally discovered conditions are shown in the supplementary information for information purposes only (Fig. S1).
Reconstruction of brain conductivity for two different volunteers (a, b) with their correspondent segmentation and reference maps. Note here the lack of conductivity matching in the ventricles (CSF) due to the insufficient signal in the UTE magnitude map. Similarly, torso conductivity maps (c, d) for another two volunteers, segmentation and reference maps, as well as estimates of fat fraction. Boundary artefacts at the periphery of the two volumes are clearly visible.
Population values
Means, medians, and SD of conductivity for all selected organs are shown in Fig. 4a. We also provide reference conductivity values from the ex vivo literature for comparison when available. The obtained EP values are close yet not equal to the reported literature values. Significant deviations are observed in some ROIs but no obvious pattern seems to emerge, in particular we do not observe a consistent bias between in vivo and ex vivo values. The example of a histogram associated with the estimation of conductivity in the WM in two different subjects is also shown (Fig. 4b), which suggests the near-normality of the data, and the validity of the analysis in terms of mean/median. As suggested earlier, we noticed that the median tends to be more stable over organs, likely due to the weight of certain outliers in the calculation of the means. Therefore the median values were used for the statistical analyses (Fig. 4c). A focus on standard deviations and the average number of voxels helps identify the most reliable regions (e.g. GM, WM, Spleen, Liver, and Pancreas).
Population statistics. Boxplot representations (a) of the population distribution of mean conductivity in each organ, median conductivity, standard deviation as well as the associated mean number of voxels and volume fat fraction. Colored diamonds represent ex vivo reference conductivity values. Example histograms (b) for a given ROI (WM) in two different volunteers show limited deviation from a Gaussian distribution. Summary table (c) of the population average of the median conductivity values in each organ compared with the corresponding reference value on which linear regressions are performed (d). The regression equations are shown in red and 95% confidence bounds are depicted in dotted lines. Each prediction variable is tested for each organ in a similar way.
Excluding outliers, the ranges of ROIs median conductivities were as follows (min–max): Grey Matter, 0.65–0.85 S/m; White Matter, 0.60–0.74 S/m; Cerebrospinal Fluid, -0.03–0.96 S/m; Spleen, 0.19–0.68 S/m; Right Kidney, 0.51–0.74 S/m; Left Kidney, 0.40–0.68 S/m; Gallbladder, 0.20–0.84 S/m; Esophagus, 0.30–1.10 S/m; Liver, 0.50–0.94 S/m; Stomach, 0.27–0.97 S/m; Postcava, 0.29–0.76 S/m; Pancreas, 0.35–0.87 S/m; Duodenum, 0.28–0.80 S/m; Heart, 0.51–0.80 S/m; Lung, -0.67–0.00 S/m, Visceral Adipose Tissue (VAT), 0.10–0.67 S/m; Aorta, 0.33–0.72 S/m. Negative values reflect the limits of the technique when SNR is very low, as in the lungs, or at the edge of the reconstruction domain, as in the CSF.
Regressions and correlations
Univariate linear regression models allow a quick account of general trends, as shown in Fig. 4d. We observe, for example, that conductivity seems to evolve positively with age in the white matter (p = 0.002), negatively with FVF in the liver (p = 0.001, r = 0.70), and positively in the heart (p = 0.013, r = 0.77) for men compared to women (M to F, r = 0.77). All these trends are summarized in Table 2, with the corresponding p-value indicated. Significant correlations are shown in bold and the curves associated are provided as additional information (Fig. S2). Results for multivariate models are presented as additional information when more than one predictor was significant (Table S1). These results must also be balanced with the trust placed in population values. FVF values in the lung were excluded due to the SNR of the DIXON images being nearly zero. Interestingly, conductivity of the three major brain areas is positively and significantly correlated with age, while that of other organs is always negatively correlated. For seven of them, the link is significant or close to it; a larger cohort would allow these findings to be refined. We also note a discrepancy between the two kidneys, which could indirectly reflect a functional asymmetry in our population. FVF seems to be a particularly good predictor of liver and spleen conductivity unlike BMI, which relates only to the duodenum. Finally, sex seems to have a discriminating effect on certain organs, notably the liver and pancreas.
