Abstract
Among parasites of the digestive tract of the black-headed gull (Chroicocephalus ridibundus) in Poland, the best known are species of digenetic trematodes and cestodes. Nematodes of this bird species are not well known. Black-headed gulls, due to their varied diet, migration, life in a flock, and changes of habitat, can become infected with various species of helminths, and like synanthropic birds, they can spread the dispersal stages of parasites across urban and recreational areas. In the present study, an attempt was made to identify the helminth fauna of C. ridibundus from breeding colonies in north-central Poland. The aim of the study was to describe the taxonomic structure of parasites of the digestive tract of the black-headed gull and determine the quantitative parameters of their occurrence. A total of 43 black-headed gulls were examined post-mortem for gastrointestinal helminths, resulting in the identification of four cestodes (Paricterotaenia porosa, Lateriporus clerci, Anomotaenia micracantha, and Wardium fusum), three trematodes (Diplostomum pseudospathaceum, Plagiorchis laricola, and Apophallus muehlingi), and three nematodes (Eucoleus contortus, Cosmocephalus obvelatus, and Porrocaecum ensicaudatum). Lateriporus clerci (in adult form), C. obvelatus and P. ensicaudatum (in larval form) were recorded for the first time in the black-headed gull in Poland.
Similar content being viewed by others
Introduction
The parasite fauna of the digestive tract of black-headed gull Chroicocephalus ridibundus (L.) is characterized by relatively high species richness. Among the helminth fauna of this bird in Poland, the presence of 14 species of cestodes and as many as 19 species of digenetic trematodes has thus far been confirmed1. The nematode fauna of black-headed gulls in Poland is not well known. Only two species of nematodes, Cyathostoma lari Blanchard, 1849 and Eucoleus contortus (Creplin, 1839), have been recorded in black-headed gulls in Poland2,3. Parasitological examination of the droppings of black-headed gulls has been carried out by Indykiewicz and Janiak4 who detected nematodes of the genus Capillaria spp.
The cestode fauna of the black-headed gull includes parasitic worms belonging to the family Diphyllobothridae, including Diphyllobothrium dendriticum (Nitzsch, 1824), Ligula intestinalis (Linnaeus, 1758), L. colymbi Zeder, 1803, Schistocephalus solidus (Müller, 1776), and S. pungitii Dubinina, 1959; the family Dilepididae, including Anomotaenia micracantha (Krabbe, 1869), A. hydrochelidonis Dubinina, 1953, Lateriporus clerci (Johnston, 1912), and Paricterotaenia porosa (Rudolphi, 1810); and the family Hymenolepididae: Aploparaksis larina (Fuhrmann, 1925), Echinocotyle multiglandularis (Baczyńska, 1914), Sobolevicanthus octacanthoides (Fuhrmann, 1906), Wardium fusum (Krabbe, 1869), and W. cirrosum (Krabbe, 1869).
Trematodes occurring in the black-headed gull are well known in Poland and around the world, and knowledge concerning them—both faunistic and ecological aspects—is updated on an ongoing basis1,5,6,7,8,9. Most trematode species thus far recorded in the black-headed gull in Poland are associated with freshwater ecosystems, belonging to the following families: Diplostomidae (Diplostomum baeri Dubois, 1937, Diplostomum paracaudum (Iles, 1959) Shigin, 1977 and Diplostomum spathaceum (Rudolphi, 1819) Olsson, 1876); Strigeidae (Ichthyocotylurus erraticus (Rudolphi, 1809) Odening, 1969, I. pileatus (Rudolphi, 1802) Odening, 1969, I. platycephalus (Creplin, 1825) Odening, 1969, and I. variegatus (Creplin, 1825) Odening, 1969); Echinostomatidae (Echinoparyphium clerci Skrjabin, 1915, E. nordiana Baschkirova, 1941, E. recurvatum (von Linstow, 1873) Lühe, 1909), and Echinostoma revolutum (Fröhlich, 1802) Looss, 1899); Heterophyidae (Apophallus muehlingi (Jägerskiöld, 1899) Lühe, 1909, Schistosomatidae (Gigantobilharzia mazuriana Khalifa, 1974 and G. monocotylea Szidat, 1930); Plagiorchiidae (Plagiorchis laricola Skrjabin, 1924 and P. moravicus Sitko 1993); Prosthogonimidae (Prosthogonimus ovatus (Rudolphi, 1803) Lühe, 1899; and Eucotylidae (Tanaisia fedtschenkoi Skrjabin, 1924)1.
The ecology of the black-headed gull, including its diet, foraging and breeding habitats, and migration, is conducive to the acquisition of internal parasites. The food of these omnivorous birds consists mainly of invertebrates, and thus potential intermediate hosts for helminths, primarily earthworms and insects (e.g. Scarabaeidae, Carabidae, Chrysomelidae, Staphylinidae, Tipulidae, Culicidae, Simuliidae, Ephemeroptera, Trichoptera and Odonata), molluscs (terrestrial species—keelback slugs of the family Limacidae and roundback slugs Arionidae—and freshwater species Cardium sp., Tellina sp., and Bithynia sp.), seeds, fruits, and carrion, as well as food produced by humans. The black-headed gull breeds mainly on islands at various types of water bodies: dam reservoirs, natural lakes, fish ponds, gravel pits, or rivers. The species nests at high densities, and colonies often comprise more than 100 pairs10,11. Black-headed gulls are long-lived birds, with an adult annual survival rate of 0.9012 and a maximum recorded age of 33 years13. Their reproductive output is usually about 1.0–1.5 fledglings per breeding pair14, making adult survival one of the major components of population dynamics15. Black-headed gulls from breeding colonies in Poland winter in Western and Southern Europe16,17. Black-headed gulls are believed to contribute to the spread of parasites which can pose a threat to the health of companion animals (cats and dogs), farm animals, and humans4.
