Abstract
The distribution and composition of the vegetation are greatly affected by the edaphology and topography of an area. The current study explores the vegetation structure of the herbaceous layer at various habitats of district Kohat for the first time. A survey was conducted during the spring seasons of 2021, 2022 and 2023 selecting 40 sites on the basis of edaphology, topography, altitude, aspect and status. Data was collected via quadrat approach to establish plant communities by species Importance Value (IV), point out dominant species by Total IV (TIV) and dominant families via Total Family IV (TFIV). The quantitative biological spectrum was also calculated. Communities’ phytosociological characteristics were analyzed via various diversity indices (Shannon’s Index (H), Simpson’s Index (D), Species Richness (SR), Evenness (E) and Maturity index (Mi)) while similarity between the communities was calculated by using Sorensen’s Index. The findings revealed a total of 253 species belonging to 57 families having the dominant species Cynodon dactylon (L.) Pers. (TIV, 484.3) followed by Saussuria heteromalla (D. Don) Hand. (TIV, 360.4), Anagallis arvensis L. (TIV, 353.2) and Aristida adscensionis L. (TIV, 349.65). Among 40 plant communities, Poaceae (TFIV, 2706.6), Asteraceae (TFIV, 2018.8), Fabaceae (TFIV, 1071.5) and Brassicaceae (TFIV, 825.9) were the dominant families. Therophytes (TIV, 7882) class was the dominant life form class followed by hemicryptophytes (TIV, 2517) while microphylls (TIV, 4669) class was the dominant leaf size class followed by nanophylls (TIV, 5469). Environmental factors i.e. topography and edaphic characteristics, showed significant effects on the diversity of the communities. The study concludes in a diverse pattern of distribution with a rich flora in the area warranting its documentation which will preserve the valuable species opening vistas for future research.
Similar content being viewed by others
Introduction
The study of plant community structure and the influences of different ecological factors on the structuring of communities is known as Eco-systematics1. It is important to identify and to describe spatial distribution and composition of the plant species of an area2. The spatial distribution of the plant species depends upon the impact of certain ecological factors3. The vegetation structure is greatly influenced by the existing environmental factors. Biotic, abiotic and anthropogenic factors affect the diversity and distribution of plant species and communities4,5. There is growing trend in the field of ecology to assess the relationship between biotic and abiotic components of an ecosystem. The components of environmental gradient influence the vegetation dynamics and plant community structure6. Species having structural and floristic traits with definite environmental tolerance are grouped into identifiable plant communities7. The edaphic and topographic (altitude and aspect) characteristics play key role in the structuring of the communities8. Thus, it is necessary to know about the driving environmental factors of an area which affect the plant species composition, distribution and community structure9,10.
Diversity of an area is an important ecological attribute which is strongly correlated with the prevailing environmental and anthropogenic variables11. It reflects the health of the vegetation besides pointing out the productivity12. The loss and degradation of the habitats decline the community diversity2. A sufficient observed model of the vegetation structure is obtained through detailed field urveys which may express the actual readily recognizable vegetation units of the area13,14. A community the plants can be classified into different life form and leaf size classes which specifies the prevailing climatic conditions at a specific habitat15.
A large proportion of the plant biodiversity of an area is composed of the herbaceous layer16. To ascertain the extent of species composition and diversity for conservation interests, eco-systematic explorations are often highly recommended17. Understory herbaceous stratum constitute major part of the cumulative floristic diversity18. The diversity and composition of the herb stratum, taxonomically and ecologically are heterogenous, which is governed by many ecological drivers.
The subtropical areas with semi-arid conditions are marked by strong environmental conditions, among which altitude and topography play significant role in the assemblage of plant communities. At local scales, however other environmental variables such as soil texture, soil moisture content, physical and chemical properties of the soil and anthropogenic activities also play key roles in such communities assembly17. Studies in the subtropical ranges have revealed a distinct pattern of plant distribution, characterized by variation in the altitude19. In particular, altitude and topography cause for the changes in soil texture, physical and chemical properties which act as important environmental filters determining variation in plant compositions20. Ultimately such filtering causes niche partitioning and overlapping which is reflected as a strong species turnover along the gradient21. For example, from the relatively higher altitude areas, the gravity-driven nutrient transport downslope results in higher soil fertility at the lowest altitude, which promotes the presence of some species while excluding some others22. Thus, altitudinal gradient can be regarded as heterogenous system, across which a broad array of ecological factors regulates the species diversity patterns23.
Similar exploration studies of the vegetation structure were conducted by several researchers on different regions, such as Zaman and Badshah24 on Terich valley Chitral, Ali et al.13 on the catchment areas of river Chenab, Ullah et al.10 on Yakhtangy Hill of Hindu-Himalayan range and Hussain et al.25 on Gadoon Hills of district Swabi.