Discussion
This study provides new insights for tissue conductivity characterization with MRI, by focusing on macroscopic measurements and their dependence on general individual factors. It shows that it is possible to identify trends by looking at the macroscopic conductivity of each organ. We chose the median as a simple preferred metric as it seemed more robust than the classical mean, but a next step might be to look closer at the histograms within each ROI45. The use of a single UTE sequence and the associated simple EPT formulation also makes post-processing easier, as well as its integration into a standard imaging protocol.
Regarding the reconstruction method used in this work15,75, the ZTE/UTE-based framework has the advantage of cleverly reducing the weight of certain assumptions made in the more conventional MR-EPT models. Firstly, it avoids the need for the so-called “transceive phase assumption” (TPA) as the total RF phase is used and not an estimate of the transmit phase which is not directly measurable10,14. In theory, this means that the method can be used with any combination of transmit/receive antennas, provided that the combination is optimal and the SNR is sufficient15,76. The TPA suffers especially from geometric asymmetries as it works best for cylindrically symmetrical setups, and becomes increasingly invalid as the tissue is further away from the magnet's center16. The use of formulations involving the product of RF fields, and not \({\text{B}}_{1}^{+}\) and/or phase alone, enables reconstructions that are more accurate because they better compensate for inhomogeneities between transmit and receive fields while reducing errors associated with model simplification in each case15,19. In this study, the pipeline applied to the brain fulfilled these geometric conditions better than for the torso, where estimation deteriorated more markedly in the peripheral regions. This is also why small or tortuous structures, such as the esophagus or duodenum for which the SD is very large, are less amenable to this kind of analysis. Finally, unlike computationally intensive reconstruction schemes28,33,35,37, the implementation of our algorithm is fairly light: the calculation time for a brain volume, for example, is of the order of 5 min.
In addition, a recent study showed that the selected MR-EPT formulation provides satisfactory conductivity values compared with an external absolute probe reference in large homogeneous regions32. Its in vivo use requires proper boundary error control, which remain a serious issue for clinical applications18,21. As for Laplacian estimation, the use of a second order Savitzky-Golay kernel has been shown theoretically to be the best linear method to avoid noise amplification17. By constraining it to an anatomical prior, we artificially recreate the homogeneity criterion that "forces" the conditions of application of the model23,55. The use of both a large selection window31 and a restricted calculation window with a constant number of voxels (brain: n = 153; torso: n = 233), further limits potential differences between central and peripheral voxels within the same ROI. Our simulation study shows that this procedure works as long as the conductivity model is piecewise constant and we might expect this not to be true of extended structures such as white matter or the liver. The piecewise-constant assumption is nevertheless a preliminary prerequisite for deriving consistent macroscopic values and taking a step towards standardization for more realistic EM models. The consistency of conductivity values in the WM across our population (0.66 + /- 0.04 S/m) is a valuable piece of evidence in the validation of in vivo conductivity measurements. One problem, however, lies in estimating and eliminating the contrast term \({I}_{0}\), which could temper the latter. Filtering strategies can be used at the cost of degraded resolution19.
To calculate the SAR accurately, the permittivity must also be known. However, it can be estimated with a slight underestimation using conductivity alone and a theoretical permittivity model77. From a practical point of view, and even if we have not studied permittivity in this work, our pipeline makes it possible to consider its simultaneous reconstruction, as well as that of complex admittivity, which could also be a useful EM biomarker19. Extending the database will reduce the uncertainty regarding these quantities.
In terms of estimated values, we first observe that we tend to overestimate conductivity values in GM and WM compared to previous studies10,16,44,55,56. While GM conductivity is still greater than WM conductivity, it is about 0.3 S/m greater than the expected values and the gap between the two tissue types is reduced3. These discrepancies could be explained by a systematic error propagated in our reconstruction pipeline : depending on whether the phase is used alone or not, many authors point to under- or over-estimates compared to reference values10,50. In our case, the overestimation, already found in in reconstructions from ZTE images15, could be related to Gibbs-ringing51. Future work will quantify this effect, by adjusting reconstruction matrix sizes. The low standard deviation for these two regions in our population nevertheless shows that these measurements are reliable and can be used to identify statistical trends. The values found for CSF, on the other hand, appear unreliable due to image edge effects and are probably subject to the pulsatile effect described previously78. Regarding values for the torso regions, we also note an overestimation of 0.3 S/m compared with previous published results for the largest ROIs, i.e. the liver79 and visceral adipose tissue. A value close to the literature is found for the bulk heart80, without differentiating between muscle tissue and blood. The low SNR of the UTE in the lung region results in negative conductivity values60. We are not aware of any in vivo reference values for the other tissues listed in our study and we observe that the conductivity values encountered tend to be lower than the ex vivo values in these regions. All values should be treated with caution, e.g. considering the number of voxels or the ROI-wise standard deviation as a surrogate confidence metric as we suggested, and will benefit from comparative studies with more advanced methods from different sequences and manufacturers. Finally, with regard to the trends observed, we emphasize the increase in conductivity in all cerebral compartments with age which is at odds with published work on animal excised pieces81. In our working frequency range, the body is usually considered a as mixture of particles in the aqueous extracellular space which then reflects most of its EM behavior. Increased conductivity may then suggest an increase in ionic concentration, fluid content42 or even a change in the structure of the extracellular space over time.