In this paper, an attempt was made to identify the helminth fauna of C. ridibundus from breeding colonies situated in north-central Poland. The aim of the study was to describe the taxonomic structure of parasites of the digestive tract of the black-headed gull and determine the quantitative parameters of their occurrence.
Materials and methods
The material for the research consisted of nematodes, trematodes and cestodes isolated from the gastrointestinal tracts of 36 adult black-headed gulls. Parasitological examination was performed in 2017–2019 on 43 black-headed gulls found dead in breeding colonies situated in north-central Poland, in the Kuyavian–Pomeranian Voivodeship, on small islands in lakes Kusowskie, Koronowskie, and Pakoskie, as well as on the River Brda flowing through Bydgoszcz and on an island in the active gravel pit Skoki Duże, a Natura 2000 protected area for birds. A detailed description of the islands and the size of the colonies are presented in works by Kitowski et al.18, Jakubas et al.19 and Indykiewicz et al.20. Parasites were identified using standard methods, including fixation, staining, and preparation of microscope slides. Cestodes were fixed in 75% ethyl alcohol, and trematodes and nematodes in 70% ethyl alcohol. Cestodes were stained with iron-acetocarmine prepared according to the formula given by Georgiev21. Slides of selected cestode specimens were prepared in Hoyer’s medium22. Trematodes were stained with carmine alum, cleared in clove oil, and mounted in Canada balsam23,24. Unfixed nematode specimens cleared in glycerine were examined under a microscope24.
Helminth species were identified using available keys and numerous original works. The taxonomic affiliation of cestodes was presented according to Pojmańska and Cielecka25 and Pojmańska et al.1. Species identification of trematodes was carried out using keys given by Niewiadomska26, Niewiadomska and Pojmańska27, Yamaguti28,29, Gibson et al.30, Jones et al.31, and Bray et al.32, as well as original works by Bychovskaja-Pavlovskaja33, Niewiadomska34, and Sonin35. The taxonomic affiliation of nematodes was determined according to Baruš and Sergeeva36, Stapf et al.37, Kim et al.3, McNeill and Anderson39,40 and Smogorzhevskaya41. The taxonomic affiliation of 17 cestode and 24 trematode specimens was not determined, and 39 nematode specimens were identified only to subfamily. This was due to severe damage to the bodies of the parasites, most likely resulting from the substantial degree of decomposition of several of the birds. Quantitative parameters of the occurrence of parasites, i.e. prevalence and intensity of infection (mean intensity and range of intensity), were calculated according to definitions given by Bush et al.42 In this study, prevalence refers to the percentage of infected gulls among all tested gulls; mean intensity refers to the number of parasites divided by the number of infected gulls; and range of intensity refers to the minimum and maximum number of parasites infecting a single gull.
A statement confirming the permission for collecting the dead birds: Resolution of the Local Bioethical Commission for Experiments on Animals in Bydgoszcz No 13/2017 of 21.03.2017 and Łódź No 40/ŁB66/2017 of 05.06.2017 and Decision of the Regional Environmental Protection Directorate in Bydgoszcz No WPN.6401.1.118.2017.RS of 03.04.2017, WPN.6401.1.96.2017.MP of 24.07.2017, and WPN.6401.11.105.2018.RS of 06.04.2018.
All experiments were performed in accordance with relevant guidelines and regulations.
Results
The prevalence of infection in the gulls was 83.7% (36 gulls infected with helminths among 43 examined). A total of 530 specimens of all helminth species were collected.
There were 45 cestode specimens isolated from the digestive tracts of gulls. Four cestode species were recorded: Paricterotaenia porosa (Rudolphi, 1810), Lateriporus clerci (Johnston, 1912), Anomotaenia micracantha (Krabbe, 1869) (Family Dilepididae), and Wardium fusum (Krabbe, 1869) (family Hymenolepididae) (Table 1).
Eleven gulls were infected with trematodes, which were isolated from the digestive tracts. Three trematode species were recorded: Diplostomum pseudospathaceum Niewiadomska 1984 (Family Diplostomidae), Apophallus muehlingi (Jägerskiöld, 1899) (family Heterophyidae), and Plagiorchis laricola Skryabin, 1924 (family Plagiorchiidae) (Table 1). D. pseudospathaceum was clearly dominant in terms of abundance (number of individuals, intensity of infection).