District Kohat as a diverse ecological/ecotonal region, possesses a rich flora at various ecological habitats. Till date, the spring herbaceous vegetation of the said district is not explored so as the current study is aimed to explore the spring herbaceous layer to record the present vegetation structure. The study is specifically intended to develop an empirical vegetation model to characterize and designate the vegetation groups and present them in an ecological pattern to recognize dominants for specific micro-environmental conditions.
Specifically, we pose and attempt to address: (Q1) What are the dominant and co-dominant species of each habitat? (Q2) Whether the diversity of the herb layer varies among various sites of the area and how is the effect of environmental factors on the diversity of the communities? (Q3) Whether the species composition of the herb layer varies among different sites of the area? To address the 1st question, each plant species IV was calculated. For 2nd question, we used Alpha diversity indices to know the differences in diversity, and regression analysis to check the environmental predictors which have significant impact on the diversity of the communities. To answer the 3rd question, we used Sorenson’s similarity index for the comparison of species composition among various communities of the area.
Based on the diverse ecological nature of the present study region, we hypothesized that the diversity and species composition must vary among the plant communities with altitude and topography as the most significant ecological determinants, because they affect the micro-environment which supports the survival of herbaceous vegetation.
Materials and methods
Study area
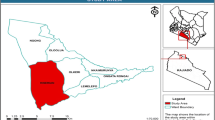
District Kohat is located in the southern part of Khyber Pakhtunkhwa (Fig. 1). It extends from 33.0676° to 33.7405° N latitude and from 71.0663° to 72.0084° E longitude. It is a subtropical area with semi-arid conditions. The altitude of various sites of the district is ranged from 270 to 1300 m. The study area is ecotonal in ecological terms i.e. between the tropical and subtropical regions. The northern and north-western part of the district is comprised of subtropical hills while the rest gradually falls to tropical region. Because of this unique topography and the significant variation in the altitudinal range, the area harbors a variety of ecological habitats and possesses several unique patterns of community structure.
Locational map of the study area showing district Kohat (top), Khyber Pakhtunkhwa (bottom right) and Pakistan (bottom left) (ArcGIS 10.8.2 https://www.esri.com/en-us/home).
Data
The district was divided into forty sites on the basis of the existing ecological condition for the herb community survey during spring seasons of 2021, 2022 and 2023. The first seven sites (S1–S7) were belonging to hydrated habitats, S8–S12 were the irrigated areas, S13–S20 were non irrigated areas, S21–S24 were non-protected rangelands, S25–S27 were protected wildlife rangelands parks, S28–S35 were the south expositions and S36–S40 were the north expositions of the hills.
Soil data and analysis
To understand the impact of soil on the vegetation structure of the area, a composite soil sample was collected from each site and provided to Agriculture Research Institute Tarnab, Peshawar for the physicochemical analysis.
Methods
Quadrat method was used to collect the data during field survey and a total of 400 quadrats (10 quadrats in each site) were applied in the area in order to investigate the spring herbaceous vegetation structure. The size of the quadrat was 1 m2. Phytosociological attributes such as Relative Density (RD), Relative Frequency (RF) and Relative Cover (RC) were calculated. Importance value (IV) was obtained by summing up the relative values (IV = RD + RF + RC). The communities were established on the basis of IV. The quantitative biological spectrum was evaluated followed the methods of Malik et al.26. Family Importance Value (FIV) were obtained by adding the IVs of species which belongs to the same family25.
Similarity between the communities was evaluated by using the Sorenson’s Similarity Index (SI)27 using Eq. (1):
Where C is the common species between the two sites, a is the number of species of site 1 and b is the number of the species of site 2.
The diversity of the communities was analyzed by via various diversity indices.
1. Shannon’s Diversity Index28 represented by H as given in Eq. (2).
Where S is the total number of species, pi is the proportion of species i in population and ln stands for natural log.
2. Simpson’s Diversity Index29 represented by D as given in Eq. (3).
where N is the number of individuals of all species and n is the number of individuals of a species.
Species Richness Index30 was calculated by using Eq. (4).
where S is the total number of species in a stand while N is the total number of individuals in a stand.
Evenness Index31 was calculated by using Eq. (5).
where H is Shannon’s diversity index and S is total number of species in a stand.
Maturity Index32 represented by Mi as given in Eq. (6).
Chao’s iNEXT online software was used for the generation of cover-based rarefaction and extrapolation curves, and Hill’s numbers33.
Distribution/Correlation analysis
Normality of the environmental data comprising 13 variables (Table 2) regarding distribution pattern was tested via Shapiro–Wilk method (See Equation below). The environmental variables were correlated following Pearson correlation analysis.
Where Normality,
where the xi are the sample data, sorted by size ordered and ai are constants obtained from the covariance, variance and means to be evaluated.
Regression analysis of environmental data vs diversity indices
The effect of edaphic and topographic factors on the diversity indices was examined using simple and multivariate linear regression. The diversity indices were regressed on the edaphic and topographic factors of the habitats and then plotted in R 4.3.0 using ggplot2.