While the biology of the aging brain is a complex process, some studies indicate an increase in brain water content, which is associated with neurodegenerative processes and cognitive decline82,83 while others suggest a decrease in an animal model84. These variations are closely linked42,55 to global conductivity in gray or white matter, for example, which would then be added to the list of parameters of interest for quantitative MRI applied to longitudinal studies. Furthermore, and this is one of the limitations of our study, we need to be able to say whether the evolution of EPs over time, and therefore of their physiological bases, is linear or not. Furthermore, the decrease in EEG power with age could be another indication of the overall increase in cerebral conductivity, even at low frequencies, with the addition of concomitant shunting effects85. The drop in conductivity in one of the two kidneys could also reflect these changes in free water concentration associated with the dehydration more frequently encountered in the elderly while the drop for aortic blood could be related to augmented whole blood viscosity86. The decreases in conductivity in several organs with respect to the fat fraction are consistent with the low theoretical conductivity of fat, whose increased level lowers simultaneously water content and ionic mobility. Finally, gender seems to be a good predictor of a difference in conductivity for the liver in particular. If the difference is real, it is once again probably associated with ionic substrates, such as iron content, or physico-chemical substrates, such as fat content87. Multi-variate regression provides initial clues to the potential independence of certain predictors (Table S1), such as age and FVF for liver. In contrast, there is a strong correlation between gender and age for the pancreas and aorta estimates, partly because older women are under-represented in our small cohort.
In conclusion, the main aim of our study was to establish a reliable protocol for estimating in vivo conductivity in several organs, compare our population values with reference ex vivo values and identify trends in relation to their characteristics. Our cohort is small and the results are preliminary but they suggest that in vivo conductivity values may deviate from the ex vivo reference values, and that inter-individual variations (e.g. vs. age or sex) may not be negligible. Beyond statistical power, the limitations of our study include the use of a simple reconstruction method and the low SNR/contrast in certain organs (such as lung or CSF) with the UTE sequence which could lead to estimation bias. As the variability of in vivo EPs has not been addressed extensively by existing studies, there is still no ground truth available for absolute quantification of conductivity in vivo, so our results will have to be confirmed by larger studies using different methods and MRI scanners. Should these correlations were to prove significant for larger cohorts, they might provide a new tool for studying structural and functional differences between individuals.
Overall, we believe that electromagnetic models for SAR management and patient safety, as well as the addition of electromagnetic parameters to clinical studies, will benefit greatly from these in vivo studies.
Data availability
Data are available from the corresponding author upon reasonable request.
References
Gabriel, C., Gabriel, S. & Corthout, E. The dielectric properties of biological tissues: I. Literature survey. Phys. Med. Biol. 41, 2231–2249 (1996).
Pethig, R. Dielectric properties of body tissues. Clin. Phys. Physiol. Meas. Off. J. Hosp. Phys. Assoc. Dtsch. Ges. Med. Phys. Eur. Fed. Organ. Med. Phys. 8(Suppl A), 5–12 (1987).
IT’IS Foundation. Tissue Properties Database V4.1. IT’IS Foundation https://doi.org/10.13099/VIP21000-04-1 (2022).
Gabriel, S., Lau, R. W. & Gabriel, C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 41, 2251 (1996).
Joines, W. T., Zhang, Y., Li, C. & Jirtle, R. L. The measured electrical properties of normal and malignant human tissues from 50 to 900 MHz. Med. Phys. 21, 547–550 (1994).
Holder, D. S. Electrical Impedance Tomography: Methods, History, and Applications (Institute of Physics Medical Physics Series) (Institute of Physics Publishing, 2005).