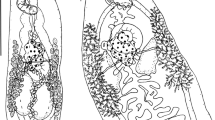
There were 26 black-headed gulls infected with nematodes. The isolated nematodes were assigned to three species: Eucoleus contortus (Creplin, 1839), Cosmocephalus obvelatus (Creplin, 1825) Seurat, 1919, and Porrocaecum ensicaudatum (Zeder, 1800) (Table 1). Nematodes of the species E. contortus and those identified to the subfamily Capillariinae spp. colonized the oesophagus of the hosts. C. obvelatus was isolated from the proventriculus. All P. ensicaudatum specimens were in the third larval stage and located under the stratum corneum at the site where the proventriculus passes into the gizzard. The first, highly characteristic morphological character of the larvae, making them easy to spot on the background of the dissected gizzard, is a black intestine visible through the colourless body. The anterior segment of the body is rounded. The three labia around the mouth typical of sexually mature forms are absent. However, there is a smooth ring of cuticle, visible on the optical microscope slide as two globules situated on each side of the anterior end (Fig. 1). In some specimens, a cap of arcadial tissue was observed below the mouth. Inside the body of the larvae, an oesophagus and a ventriculus could be seen. Their dimensions and those of other parts of the body are presented in Table 2. The posterior end of the body terminates in a conical tail (Fig. 2). There are no male or female sex organs.
Discussion
Among the helminth fauna of the black-headed gull, nematodes were the group with the highest prevalence, followed by cestodes, while the fewest birds were infected with trematodes. This pattern of prevalence is in agreement with the findings of Indykiewicz and Janiak4.
This is the first record of an adult specimen of the species Lateriporus clerci (Johnston, 1912) in Poland. Gulls are definitive hosts of this parasite, while amphipod crustaceans of the genus Gammarus function as intermediate hosts25. The life cycle of the cestode may also include ducks as a paratenic host. It has been recorded in Europe and Asia, e.g. in C. ridibundus, Larus canus, and Larus argentatus ssp. mongolicus from Lake Baikał in south-eastern Siberia, as well as in Chroicocephalus philadelphia from Cooking Lake in Alberta, Canada43,44. In Poland, Czapliński et al.45 detected larvae of this cestode in Gammarus pulex in the Masurian Lake District.
The black-headed gull is a definitive host for all cestode species recorded in the study. The species identified in the research material had previously been recorded (except for L. clerci) among the cestode fauna of these birds in Poland and other parts of Europe1,46,47. The material was not confirmed to include cestodes of the genus Hymenolepis, whose eggs were previously found in 60 adult black-headed gulls from breeding colonies on an island in a lake in Myślęcinek, in the Kuyavian-Pomeranian Voivodeship4.
Paricterotaenia porosa (syn Choanotaenia porosa Rudolphi, 1810) is a widespread parasite in Europe, Asia, and North and Central America. Mature specimens have been recorded in the black-headed gull, common gull, great black-backed gull, and common tern. The intermediate host in its life cycle is unknown25. In Poland, cestodes of this species have been identified in gulls on the Baltic Coast48,49, in the Masurian Lake District50,51, in the Wielkopolsko-Kujawska Lowland52,53, and in eastern Poland54. In Europe, P. porosa has been recorded in Chroicocephalus ridibundus in Denmark (in 16 gulls among 111 examined)55 and in northern Karelia in Russia (7 cestodes in one black-headed gull)5. In Great Britain, cestodes identified only to genus as Paricterotaenia sp. were detected only in a single five-day-old black-headed gull chick56.
Gulls and terns function as definitive hosts of Anomotaenia micracantha (syn Anomotaenia micracantha micracantha Krabbe, 1869). Its intermediate host is unknown. It has been recorded in Europe and North America25. Cestodes of this species have been recorded in Poland in the black-headed gull and black tern in the Masurian Lake District50 and the Wielkopolsko-Kujawska Lowland53.
Gulls are a definitive host of Wardium fusum, syn. Aploparaksis fusus (Krabbe, 1869), and the intermediate host is the crustacean Artemia salina25. The species has been recorded in Europe and Asia, and in Poland in the black-headed gull on the Baltic Coast48,49 and in the Wielkopolsko-Kujawska Lowland53.
The black-headed gull is a host for all species of trematodes recorded in the study. Trematodes are common parasites of black-headed gull, recorded earlier in this bird in Poland, with broad host specificity1. Diplostomum pseudospathaceum, Plagiorchis laricola and Apophallus muehlingi are associated through their life cycles with freshwater environments. The fact that these trematodes were recorded in a synanthropic environment in the region of the Vistula is indicative of foraging by gulls in the inland ecosystem and of a diet consisting in part of natural food which included the hosts of trematodes, which were present in them in larval form (metacercariae). These include fish, insect larvae, crustaceans, and molluscs. This finding indirectly demonstrates the occurrence of invertebrates in the foraging grounds of gulls—snails of the genera Lymnaea, Stagnicola, and Lithoglyphus, which in the life cycles of trematodes are hosts of parthenogenetic forms of trematodes. The three trematode species identified in the material have also been recorded in black-headed gulls in the Czech Republic7. In northern Karelia in Russia, as well as in Great Britain and Denmark, the occurrence of other trematode species belonging to the same genera as those recorded in the present study has been described5,55,56.