Results
Community structure
Communities were established in herb layer for each site via IVs of plant species. Owing to the habitats selected, the structure of these communities varied significantly. The pattern of family dominancy, on the basis of FIV and the quantitative biological spectrum of each community, was calculated. A total of 253 herb species which belongs to 57 families were recorded during the present survey (Fig. 2). The structure of each community is given in Table 1.
Dominant species (TIV), dominant family (TFIV) and quantitative biological spectrum
TIV was calculated for each species which revealed that Cynodon dactylon (TIV, 484.3), Saussuria heteromalla (TIV, 360.4), Anagallis arvensis (TIV, 353.2), Aristida adscensionis (TIV, 349.65), Brachypodium distachyon (TIV, 298.4), Linum perenne (TIV, 270.9), Bromus pectinatus (TIV, 241.7), Calendula arvensis (TIV, 211.5), Argyrolobium stenophyllum (TIV, 179.04) and Fagonia indica (TIV, 179.01) were the dominant species of the area (Table S1 and Fig. 3a). On the basis of TFIV Poaceae (TFIV, 2706.6), Asteraceae (TFIV, 2018.8), Fabaceae (TFIV, 1071.5) and Brassicaceae (TFIV, 825.9) were the dominant families (Fig. 3b). The quantitative biological spectrum showed that therophytes (TIV, 7882) was the dominant life form class followed by hemicryptophytes (TIV, 2517) while microphylls (TIV, 4669) was the dominant leaf size class followed by nanophylls (TIV, 5469) (Fig. 3c).
Edaphology
The analyses of the composite soil samples from each site revealed a significant variation in the edaphic characteristics of the area. The silt particles in the soil samples of the area ranges from 14% to 76%, sand particles ranges from 13% to 75%, pH ranges from 7.9 to 8.9, electrical conductivity (EC) ranges from 0.05 ds m−1 to 0.23 ds m−1, total soluble salts (TSS) ranges from 0.016% to 0.073%, CaCO3 ranges from 6.5% to 10%, organic matters (OM) ranges from 0.34% to 3.1%, Nitrogen ranges from 0.017 mg kg−1 to 0.155 mg kg−1, Phosphorus ranges from 2.4 mg kg−1 to 12.9 mg kg−1, Potassium (K) ranges from 68 mg kg−1 to 562 mg kg−1 and soil moisture content (SMC) ranges from 7.8% to 47.2% (Table 2) (Table S2).
Distribution and correlation among the environmental variable of the area
The normality test (N-value) showed the normal distribution (Above 0.5) of the environmental factors among all the sites (Table 2) while Pearson’s correlation analysis among the existing environmental variables showed that the plain topography showed negative correlation with higher altitude. The plain topography showed positive correlation with silt concentration. The higher silt concentration showed positive correlation with EC, TSS, OM, SMC, N and P while altitude showed positive correlation with pH and sand. The higher sand concentration of soil showed negative correlation with EC, TSS, OM, SMC, N and P (Fig. S1).
Alpha taxonomic diversity index
Different diversity indices were calculated for each community. The results of Shannon’s diversity index revealed that S3 (H, 3.65) community was the most diverse community of the area followed by S5 (H, 3.58) and S10 community (H, 3.50) while S7 community (H, 1.90) showed the lowest diversity followed by S31 (H, 1.91) and S39 community (H, 1.96) (Table 3). The Simpson’s diversity index (D) almost showed the same pattern of diversity (Table 3).
Species richness results revealed that S3 community has the highest species richness (SR, 2.064) followed by S20 (SR, 1.93) and S18 community (SR, 1.97) while lowest species richness values were calculated for S7 community (SR, 0.537), S25 (SR, 0.751) and S27 community (SR, 0.904) (Table 3).
Evenness index results showed that highest evenness value was recorded for S33 community (E, 0.958) followed by S39 (E, 0.944) and S5 community (E, 0.943) while low evenness values were recorded for S37 (E, 0.735), S25 (E, 0.751) and S6 community (E, 0.790) (Table 3).
Maturity index results showed that highest maturity index was recorded for S7 (Mi, 64) followed by S23 (Mi, 39.26) and S27 community (Mi, 39.2) while lowest maturity values were observed for S20 (Mi, 23.54), S28 (Mi, 23.57) and S10 community (Mi, 23.57) (Table 3).
We analyzed the abundance data through iNEXT online software. The Hill numbers confirmed the variation and species diversity among the different habitats of the area. The habitats which have plain landscape (Hydrated habitats, Irrigated, non-irrigated and rangelands) clearly showed the highest effective numbers of species diversity at different orders q0, q1 and q2 whereas the habitats with hilly topography showed the lowest species diversity (Fig. 4).