Griffiths, H. Magnetic induction tomography. Meas. Sci. Technol. 12, 1126 (2001).
Haacke, E. M., Petropoulos, L. S., Nilges, E. W. & Wu, D. H. Extraction of conductivity and permittivity using magnetic resonance imaging. Phys. Med. Biol. 36, 723–734 (1991).
Wen, H. 2003 Noninvasive quantitative mapping of conductivity and dielectric distributions using RF wave propagation effects in high-field MRI in Medical Imaging. Phys. Med. Imaging 5030, 471–477 (2003).
Voigt, T., Katscher, U. & Doessel, O. Quantitative conductivity and permittivity imaging of the human brain using electric properties tomography. Magn. Reson. Med. 66, 456–466 (2011).
Katscher, U. et al. Determination of electric conductivity and local SAR Via B1 mapping. IEEE Trans. Med. Imaging 28, 1365–1374 (2009).
Collins, C. M. & Wang, Z. Calculation of radiofrequency electromagnetic fields and their effects in MRI of human subjects: RF Field Calculations in MRI. Magn. Reson. Med. 65, 1470–1482 (2011).
Jin Keun Seo & Eung Je Woo. Electrical tissue property imaging at low frequency using MREIT. IEEE Trans. Biomed. Eng. 61, 1390–1399 (2014).
Katscher, U., Kim, D.-H. & Seo, J. K. Recent progress and future challenges in MR electric properties tomography. Comput. Math. Methods Med. 2013, 1–11 (2013).
Lee, S.-K. et al. Tissue electrical property mapping from zero echo-time magnetic resonance imaging. IEEE Trans. Med. Imaging 34, 541–550 (2015).
van Lier, A. L. H. M. W. B. et al. Phase mapping at 7 T and its application for in vivo electrical conductivity mapping. Magn. Reson. Med. 67, 552–561 (2012).
Lee, S.-K., Bulumulla, S. & Hancu, I. Theoretical investigation of random noise-limited signal-to-noise ratio in MR-based electrical properties tomography. IEEE Trans. Med. Imaging 34, 2220–2232 (2015).
Mandija, S., Sbrizzi, A., Katscher, U., Luijten, P. R. & van den Berg, C. A. T. Error analysis of helmholtz-based MR-electrical properties tomography. Magn. Reson. Med. 80, 90–100 (2018).
Soullié, P., Missoffe, A., Ambarki, K., Felblinger, J. & Odille, F. MR electrical properties imaging using a generalized image-based method. Magn. Reson. Med. 85, 762–776 (2021).
Seo, J. K. et al. Error analysis of nonconstant admittivity for mr-based electric property imaging. IEEE Transact. Med. Imaging 31(2), 430–437 (2011).
Duan, S. et al. Quantitative analysis of the reconstruction errors of the currently popular algorithm of magnetic resonance electrical property tomography at the interfaces of adjacent tissues. NMR Biomed. 29, 744–750 (2016).
Shin, J., Kim, J.-H. & Kim, D.-H. Redesign of the Laplacian kernel for improvements in conductivity imaging using MRI. Magn. Reson. Med. 81, 2167–2175 (2019).
Katscher, U. et al. Estimation of breast tumor conductivity using parabolic phase fitting. Proc. 20th Sci. Meet. Int. Soc. Magn. Reson. Med. (2012).
He, Z., Chen, B., Lefebvre, P. & Odille, F. An Adaptative Savitzky-Golay Kernel for Laplacian Estimation in Magnetic Resonance Electrical Property Tomography. In: 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC) 1–4 (IEEE, Sidney, France, 2023). https://doi.org/10.1109/embc40787.2023.10341200.
Liu, C. et al. MR-based electrical property tomography using a modified finite difference scheme. Phys. Med. Biol. 63, 145013 (2018).
Katscher, U. & van den Berg, C. A. T. Electric properties tomography: Biochemical, physical and technical background, evaluation and clinical applications. NMR Biomed. 30, e3729 (2017).
Marques, J. P., Sodickson, D. K., Ipek, O., Collins, C. M. & Gruetter, R. Single acquisition electrical property mapping based on relative coil sensitivities: a proof-of-concept demonstration. Magn. Reson. Med. 74, 185–195 (2015).
Liu, J., Zhang, X., Schmitter, S., Van de Moortele, P.-F. & He, B. Gradient-based electrical properties tomography (gEPT): a robust method for mapping electrical properties of biological tissues in vivo using magnetic resonance imaging. Magn. Reson. Med. 74, 634–646 (2015).