Birds of the family Laridae can be parasitized by several species of the genus Diplostomum57,58,59,60. Pojmańska et al.1 stress that some published data on Diplostomum spathaceum may in fact refer to D. pseudospathaceum, as in the past these two species were not distinguished34. Cercariae of D. pseudospathaceum develop in the great pond snail Lymnaea stagnalis and the marsh pond snail Stagnicola palustris. Metacercariae are found in the eye lens of many species of fish, including cyprinids and percids26. Diplostomum spathaceum has often been recorded in fish in Poland, but according to Niewiadomska61, it is a species complex which includes D. pseudospathaceum. It is difficult to distinguish Diplostomum species on the basis of the morphology and morphology of metacercariae due to their close similarity and individual variation. Adult forms of these trematodes do not exhibit such high variation of taxonomic characters as the metacercariae. In a study by Morozińska-Gogol62, in order to determine the Diplostomum species found in three-spined stickleback Gasterosteus aculeatus on the Baltic coast, black-headed gulls were experimentally infected with these metacercariae. The adult trematodes obtained in this manner were predominantly identified as D. spathaceum, indicating that this trematode is the most numerous parasite of the eye of three-spined stickleback in the Baltic62.
Plagiorchis laricola is a widespread species and is found in many species of birds, including storks, plovers and gulls. The host for the sporocysts and cercariae are the great pond snail and the wandering snail Radix balthica, and the metacercariae develop in insect larvae, crustaceans, and molluscs27.
Apophallus muehlingi is a parasite of piscivorous birds. In Poland it has been recorded in gulls of the genus Larus and Gavia stellata. It has also been found in some mammals that feed on fish1. The host of the rediae and mother sporocysts is the gravel snail Lithoglyphus naticoides. The metacercariae develop in cyprinid fish63. The role of the gravel snail as a host is confirmed by the results of research and analyses by numerous authors64,65,66,67. In Poland, larval forms of A. muehlingi occurring in gastropods have not been described. The gravel snail is an alien species in Poland, from the Pontic-Caspian steppe region. At the same time it is one of the endangered species listed in the Polish Red Book of Animals. Its occurrence has been described at many river sites in Poland, including the Vistula68,69. In the light of current knowledge of the role of L. naticoides in the life cycle of the trematode A. muehlingi, this snail can be said to contribute to the persistence of the A. muehlingi population in the area where the gulls for the research were obtained.
The presence of two nematode species has thus far been recorded in Poland: Cyathostoma lari and Eucoleus contortus1. The present study confirmed the occurrence of E. contortus (subfamily Capillariinae), and additionally nematodes Cosmocephalus obvelatus and Porrocaecum ensicaudatum were detected. The presence of C. obvelatus is the first recorded case in the gull C. ridibundus in Poland. This species is found in gulls and other birds associated with the aquatic environment (e.g. Mergus serrator, Sterna arctica, Gavia arctica, Podiceps cristatus, and Alca torda)38,41,70. Adult forms parasitize the oesophagus or proventriculus of the definitive host. The life cycle includes an intermediate host—invertebrates of the order Amphipoda (genera Gammarus, Hyalella, and Crangonyx)71. In Poland, the presence of this nematode species has been described in Phalacrocorax carbo sinensis1.
Porrocaecum ensicaudatum in the form of third-stage larvae is the first confirmed case in the black-headed gull in Poland. Sexually mature nematodes parasitize birds of the order Passeriformes. They develop to the invasive L3 larval form in the intermediate host, which is earthworms39. In birds of the order Charadriiformes (the genus Larus), the development of L3 larvae is inhibited. Birds of the species C. ridibundus are considered to be atypical hosts. P. ensicaudatum larvae live under the stratum corneum at the site where the proventriculus passes into the gizzard. A morphologically similar species for which the black-headed gull and other birds of the order Charadriiformes are recognized as definitive hosts is P. semiteres. Both of these nematode species can parasitize the definitive host in the invasive larval stage. The two species can be distinguished by calculating the ratio of the ventriculus length to the intestinal caecum length70,72,73. The length of the caecum of P. ensicaudatum larvae is no more than half the length of the ventriculus74. Analysis of measurements of the length of the intestinal caecum and ventriculus reveals a ratio of 1:0.29–0.35. The results are similar to the values obtained by Supryaga and Supryaga75.
E. contortus had the highest prevalence and intensity among all recorded nematodes. Indykiewicz and Janiak4 also reported a predominance of nematode eggs of the genus Capillaria (subfamily Capillariine) in the droppings of black-headed gulls in comparison to other groups of helminths. Infection of gulls with nematodes E. contortus confirms the presence of earthworms in the diet of these birds. Earthworms (Oligocheta) are intermediate hosts of nematodes of the genera Capillaria and Eucoleus37,70. The black-headed gull is a typical host for the nematode species found in the present study. Their presence has been described in the black-headed gull in countries such as the Czech Republic73 and Great Britain76.
As colonial birds, gulls have higher species richness of parasitic worms than non-colonial birds77. The species richness of the helminth fauna of birds of the order Charadriiformes is primarily determined by the habitat (freshwater and/or marine) and diet (omnivory, invertebrates, fish-based diet)78. Black-headed gulls are hosts for nearly 200 species of parasitic worms, with trematodes believed to have the highest species richness (120 species detected) (London Natural History Museum host-parasite database79). This is confirmed by research by Gutiérrez et al.78. The authors noted that the species richness of trematodes is higher in birds whose diet includes mixed food sources (invertebrates and fish) or in omnivorous birds occupying marine or freshwater/marine habitats. The number of species of nematodes, cestodes and acanthocephalans is not influenced by the host diet or habitat, but in the case of nematodes, they predominate over other helminths in the parasite fauna of birds which are habitat generalists. The number of trematode species also increases with geographic range, while the species richness of cestodes increases with geographic latitude. The species richness of nematodes decreases as migration distance increases78.