Cover-based rarefaction and extrapolation curves with 95% confidence intervals comparing species diversity across 7 different habitats (H1: Hydrated habitats, H2 : Wetlands, H3 : Irrigated plain areas, H4 : Non-Irrigated plain areas, H5 : Rangelands, H6 : High altitude hills, H7 : Low altitude hills).
Impact of environmental factors on the diversity of the communities
Shannon index was linearly regressed with species richness and evenness. Shannon index showed positive correlation with species richness (p = 1.012e−11) and evenness (p ≤ 0.05). The simple and multivariate linear regression plots showed that topography and altitude showed significant (p ≤ 0.05) correlation with Shannon index. The topography (plain) showed strong positive correlation with the diversity while the altitude showed significant negative correlation with diversity (Table 4, Fig. 5). The pH of the soil also showed negative correlation with the diversity (p ≤ 0.04). Generally, potassium, moisture content, TSS, EC and silt showed positive correlation with Shannon index values while CaCO3, pH and sand showed negative correlation (Table 4). The species richness values of the communities showed positive correlation with EC and TSS while it showed negative correlation with pH and CaCO3. Sand, pH, TSS and Phosphorus showed positive correlation with evenness values while silt and nitrogen showed negative correlation (Table 4, Fig. S2). The maturity index (Mi) values were affected by the status of the habitats. The protected habitats, forested habitats, showed positive relation while the plains areas were mostly cultivated which showed negative relation with the Mi values (Fig. 6).
Sorenson’s similarity index
Sorenson’s similarity index results showed that higher similarity was found between the S3 and S4 community. The calculated Sorenson’s similarity index value for these two communities was 0.752. While the other communities, which showed higher similarity (above 0.60 SI) between them were, S14 and S19 communities (SI, 0.72), S4 and S8 communities (SI, 0.675), S22 and S18 communities (SI, 0.658), S3 and S8 communities (SI, 0.604), S8 and S5 communities (SI, 0.605), S10 and S9 communities (SI, 0.675), S4 and S5 communities (SI, 0.637), S26 and S25 communities (SI, 0.603), S12 and S10 communities (SI, 0.627) and, S3 and S5 communities (SI, 0.68) (Fig. 7). A total of 105 values in the similarity figure (Fig. 7) are 0 which show that there is no similarity between the concerned two communities. Rest of the Sorenson’s similarity index values between the communities are in the range of 0–0.599 (Fig. 7).
The similarity among forty study sites was established via Sorenson’s index resulting in seven habitats. Further the habitats were tested for similarity via the same Sorenson’s index which revealed Hydrated habitats and Wetlands, Irrigated and Non-Irrigated plains, Non-Irrigated plains and Rangelands showed higher similarity (above 0.60 SI) between them (Table S3).
Discussion
Ecological studies especially the plant community structure exploration are important to recognize the true image of the vegetation. It also describes species compositional variations and the driving environmental variables along both time and space in a specific area2 especially in a changing climatic region34,35. The early recognition that plant species sorting does not merely depends on random process was an important turning point from which deterministic process was defined by later successfully incorporated through theoretical framework to improve the general understanding of species assemblages20. Environmental factors and associated functional diversity metrics have helped immensely in such attempts to quantify community structure as a deviation from randomness36. Altitudinal variation has long been used and realized as natural experiments to study biodiversity patterns and underlying mechanisms37. Elevational gradients have much steeper environmental gradients in a shorter geographical distance and represent a large variety of microclimatic conditions in a specific zone which were responsible for the determination of discrete community structures in different microclimatic ecosystems38.
During the present study the community structure of the spring herbaceous vegetation were studied in 40 sites of district Kohat. The sites were harboring variable climatic, edaphic and topographic conditions resulting in diverse community structure. The plant species IVs give a total picture of the social structure of the species in a community and can be used to form an association of dominant species39. Sonchus asper, Alopecrus myosuroides, Agrostis viridis, Nasturtium officinale, Bolboschoenus affinis, Persicaria hydropiper, Polypogon monspeliensis, Mentha longifolia, Rumex dentatus, Phalaris minor, Medicago polymorpha, Veronica anagllis-aquatica, Stellaria media and Pulicaria undulata were the dominant species of the hydrated habitats. All the mentioned species were typical hydrophytes as they are found abundantly in these habitats. The distribution of these species was greatly influenced by the high percentage of moisture. Ali et al.13 studied the vegetation structure of the river Chenab catchments; they also have the similar type of dominant species at the similar type of soil (Loamy soil). Ali et al.40 also reported these species which grows immensely in the similar types of habitats.