Wang, Y., Van de Moortele, P.-F. & He, B. Automated gradient-based electrical properties tomography in the human brain using 7 Tesla MRI. Magn. Reson. Imaging 63, 258–266 (2019).
Gurler, N. & Ider, Y. Z. Gradient-based electrical conductivity imaging using MR phase. Magn. Reson. Med. 77, 137–150 (2017).
Hafalir, F. S., Oran, O. F., Gurler, N. & Ider, Y. Z. Convection-reaction equation based magnetic resonance electrical properties tomography (cr-MREPT). IEEE Trans. Med. Imaging 33, 777–793 (2014).
He, Z. et al. Phantom evaluation of electrical conductivity mapping by MRI: Comparison to vector network analyzer measurements and spatial resolution assessment. Magn. Resonance Med. 91(6), 2374–2390 (2024).
Serralles, J. E. C. et al. Noninvasive estimation of electrical properties from magnetic resonance measurements via global maxwell tomography and match regularization. IEEE Trans. Biomed. Eng. 67, 3–15 (2020).
Borsic, A., Perreard, I., Mahara, A. & Halter, R. J. An inverse problems approach to MR-EPT image reconstruction. IEEE Trans. Med. Imaging 35, 244–256 (2016).
Balidemaj, E. et al. CSI-EPT: a contrast source inversion approach for improved mri-based electric properties tomography. IEEE Trans. Med. Imaging 34, 1788–1796 (2015).
Ammari, H., Kwon, H., Lee, Y., Kang, K. & Seo, J. K. Magnetic resonance-based reconstruction method of conductivity and permittivity distributions at the Larmor frequency. Inverse Probl. 31, 105001 (2015).
Mandija, S., Meliadò, E. F., Huttinga, N. R. F., Luijten, P. R. & van den Berg, C. A. T. Opening a new window on MR-based electrical properties tomography with deep learning. Sci. Rep. 9, 8895 (2019).
Jung, K.-J. et al. Data-driven electrical conductivity brain imaging using 3 T MRI. Hum. Brain Mapp. 44, 4986–5001 (2023).
Inda, A. J. G. et al. Physics informed neural networks (PINN) for low snr magnetic resonance electrical properties tomography (MREPT). Diagn. Basel Switz. 12, 2627 (2022).
Hernandez, D. & Kim, K.-N. Use of machine learning to improve the estimation of conductivity and permittivity based on longitudinal relaxation time T1 in magnetic resonance at 7 T. Sci. Rep. 13, 7837 (2023).
Hampe, N. et al. Dictionary-based electric properties tomography. Magn. Reson. Med. 81, 342–349 (2019).
Michel, E., Hernandez, D. & Lee, S. Y. Electrical conductivity and permittivity maps of brain tissues derived from water content based on T1-weighted acquisition. Magn. Reson. Med. 77, 1094–1103 (2017).
Mandija, S., Petrov, P. I., Vink, J. J. T., Neggers, S. F. W. & van den Berg, C. A. T. Brain tissue conductivity measurements with MR-electrical properties tomography: an in vivo study. Brain Topogr. 34, 56–63 (2021).
Cao, J., Ball, I., Humburg, P., Dokos, S. & Rae, C. Repeatability of brain phase-based magnetic resonance electric properties tomography methods and effect of compressed SENSE and RF shimming. Phys. Eng. Sci. Med. 46, 753–766 (2023).
Tha, K. K. et al. Noninvasive electrical conductivity measurement by MRI: a test of its validity and the electrical conductivity characteristics of glioma. Eur. Radiol. 28, 348–355 (2018).
Park, J. E. et al. Low conductivity on electrical properties tomography demonstrates unique tumor habitats indicating progression in glioblastoma. Eur. Radiol. 31, 6655–6665 (2021).
Mori, N. et al. Diagnostic value of electric properties tomography (EPT) for differentiating benign from malignant breast lesions: comparison with standard dynamic contrast-enhanced MRI. Eur. Radiol. 29, 1778–1786 (2019).
Shin, J. et al. Initial study on in vivo conductivity mapping of breast cancer using MRI. J. Magn. Reson. Imaging JMRI 42, 371–378 (2015).