The helminth fauna of black-headed gulls C. ridibundus from several areas of Russia, i.e. northern Karelia, the Republic of Mordovia, and Lake Baikal in south-eastern Siberia, has been studied during the last 10 years5,44,80. Dorzhiev et al.44 examined nearly the same number of gulls as in the present study. While the total prevalence of helminths in black-headed gulls from Lake Baikal was 79.5%, in the present study it was more than 83%. There are considerable differences in the number of species of parasitic worms. Gulls from Lake Baikal were infected with 11 species of cestodes, 23 species of trematodes, 10 species of nematodes, and 3 species of acanthocephalans. In comparison with these data, the species richness of parasites of black-headed gulls from breeding colonies in north-central Poland seems quite modest: 4 species of cestodes, 3 species each of trematodes and nematodes, and no acanthocephalans. However, the species composition of helminths in gulls from these regions overlaps to only a small extent, mainly in the case of cestodes44. No nematodes were found in gulls from the Republic of Mordovia in Russia, but the birds were infected with two species of cestodes, which were also found in the material in the present study, and 4 species of trematodes, of which one, Plagiorchis laricola, was present in gulls from Poland. The helminth fauna of black-headed gulls from North Karelia was poorer in species than in the material analysed in the present study. A species common to both regions was Paricterotaenia porosa.
Other Laridae species in which similar species richness of helminths has been described are the European herring gull Larus argentatus and Larus argentatus ssp. Mongolicus44,79,81,82. The abundance of parasite species can be explained by the range of occurrence of these birds and their type of diet. In seagulls from both the Kola Bay of the Barents Sea and Lake Baikal, trematodes were represented in the highest numbers, with fewer cestodes and nematodes44,81,82.
Gulls Chroicocephalus ridibundus, like Larus argentatus, can be hosts of cestodes Diphyllobothrium dendriticum, which pose a threat to human health. Infections with D. dendriticum in humans still receive little attention, due to their sporadic occurrence. However, they are an example of a food-borne zoonosis and a consequence of the globalization of the fish trade, transport of fresh fish on ice, increased human migration, and changes in culinary habits. Recent cases of infection with D. dendriticum in the Netherlands, Switzerland and the Czech Republic indicate the need to address factors spreading parasites which are rare or do not belong to the native fauna83. The gulls mentioned above can also be hosts of the cestode Ligula intestinalis, which parasitizes cyprinid fish in the form of plerocercoid larvae44,83,84,85. This parasite does not pose a threat to humans.
Helminth species were precisely identified in the present study, but there were certain limitations, e.g. the poor state of some of the carcasses. Nevertheless, in addition to the fresh carcasses examined, birds that were not fresh were used as well, and it was possible to obtain helminths from them in accordance with literature recommendations86,87,88,89. In future endeavours, the use of molecular methods in addition to morphological methods would be of value. Molecular methods can be useful in studies of cryptic species. For example, the use of genetic methods has proven useful in identifying the cryptic diversity of Diplostomum species, including species identification of specimens obtained from intermediate and definitive hosts90,91,92,93. Researchers studying Plagiorchis sp. provide molecular evidence of the high diversity of the genus in snails and stress the need to use molecular methods in further study of this diversity, as well as the importance of further molecular testing to establish the links between various stages in the life cycle of these parasites94,95.
Conclusion
The results of the present study contribute new information regarding helminths of the black-headed gull. Given the new information about the parasite species found in black-headed gull and the dependency of their occurrence on the diet of these birds and their foraging grounds, parasitological research should be continued.
Data availability
Te datasets used and analyzed during this study are available from the first author (A.S.) upon reasonable request.
References
Pojmańska, T., Niewiadomska, K. & Okulewicz, A. Pasożytnicze helminty Polski. Gatunki, żywiciele, białe plamy (Polskie Towarzystwo Parazytologiczne, 2007).
Okulewicz, A. Nicienie ptaków siewkowatych (Charadriiformes) Dolnego Śląska. Wiad. Parazytol. 30, 331–340 (1984).
Okulewicz, A., Perec-Matysiak, A., Hildebrand, J. & Zaleśny, G. Specyficzność żywicielska krajowych nicieni. Wiad. Parazytol. 54, 11–16 (2008).
Indykiewicz, P. & Janiak, K. Black-headed gull Chroicocephalus ridibundus as a source of parasites pathogenic to man and domestic animals. In Animal Man and the City—Interactions and Relationships (eds Indykiewicz, P. & Böhner, J.) 271–278 (ArtStudio, 2014).
Lebedeva, D. I., Yakovleva, G. & Ieshko, E. P. Parasites of gulls (Larus canus L. 1758 and L. ridibundus L. 1766) from Northern Karelia. In Proceedings of the Karelian Research Center of the Russian Academy of Sciences, 1–5 (2015). https://doi.org/10.17076/bg69
Pérez del Olmo, A., Georgieva, S., Pula, H. J. & Kostadinova, A. Molecular and morphological evidence for three species of Diplostomum (Digenea: Diplostomidae), parasites of fishes and fish-eating birds in Spain. Parasit. Vectors 7, 502. https://doi.org/10.1186/s13071-014-0502-x (2014).
Šimková, A., Sitko, J., Okulewicz, J. & Morad, S. Occurrence of intermediate hosts and structure of digenean communities of the black-headed gull, Larus ridibundus (L.). Parasitology 126, 69–78. https://doi.org/10.1017/S0031182002002615 (2003).