In the irrigated areas of the district, the dominant species were Cynodon dactylon, Bromus catharticus, Phalaris minor, Rumex dentatus, Silybum marianum, Euphorbia helioscopia, Medicago polymorpha, Malva parviflora, Eruca sativa, Calendula arvensis. Most of these species flourish in these kind of habitats40. In the non-irrigated lands Cynodon dactylon, Eruca sativa, Carthamus oxycantha, Asphodelus tenuifolius, Calendula arvensis, Sisymbrium irio, Malva parviflora, Medicago polymorpha, Peganum harmala, Filago hurdwarica, Anagallis arvensis, Medicago minima, Bromus pectinatus, Stipa capensis, Brachypodium distachyon, Emex australis were found dominant. The results revealed that some of the species which found in dominant or in co-dominant position in these non-irrigated lands were also prominent in the irrigated lands which show the existence of the overlapping of the species6. Most of these species were also reported by Khan et al.41 as dominant in these kinds of non-irrigated conditions. In the rangelands Filago hurdwarica, Astragalus amherstianus, Arenaria serpyllifolia, Cynodon dactylon, Medicago minima, Cousinia prolifera, Malcolmia cabulica, Taraxacum officinale, Plantago amplexicaulis, Anagallis arvensis, Ochthochloa compressa, Brachypodium distachyon, Sedum hispanicum, Arenaria serpyllifolia, Linum perenne and Bromus pectinatus were dominant. Some of the species were fumeroles while some of the species could tolerate drought conditions and also, they were not grazed intensively, because of which these species were found dominant in the rangelands. Most of the habitats, where these species were dominant were having sandy texture. Ali et al.42 also showed the dominancy of these species in the sandy soil texture which holds low contents of moisture.
At the south expositions of the hills at different sites Scutellaria linearis, Ajuga bracteosa, Cynodon dactylon, Argyrilobium stenophylum, Tetrapogon villosus, Fagonia indica, Echinops echinatus, Aristida adscensionis, Saussuria heteromalla, Evolvulus alsinoides and Linum perenne were found dominant. At the north exposition of the hills at different sites Brachypodium distachyon, Saussuria heteromalla, Lactuca dissecta, Linum perenne, Teucrium stocksianum, Echinops echinatus, Kickxia ramosissima, Polygala erioptera, Argyrolobium roseum and Anagallis arvensis were found dominant. The dominancy of these species were also reported by Ilyas et al.8 and Majeed et al.2 in the same type of subtropical hills.
Therophytes were dominant in the present study which is the indicator of the subtropical and temperate regions43. Therophytes were also experts of occupying the newly vacant habitats due to the disturbance in the area44. Microphylls was the dominant leaf size class which expresses the xeric condition of the area. The prevailing climatic condition of the district showed that most of the area is having semi-arid condition45.
Species diversity is a measure of complexity in form and structure. Diversity of a community can be correlated with several climatic and anthropogenic factors12. Due to, ecotonal status between tropical and subtropical regions, the location of the district, we hypothesized a significant variation in the diversity of the communities. In the present study high Shannon H, ≥ 3.4, Simpson D, ≤ 0.05 and species richness SR, ≥ 1.6 were recorded for the communities which were established in the plain areas with low altitude habitats (Streambeds, irrigated, non-irrigated lands and rangelands) while low values H, ≤ 2.6; D, ≥ 0.1 and SR, ≤ 1.2 were recorded for the communities which were at the hilly landscape habitats and were at relatively high altitude. The communities which were established at low altitude with plain topography were relatively highly diverse as compared to the hilly areas with relatively high altitude. The topographic and altitudinal factors is considered to be more influential factor which modify the edaphic characteristics especially soil texture and soil moisture contents46. Three communities were established in the protected rangelands which were plain areas also, in contrast to the trend those three communities showed relatively low diversity. The possible reason for it might be the undisturbed nature of the habitat. At the herbaceous layer the diversity of disturbed habitats is greater than the undisturbed habitats43. The present diversity results also showed higher diversity as the soil moisture contents increased47. The diversity in the wetland’s during spring is low possibly due to the differences in the local microclimatic conditions (low water percentage during spring) or the degree of disturbance, this statement is supported by the finding of Khan et al.41 and Khan et al.6. The recorded evenness values were in the range of 0.735 to 0.957. Most of the communities showed greater value than E ≥ 8.7. These values showed that most of the communities were having no highly aggregated species. Except one community, all the communities showed Mi ≤ 60 which indicates that all the habitats in the area were disturbed. 34 communities showed Mi ≤ 35 which inferred the degree of the disturbance in the area. The herbaceous species of the area was greatly disturbed by many factors like human interference, grazing, disturbed habitat condition and soil erosion9. The alpha diversity indices of the various habitats of the area showed a significant variation in the diversity of the communities which were further confirmed through Hill’s numbers (iNEXT online software) and which supports our hypothesis that the established communities at various habitats (40 sites) of the district vary in their diversities due to the diverse ecological nature of the district.