Suh, J. et al. Noncontrast-enhanced MR-based conductivity imaging for breast cancer detection and lesion differentiation. J. Magn. Reson. Imaging 54, 631–645 (2021).
Lee, J. H. et al. In vivo electrical conductivity measurement of muscle, cartilage, and peripheral nerve around knee joint using MR-electrical properties tomography. Sci. Rep. 12, 73 (2022).
Balidemaj, E., van Lier, A. L. H. M. W., Nederveen, A. J., Crezee, J. & van den Berg, C. Feasibility of EPT in the Human Pelvis at 3T. In: Proc. 20th Sci. Meet. Int. Soc. Magn. Reson. Med. 3468 (2012).
Lesbats, C. et al. High-frequency electrical properties tomography at 9.4T as a novel contrast mechanism for brain tumors. Magn. Reson. Med. 86, 382–392 (2021).
Kim, J. W. et al. MR-based electrical conductivity imaging of liver fibrosis in an experimental rat model. J. Magn. Resonance Imaging 53(2), 554–563 (2021).
Gavazzi, S. et al. Accuracy and precision of electrical permittivity mapping at 3T: the impact of three mapping techniques. Magn. Reson. Med. 81, 3628–3642 (2019).
Liao, Y. et al. Correlation of quantitative conductivity mapping and total tissue sodium concentration at 3T/4T. Magn. Reson. Med. 82, 1518–1526 (2019).
Ropella, K. M. & Noll, D. C. A regularized, model-based approach to phase-based conductivity mapping using MRI. Magn. Reson. Med. 78, 2011–2021 (2017).
McCann, H., Pisano, G. & Beltrachini, L. Variation in reported human head tissue electrical conductivity values. Brain Topogr. 32, 825–858 (2019).
Gho, S.-M., Shin, J., Kim, M.-O. & Kim, D.-H. Simultaneous quantitative mapping of conductivity and susceptibility using a double-echo ultrashort echo time sequence: example using a hematoma evolution study. Magn. Reson. Med. 76, 214–221 (2016).
Katscher, U. & Börnert, P. Imaging of Lung Conductivity Using Ultrashort Echo-Time Imaging. In: Proc. 24th Sci. Meet. Int. Soc. Magn. Reson. Med. 2923 (2016).
Schweser, F. et al. Conductivity mapping using ultrashort echo time (UTE) imaging. In: Proc. 21st Sci. Meet. Int. Soc. Magn. Reson. Med. 4190 (2013).
Qian, Y. & Boada, F. E. Acquisition-weighted stack of spirals for fast high-resolution three-dimensional ultra-short echo time MR imaging. Magn. Reson. Med. 60, 135–145 (2008).
Mugler, I. J., Meyer, C., Pfeuffer, J., Stemmer, A. & Kiefer, B. Breath-hold UTE lung imaging using a stack-of-spirals acquisition. In: Proc. 26th Sci. Meet. Int. Soc. Magn. Reson. Med. 4904 (2017).
Fauveau, V. et al. Performance of spiral UTE-MRI of the lung in post-COVID patients. Magn. Reson. Imaging 96, 135–143 (2023).
Grimm, A. et al. Evaluation of 2-point, 3-point, and 6-point Dixon magnetic resonance imaging with flexible echo timing for muscle fat quantification. Eur. J. Radiol. 103, 57–64 (2018).
Panagiotelis, I. & Blasche, M. TrueFormTMTechnology. MAGNETOM Flash vol. 114 (2009).
Walsh, D. O., Gmitro, A. F. & Marcellin, M. W. Adaptive reconstruction of phased array MR imagery. Magn. Reson. Med. 43, 682–690 (2000).
Lustig, M. & Pauly, J. M. SPIRiT: Iterative self-consistent parallel imaging reconstruction from arbitrary k-space. Magn. Reson. Med. 64, 457–471 (2010).
Vishnevskiy, V., Gass, T., Szekely, G., Tanner, C. & Goksel, O. Isotropic total variation regularization of displacements in parametric image registration. IEEE Trans. Med. Imaging 36, 385–395 (2017).
Isensee, F., Jaeger, P. F., Kohl, S. A. A., Petersen, J. & Maier-Hein, K. H. nnU-Net: a self-configuring method for deep learning-based biomedical image segmentation. Nat. Methods 18, 203–211 (2021).
YUANFENG, J. Amos: A large-scale abdominal multi-organ benchmark for versatile medical image segmentation. Zenodo https://doi.org/10.5281/zenodo.7155725 (2022).