Sitko, J. Ecological relations of trematodes infesting lariform birds in the Czech Republik, Přerov. Acta Sc. Nat. Brno 27, 1–98 (1993).
Sitko, J., Faltýnková, A. & Scholz, T. Checklist of the Trematodes (Digenea) of birds of the Czech and Slovak Republics (Academia, 2006).
Wylegała, P. et al. Liczebność i rozmieszczenie lęgowej populacji śmieszki Chroicocephalus ridibundus oraz zausznika Podiceps nigricollis w Wielkopolsce w roku 2013. Ptaki Wielkopolski 3, 101–111 (2014).
Antczak, J., Sikora, A., Kajzer, Z. & Zieliński, P. 2015: Rozmieszczenie i liczebność lęgowych mew i rybitw Laridae na Pomorzu. Ptaki Pomorza 5, 5–31 (2015).
Prevot-Julliard, A. C., Lebreton, J. D. & Pradel, R. Re-evaluation of adult survival of black-headed gulls (Larus ridibundus) in presence of recapture heterogeneity. Auk 115, 85–95 (1998).
van Dijk, K., Lutterop, D., Voesten, R. & Majoor, F. A. Black-headed Gull Larus ridibundus of 33 years and re-appeal to stop using aluminium rings to mark gulls. Dutch Bird. 36, 249–252 (2014).
Viksne, J. & Janaus, M. Breeding success of the Black-headed Gull Larus ridibundus in relation to the nesting time. Ornis Fenn. 57, 1–10 (1980).
Boulinier, T. Avian influenza spread and seabird movements between colonies. Trends Ecol Evol 38, 391–395 (2023).
Cramp, S. (ed.) Handbook of the Birds of Europe, the Middle East and North Africa: the Birds of the Western Palearctic. Volume 3—Waders to Gulls (Oxford University Press, 1983).
del Hoyo, J., Elliott, A. & Sargatal, J. Handbook of the Birds of the World Vol. 3 (Hoatzin to Auks. Lynx Edicions, 1996).
Kitowski, I., Indykiewicz, P., Wiącek, D. & Jakubas, D. Intra-clutch and inter-colony variability in concentrations of elements in eggshells of the black-headed gull Chroicocephalus ridibundus in N Poland. Environ. Sci. Pollut. Res. 24, 10341–10353 (2017).
Jakubas, D., Indykiewicz, P., Minias, P., Kowalski, J. & Iciek, T. Inter-colony variation in flight characteristics of black-headed gulls Chroicocephalus ridibundus during the incubating period. Ecol Evol. 10, 5489–5505 (2020).
Indykiewicz, P., Jakubas, D. & Gerke, J. Gulls of a feather do not sleep whenever—Circadian rhythm of activity of Black-headed Gulls during the incubation period. J. Ornithol. 162, 1101–1114 (2021).
Georgiev, B., Biserkov, V. & Genov, T. In toto staining for cestodes with iron acetocarmine. Helminthologia 23, 279–281 (1986).
Cielecka, D., Salamatin, R. & Garbacewicz, A. Zastosowanie płynu Hoyera do diagnostyki i badań morfologicznych niektórych pasożytów. Wiad. Parazytol. 55, 265–270 (2009).
Sepulveda, M. S. & Kinsella, J. M. Helminth Collection and Identification from Wildlife. J Vis. Exp. 82, e51000. https://doi.org/10.3791/51000 (2013).
Dubinina, M. N. Parazitologicheskoye issledowaniye ptic. Metody parazitologicheskikh issledowaniny. (Leningrad, Academia Nauk SSSR, 1971).
Pojmańska, T. & Cielecka, D. Tasiemce (Cestoda) związane ze środowiskiem wodnym (Wydawnictwo Uniwersytetu Łódzkiego, 2001).
Niewiadomska, K. Przywry (Trematoda). Część ogólna; Cześć systematyczna—Aspidogastrea, Digenea: Strigeida 388 (Wydawnictwo Uniwersytetu Łódzkiego, 2010).
Niewiadomska, K. & Pojmańska, T. Przywry (Trematoda). Cześć systematyczna Digenea: Plagiorchiida 387 (Wydawnictwo Uniwersytetu Łódzkiego, 2018).
Yamaguti, S. Systema Helminthum. Volume I. parts 1 and 2. The Digenetic Trematodes of Vertebrates. (Interscience Publishers Inc. 1958).
Yamaguti, S. Synopsis of Digenetic Treamatodes of verebrates Vol. I and II (Keiagu Publishing Company, 1971).
Gibson, D. I., Jones, A. & Bray, R. A. Keys to the Trematoda Vol. 1 (CAB International Publishing and The Natural History Museum, 2002).
Jones, A. et al. (eds) Keys to the Trematoda Vol. 2 (Wallingford: CABI, 2005).
Bray, R. A., Gibson, D. I. & Jones, A. Keys to the Trematoda Vol. 3 (CAB International Publishing and The Natural History Museum, 2008).
Bychovskaja-Pavlovskaja, I. E. Trematoda of birds of the USSR fauna. [Trematody ptic fauny SSSR]. (Izd. AN SSSR, Moskva-Leningrad, 1962).