Our studied area differs significantly in term of environmental conditions and herbaceous vegetation characteristics. The prevailing edaphic and topographic conditions of the habitats entail solid relation with the diversity of the communities, which infer that the diversity of the vegetation of different habitats of the area was strongly influenced by the existing environmental conditions. Topography and altitude were the most significant abiotic factors39 which filter the species distribution in different areas of the district. The results of our regression analysis was also in accordance to our hypothesis that environmental variables have significant effects on the diversity of communities.
Topography and edaphology of an area are closely associated with the community’s species composition; any dissimilarity in these factors alters the species composition of a community. The similarity and dissimilarity of the species composition between the two communities are governed by ecological factors48. The similarity of species composition is usually associated with the environment and spatial distance. Two components, that are often interpreted as signals for different community assembly processes, are filtering and diffusion limitation49. The role of environmental filtering and dispersal limitations is also of importance in determining the composition variation of plants in semi-arid regions50. The present similarity analysis resulted that the habitats which have relatively similar abiotic characteristic harbored relatively similar species composition. The Sorenson similarity index revealed eleven SI values ≥ 0.60 which implied that these communities were relatively similar. The similarity values were ≥ 3.5 between the communities which were established on the ecological habitats exhibiting some of the similar abiotic traits. Based on the Sorenson’s similarity index the total district showed 6 distinct types of ecological habitats which have relatively similar abiotic factors, mainly they were having relatively same topography and having fewer differences in their altitude. After the topography and altitude, the edaphic characteristics of the sites play significant role in the assemblage of the community, specifically in our case, soil texture, pH, and soil moisture content. The distinct type of ecological niches includes, the hydrated habitats, irrigated plains, non-irrigated plains, rangelands, high altitude hills (south exposition of Merai high altitude hills of 1300 m elevation) and low altitude hills. Among these 6 major ecological habitats most of the communities showed very less similarity SI ≤ 0.25 with each other. Overlapping of niches was observed between the major habitats following a sequence in the present study, because of the similar abiotic factors. The hydrated habitats mostly showed relatively high similarity ≥ 0.2.3 with irrigated habitats, irrigated habitats mostly showed relatively high similarity ≥ 0.27 with non-irrigated zones, non-irrigated mostly showed relatively high similarity ≥ 0.30 with rangelands. Although the communities were not similar but the little similarity between these groups might be due to the existing similar abiotic factors like their topography and altitude and the differences among these community might be because of the variation in their soil moisture contents which filters the species distribution in theses habitats. The two extremes i.e. wetlands habitats and high-altitude hills (Merai high hills) mostly showed very less similarity with the communities that were established in the other habitats. This was because of the harsh prevailing condition which excludes other species of the area. The similarity and dissimilarity between the communities in the area is significantly driven by the ecological factors such as edaphic and topographic factors25. These ecological factors of the area are greatly influenced the distribution and composition of species at various ecological habitats of the area24,51. The species with definite tolerance levels coexist in similar groups7. As we hypothesized that the present area possess a variety of habitats mainly due to the elevation differences ranging from 270 to 1300 m so as the species composition at various sites must be varied.
Conclusion
The current research provides insight for the first time about the spring herbaceous community structure of district Kohat at 40 study sites (aggregated to 7 habitats). It provides a comprehensive and quantitative understanding of the species composition and diversity based on standard phtyosociological approaches and methods. The survey was conducted throughout the district during spring season and a total of 253 herbaceous species were recorded which belongs to 57 families. The study divulged that the topography and altitude of the area were the most significant factors as evident from strong correlation with the edaphic factors which have significant impact on the diversity of plants at various habitats. The similarity index results inferred that the similarities between the communities were greatly influenced by the existing ecological factors. Furthermore, the study also concludes that the diversity of the plain habitat communities with relatively low altitude was greater than the communities which were established at the hills/high altitudes.
Data availability
The raw data will be made available on request from the corresponding author.
References
Duke, J. A. Ecosystematics. Chap. 4, 53–68. (Halsted Press, 1978).
Majeed, M. et al. Vegetation analysis and environmental indicators of an arid tropical forest ecosystem of Pakistan. Ecol. Indic. 142, 109291 (2022).
Dray, S. et al. Community ecology in the age of multivariate multiscale spatial analysis. Ecol. Monogr. 82, 257–275. https://doi.org/10.1890/11-1183.1 (2012).
Akhlaq, R. et al. Species diversity and vegetation structure from different climatic zones of Tehsil Harighel, Bagh, Azad Kasmir, Pakistan analysed through multivariate techniques. Appl. Ecol. Environ. Res. 16, 5193–5211 (2018).
Brown, J. H. On the relationship beween abundance and distribution of species. Am. Nat. 124, 255–279 (1984).
Khan, N., Siddiqui, M. F., Ahmed, M., Bibi, S. & Ahmed, I. A phytosociological study of forest and non-forest vegetation of district Chitral, Hindukush Range of Pakistan. J. Biol. 2, 91–101 (2012).