Paszke, A. et al. PyTorch: An Imperative Style, High-Performance Deep Learning Library. In: Advances in Neural Information Processing Systems vol. 32 (Curran Associates, Inc., 2019).
Egger, J. et al. GBM volumetry using the 3D slicer medical image computing platform. Sci. Rep. 3, 1364 (2013).
Hoult, D. I. The principle of reciprocity in signal strength calculations?A mathematical guide. Concepts Magn. Reson. 12, 173–187 (2000).
Zhu, D. & Smith, W. A. P. Least squares surface reconstruction on arbitrary domains. Preprint at https://doi.org/10.48550/arXiv.2007.08661 (2020).
Leijsen, R., Brink, W., van den Berg, C., Webb, A. & Remis, R. Electrical properties tomography: a methodological review. Diagnostics 11, 176 (2021).
Lee, J., Shin, J. & Kim, D.-H. MR-based conductivity imaging using multiple receiver coils. Magn. Reson. Med. 76, 530–539 (2016).
Voigt, T., Homann, H., Katscher, U. & Doessel, O. Patient-individual local SAR determination: in vivo measurements and numerical validation. Magn. Reson. Med. 68, 1117–1126 (2012).
Katscher, U., Stehning, C. & Tha, K. K. The impact of CSF pulsation on reconstructed brain conductivity. In: Proc. 26th Sci. Meet. Int. Soc. Magn. Reson. Med. 0546 (2018).
Tha, K. K. et al. Higher electrical conductivity of liver parenchyma in fibrotic patients: noninvasive assessment by electric properties tomography. J. Magn. Reson. Imaging 54, 1689–1691 (2021).
Katscher, U. & Weiss, S. Mapping electric bulk conductivity in the human heart. Magnetic Res. Med. 87(3), 1500–1506 (2022).
Gabriel, C. Dielectric properties of biological tissue: Variation with age. Bioelectromagnetics 26, S12–S18 (2005).
Gullett, J. M. et al. The association of white matter free water with cognition in older adults. NeuroImage 219, 117040 (2020).
Filo, S. et al. Disentangling molecular alterations from water-content changes in the aging human brain using quantitative MRI. Nat. Commun. 10, 3403 (2019).
Gottschalk, A., Scafidi, S. & Toung, T. J. K. Brain water as a function of age and weight in normal rats. PLoS ONE 16, e0249384 (2021).
He, M. et al. Age-related EEG power reductions cannot be explained by changes of the conductivity distribution in the head due to brain atrophy. Front. Aging Neurosci. 13, 632310 (2021).
Simmonds, M. J., Meiselman, H. J. & Baskurt, O. K. Blood rheology and aging. J. Geriatr. Cardiol. JGC 10, 291 (2013).
Wáng, Y. X. J. Gender-specific liver aging and magnetic resonance imaging. Quant. Imaging Med. Surg. 11, 2893 (2021).
Acknowledgements
The study was funded by ANR-21-CE19-0040 (ELECTRA project), Métropole du Grand Nancy, CPER 2015-2020 IT2MP, FEDER-FSE Lorraine et Massif des Vosges 2014-2020. The authors thank Thomas Benkert (PhD, Application Development, Siemens Healthcare, Erlangen, Germany) for providing the UTE sequence, Bailiang Chen (PhD, CIC-IT Nancy) for advice on segmentation software, and Fabienne Antoine (CIC-IT) for the recruitment of healthy volunteers. This work was performed on a platform member of France Life Imaging network (grant ANR-11-INBS-0006).
Author information
Authors and Affiliations
Contributions
Z.H collected data, developed EPs reconstruction method, performed torso reconstructions, conducted statistical analyses and prepared figures. P.S conducted the simulation test, performed brain reconstructions, conducted statistical analyses, prepared figures and wrote the main manuscript text. F.O obtained funding, conceived and supervised the study, and revised the manuscript. P.L supervised the study and revised the manuscript. J.F obtained ethics approval for the volunteer study and revised the manuscript. K.A provided the MRI sequence prototype, and revised the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, Z., Soullié, P., Lefebvre, P. et al. Changes of in vivo electrical conductivity in the brain and torso related to age, fat fraction and sex using MRI. Sci Rep 14, 16109 (2024). https://doi.org/10.1038/s41598-024-67014-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-67014-9
- Springer Nature Limited