Niewiadomska, K. Present status of Diplostomum spathaceum (Rudolphi, 1819) and differentiation of Diplostomum pseudospathaceum nom. Nov. (Trematoda, Diplostomatidae). Syst. Parasitol. 6, 81–86 (1984).
Sonin, M. D. 1986. Guide to the Trematoda of fish-eating birds of the Palearctic region (Opisthorchiidae, Renicolidae, Strigeidae), vol. 2. [Opredelitel’ trematod rybojadnych ptic Palearttiki (opistorhidy, renikolidy, strigeidy)] (Academia NAuk SSSR, Nauka, Moskwa 1986).
Baruš, V., Sergeeva, T. P., Sonin, M. D. & Rzyhikov, K. M. Helminths of Fish Eating Birds of the Palearctic Region I. Nematoda (Praha, Academy of Sciences, 1978).
Stapf, A. N., Kavetska, K. M., Ptak, P. P. & Rząd, I. Morphological and ecological analysis of nematodes of the family Capillariidae (Neveu-Lemaire, 1936) in wild ducks (Anatinae) from the north-western Poland. Ann. Parasitol. 59, 195–201 (2013).
Kim, S. M., Park, B. K., Jung, B. D. & Kim, H. C. H. First record of Cosmocephalus obvelatus (Acuariidae) in common gulls (Larus canus) from Gangneung, Korea. Korean J Parasitol 53, 101–104 (2015).
McNeill, M. A. & Anderson, R. C. Development of Porrocaecum ensicaudatum (Nematoda: Ascaridoidea) in terrestrial oligochaetes. Can. J. Zool. 68, 1476–1483 (1990).
McNeill, M. A. & Anderson, R. C. Development of Porrocaecum ensicaudatum (Nematoda: Ascaridoidea) in starlings (Sturnus vulgaris). Can. J. Zool. 68, 1484–1493 (1990).
Smogorzhevskaya, L. A. Nematodes. Part 3. Acuarioidea. In Fauna Ukrainy (ed. Sharpilo, V. P.) 248–250 (Naukova Dumka, 1990).
Bush, A. O., Lafferty, K. D., Lotz, J. M. & Shostak, A. W. Parasitology meets ecology on its own terms: Margolis et al. revisted. J. Parasitol. 83, 575–583 (1997).
Hair, J. D. & Holmes, J. C. Helminths of Bonaparte’s gulls, Larus Philadelphia, from Cooking Lake. Alberta. Can. J. Zool. 48, 1129–1131 (1970).
Dorzhiev, T., Badmaeva, E. N. & Dugarov, Z. Ecological and faunistic analysis of helminthes of water-marsh birds of Baikal Siberia: 3. Gulls Laridae. Nat. Inn. Asia 1, 66–78 (2020).
Czapliński, B., Sulgostowska, T. & Czaplińska, D. Tasiemce—Cestoda. Katalog Fauny Pasożytniczej Polski. Cz. IV. Pasożyty ptaków 1–186 (Pol. Tow. Parazyt., Kom. Faunistyczna, 1992).
Okulewicz, A. New records of helminth species and their hosts in Poland. Wiad. Parazytol. 57, 3–9 (2011).
Okulewicz, A. Helminths in migrating and wintering birds recorded in Poland. Ann. Parasitol. 60, 19–24 (2014).
Rybicka, K. Tasiemce ptaków niekaczkowatych (exclusive Anseriformes) jezioro Drużno. Wiad. Parazytol. 2(1), 195–198 (1956).
Rybicka, K. Tasiemce ptaków (excl. Anseriformes) jezioro Drużno (Parazytofauna biocenozy jeziora Drużno. Część IV. Acta Parasitol. Pol. 6, 143–178 (1958).
Korpaczewska, W. Tapeworms of aquatic birds in some Mazurian lakes. Acta Parasit. Pol. 11, 315–336 (1963).
Korpaczewska, W. & Sulgostowska, T. Materiały do helmintofauny ptaków wodnych Mazur (jezior Warnołty). Wiad. Parazytol. 13, 737–744 (1967).
Sulgostowska, T. & Korpaczewska, W. Helminth fauna of birds two pond system of the Milicz pond reserve. Acta Parasitol. Pol. 20, 75–94 (1972).
Pojmańska, T., Machalska, J. & Niewiadomska, K. Parasites of birds from the lake Gopło and heated lakes of the Konin region. Acta Parasiolt. Pol. 29, 227–290 (1984).
Bezubik, B. Materiały do helmintofauny ptaków wodnych Polski. Acta Parasitol. Pol. 4, 59–88 (1956).
Guidal, J. A. Some qualitative and quantitative investigations on the endoparasitic fauna of the scandinavian population of the black-headed gull (Larus ridibundus L). In Proceedings of the first international congress of parasitology, vol. 1, 517–519 (1966).
Jennings, A. R. & Soulsby, E. J. Disease in a colony of black-headed gulls Larus ridibundus. Ibis 100, 305–312 (1958).
Sitko, J. Trematodes of birds of the family Laridae in Czechoslovakia. Acta Soc. Zool. Bohemoslov. 32, 275–292 (1968).
Höglund, J. & Thulin, J. Identification of Diplostomum spp. in the retina of perch Perca fluviatilis and the lens of roach Rutilus rutilus from the Baltic Sea—An experimental study. Syst. Parasitol. 21, 1–19 (1992).