Mumshad, M. et al. Phyto-ecological studies and distribution pattern of plant species and communities of Dhirkot, Azad Jammu and Kashmir, Pakistan. PLoS One 16, 10 (2021).
Ilyas, M., Qureshi, R., Akhtar, N., Ziaul-Haq, Z. & Khan, A. M. Floristic diversity and vegetation structure of the remnant subtropical broad leaved forests from Kabal Valley, Swat, Pakistan. Pak. J. Bot. 50, 217–230 (2018).
Rahman, I. U. et al. Environmental variables drive plant species composition and distribution in the moist temperate forests of Northwestern Himalaya, Pakistan. PLoS One 17, 2 (2022).
Ullah, H. et al. Vegetation assessments under the influence of environmental variables from the Yakhtangay Hill of the Hindu-Himalayan range, North Western Pakistan. Sci. Rep. 12, 1 (2022).
Ahmad, I. et al. Spatiotemporal aspects of plant community structure in open scrub rangelands of sub-mountainous Himalayan Plateaus. Pak. J. Bot. 42, 3431–3440 (2010).
Amjad, M. S., Arshad, M. & Chaudhari, S. K. Structural diversity, its components and regenerating capacity of lesser Himalayan forests vegetation of Nikyal valley District Kotli (A.K) Pakistan. Asian Pac. J. Trop. Med. 7, S454–S460 (2014).
Ali, M. A. et al. Plant species diversity assessment and monitoring in catchment areas of River Chenab, Punjab, Pakistan. PLoS One 17, 8 (2022).
Weber, H. E., Moravec, J. & Theurillat, J.-P. International code of phytosociological nomenclature. J. Veg. Sci. 11, 739–768 (2000).
Haq, F., Ahmad, H. & Iqbal, Z. Vegetation description and phytoclimatic gradients of subtropical forests of Nandiar Khuwar catchment District Battagram. Pak. J. Bot. 47, 1399–1405 (2015).
Ayub, A., Ahmad, S. S. & Bashir, H. A Multivariate assessment of herbaceous vegetation at Shinghar Vally, District Zhob, Pakistan. AEJAES. 16, 713–718 (2016).
Ramos, M. B. et al. The role of edaphic factors on plant species richness and diversity along altitudinal gradients in the Brazilian semi-arid region. J. Trop. Ecol. 36, 199–212 (2020).
Dar, A. A. & Parthasarathy, N. Herb stratum diversity and community structure in Gurez valley of Kashmir Himalaya: Application of multivariate techniques in community analyses. Geol. Ecol. Landsc. https://doi.org/10.1080/24749508.2023.2216532 (2023).
Almeida, H. A., Ramos, M. B., Diniz, F. C. & Lopes, S. D. What role does elevational variation play in determining the stock and composition of litter?. Floresta Ambient 27, e20180196 (2020).
Kermavnar, J. & Kutnar, L. Patterns of understory community assembly and plant trait-environment relationships in temperate SE European Forests. Diversity 12, 91 (2020).
Münkemüller, T. et al. Dos and don’ts when inferring assembly rules from diversity patterns. Glob. Ecol. Biogeogr. 29, 1212–1229 (2020).
Pinto-Junior, H. V., Villa, P. M., de Menezes, L. F. T. & Pereira, M. C. A. Effect of climate and altitude on plant community composition and richness in Brazilian inselbergs. J. Mt. Sci. 17, 1931–1941 (2020).
Ahmad, M. et al. Patterns of plant communities along vertical gradient in Dhauladhar Mountains in Lesser Himalayas in North-Western India. Sci. Total Environ. 716, 136919 (2020).
Zaman, A. & Badshah, L. Vegetation dynamics along an elevational gradient in Terich valley, Chitral Hindukush range, northern Pakistan. Appl. Ecol. Environ. Res. 18, 6099–6119 (2020).
Hussain, F. et al. Floristic and vegetation diversity of Gadoon hills outer Himalayas district Swabi, Pakistan. Pak. J. Bot. 55, 257–276 (2023).
Malik, Z., Hussain, F. & Malik, N. Life form and leaf size spectra of plant communities Harbouring Ganga Chotti and Bedori Hills during 1999–2000. Int. J. Agric. Biol. 9, 833–838 (2007).
Sorensen, T. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analyses of the vegetation on Danish commons. Biol. Skr. 5, 1–34 (1948).
Takada, Y. On the mathematical theory of communication. Jpn. J. Psychol. 25, 110–117 (1954).
Simpson, E. H. Measurement of diversity. Nature 163, 688 (1949).
Menhinick, E. F. A comparison of some species-individuals diversity indices applied to samples of field insects. Ecology 45, 859–861 (1964).
Pielou, E. C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 15, 177 (1967).
Pichi-Sermolli, R. E. An index for establishing the degree of maturity in plant communities. J. Ecol. 36, 85 (1948).
Chao, A., Ma, K. H. & Hsieh, T. C. User’s guide for iNEXT online: Software for interpolation and Extrapolation of species diversity. Code 30043, 1–14 (2016).
Shah, I. A., Muhammad, Z. & Khan, H. Impact of climate change on spatiotemporal variations in the vegetation cover and hydrology of district Nowshera. J. Water Clim. Chang. 13, 3867–3882 (2022).
Shah, I. A. et al. Evaluation of climate change impact on plants and hydrology. Front. Environ. Sci. 12, 1–16 (2024).
Carlucci, M. B., Streit, H., Duarte, L. D. S. & Pillar, V. D. Individual-based trait analyses reveal assembly patterns in tree sapling communities. J. Veg. Sci. 23, 176–186 (2012).
Zheng, J. et al. Plant community assembly is jointly shaped by environmental and dispersal filtering along elevation gradients in a semiarid area, China. Front. Plant Sci. 13, 1041742 (2022).
Haq, F., Ahmad, H. & Iqbal, Z. Vegetation composition and ecological gradients of subtropical-moist temperate ecotonal forests of Nandiar Khuwar catchment, Pakistan. Bangladesh J. Bot. 44, 267–276 (2015).
Aye, T. H. & Shibata, S. Vegetation structure and tree species diversity inside and outside a newly established Zalon Taung national park in northwest Myanmar. Int. J. For. Res. 2023, 1–18 (2023).
Ali, A., Badshah, L. & Hussain, F. Vegetation structure and threats to montane temperate ecosystems in Hindukush Range, Swat, Pakistan. Appl. Ecol. Environ. Res. 16, 4789–4811 (2018).
Khan, N., Ahmad, M., Wahab, M. & Ajaib, M. Phytosociology, structure and physiochemical analysis of soil in Quercus baloot Griff, Forest District Chitral Pakistan. Pak. J. Bot 42, 2429–2441 (2010).
Ali, H., Muhammad, Z., Ahmad, Z. & Khan, S. M. Environmental determinants of vegetation in district Malakand, a sub-tropical zone of the outer Hindu Kush Mountain Range. Appl. Ecol. Environ. Res. 20, 339–361 (2022).
Dar, M. E. U. I. et al. Comparative analysis of vegetation from eroded and non-eroded areas, a case study from Kashmir Himalayas, Pakistan. Appl. Ecol. Environ. Res. 16, 1725–1737 (2018).
Pyšek, P. et al. Effects of abiotic factors on species richness and cover in Central European weed communities. Agric. Ecosyst. Environ. 109, 1–8 (2005).
Kumar Kar, P., Lochan Barik, K., Kar, P. K., Biswal, A. K. & Barik, K. L. Floristic composition and biological spectrum of a grassland community of Rangamatia in the district of Mayurbhanj, Odisha. J. Curr. Sci. 15, 465–469 (2010).
Wang, H. D. et al. Effect of soil and topography on vegetation restoration in an opencast coal mine dump in a loess area. Shengtai Xuebao 36, 5098–5108 (2016).
Danin, A. Desert rocks as plant refugia in the Near East. Bot. Rev. 65, 93–170 (1999).
Biondi, E. Phytosociology today: Methodological and conceptual evolution. Plant Biosyst. 145, 19–29 (2011).
Nishizawa, K., Shinohara, N., Cadotte, M. W. & Mori, A. S. The latitudinal gradient in plant community assembly processes: A meta-analysis. Ecol. Lett. 25, 1711–1724 (2022).
He, X. et al. Plant diversity patterns along an elevation gradient: The relative impact of environmental and spatial variation on plant diversity and assembly in arid and semi-arid regions. Front. Environ. Sci. 11, 1021157 (2023).
ur Rahman, M., Muhammad, Z., Ullah, R. & Khan, W. M. Floristic composition, altitudinal distribution, seasonality and biological spectrum of the vascular flora of Zaini Pass, District Chitral, Pakistan. Mitteilungen Klosterneubg (2021).
Acknowledgements
The manuscript is a part of the first author’s (Tahseen Ullah) Ph.D. thesis submitted to University of Peshawar via Higher Education Commission, Pakistan. The authors extend their appreciation to the Researchers Supporting Project number (RSPD2024R686) King Saud University, Riyadh, Saud Arabia.
Funding
This work is financially supported by the Researchers Supporting Project number RSPD2024R686, King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
T.U.: Conceptualization, Methodology, Writing- Original draft preparation. Z.M., A.-R.Z.G.: Supervision, Visualization, Investigation, Validation. I.A.S., M.B., Y.A.Y., S.I.: Writing- Reviewing and Editing, Data curation, Software.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ullah, T., Muhammad, Z., Shah, I.A. et al. Eco-systematic assessment of the spring herbaceous vegetation under edaphic and topographic effects. Sci Rep 14, 16920 (2024). https://doi.org/10.1038/s41598-024-66816-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66816-1
- Springer Nature Limited