Valtonen, E. T. & Gibson, D. I. Aspects of the biology of diplostomid metacercarial (Digenea) populations occurring in fishes in different localities of northern Finland. In Annales Zoologici Fennici, 47–59 (Finnish Zoological and Botanical Publishing Board, 1997).
Rolbiecki, L., Rokicki, J., Morozińska-Gogol, J. & Chibani, M. Larval stages of helminths in fish from the Vistula Lagoon and the Gulf of Gdańsk in relation to bird occurrence. Bull. Sea Fish. Inst. 2, 51–60 (1999).
Niewiadomska, K. 2003. Pasożyty ryb Polski (klucze do oznaczania). Przywry – Digenea. Monografie Parazytologiczne, vol 15. (Polskie Towarzystwo Parazytologiczne, 2023).
Morozińska-Gogol, J. Experimental infection of the black-headed gull Larus ridibundus (L.) with eye-flukes parasites of the Larus ridibundus (L.) with eye-flukes parasites of the three-spined stickleback Gasterosteus aculeatus (L.). Balt. Coast. Zone 11, 59–64 (2007).
Wierzbicka, J. & Wierzbicki, K. Metacerkariae of the genus Apophalus Luhe, 1909 (Trematoda: Heterophyidae) in Western Pomerania of Poland. Acta Ichth. Pisc. 3, 75–89 (1973).
Cichy, A., Faltýnková, A. & Zbikowska, E. Cercariae (Trematoda, Digenea) in European freshwater snails—A checklist of records from over one hundred years. Folia Malacol. 19, 165–189. https://doi.org/10.2478/v10125-011-0023-6 (2011).
Izvekova, G. I. & Tyutin, A. V. Occurrence of partenites in mollusks and the influence that metacercaria of Apophallus muehlingi (Jagerskiold, 1898) and Posthodiplostomum cuticola (Nordmann, 1832) has on some biochemical parameters in fish. Inland Water Biol. 4, 367–372 (2011).
Tyutin, A. V. & Izvekova, G. I. Infection of mollusks and fish by the trematode Apophallus muehlingi (Jagerskiold, 1898) and its interrelations with intermediate hosts. Inland Water Biol. 6, 52–56 (2013).
Vojtek, J. Prispevek k poznani Helmintofauny ryb v okoli Komarna. Publ. Fac. Sci. Univ. Brno, 407, 437–465. [A contribution to the knowledge of the helminth fauna of fishes near Komarno]. Pub. Fae. Sci. Univ. Brno, Tchecoslovaquie, 407: 437–465 (1959).
Piechocki, A. Lithoglyphus naticoides (C. Pfeiffer, 1828). In Polska czerwona księga zwierząt Bezkręgowce (eds Głowaciński, Z. & Nowacki, J.) 318–319 (IOP PAN i AR im. Cieszkowskiego, 2004).
Kołodziejczyk, A. Gatunki obce w faunie Polski: Namułek pospolity. (IOP PAN, 2008). http://www.iop.krakow.pl/gatunkiobce/default.asp?nazwa=opis&id=11&je=pl
Barus, V., Sitko, J. & Tenora, F. Nematoda and Pentastomida parasitizing gulls (Aves: Laridae) in Bohemia and Moravia. Acta Univ. Agric. Brno Fac. Agron. 26, 169–182 (1978).
Wong, P. L. & Anderson, R. C. The transmission and development of Cosmocephalus obvelatus (Nematoda: Acuarioidea) of gulls (Laridae). Can. J. Zool. 60, 1426–1440 (1982).
Frantova, D. On the morphology and surface ultrastructure of some parasitic nematodes (Nematoda) of birds (Aves). Acta Soc. Zool. Bohem. 66, 85–97 (2002).
Stapf-Skiba, A. Pasożytnicze nicienie Porrocaecum ensicaudatum (Zeder, 1800) u ptaków z rzędu Passeriformes i Charadriiformes. In Środowiskowe i genetyczne uwarunkowania zdrowia ludzi i zwierząt (eds Pilarczyk, B. et al.) 223–231 (Wydawnictwo Uczelniane Zachodniopomorskiego Uniwersytetu Technologicznego w Szczecinie, 2020).
Li, L. & Scholz, T. Redescription of Porrocaecum semiteres (Zeder, 1800)(Nematoda: Ascaridida) from the Song Thrush Turdus philomelos (Passeriformes: Turdidae). Acta Parasitol. 64, 1–6 (2019).
Suprjaga, V. G. & Suprjaga, A. M. K diferencjalnoj diagnostike liczinok nematod roda Porrocaecum Railliet et Henry, 1912 iz promeżutocznych chozjajev—dożdevych czervej. Trudy Gelan. 22, 200–203 (1971).
Pemberton, R. Helmint parasites of three species of british gulls, Larus argentatus Pont., L. fuscus L. and J. ridibundus L. J. Helminthol. 37, 57–88. https://doi.org/10.1017/S0022149X00019660 (1963).
Gutiérrez, J. S., Piersma, T. & Thieltges, D. W. Micro- and macroparasite species richness in birds: The role of host life history and ecology. J. Anim. Ecol. 88, 1226–1239 (2019).
Gutiérrez, J. S., Rakhimberdiev, E., Piersma, T. & Thieltges, D. W. Migration and parasitism: habitat use, not migration distance, influences helkminth species richness in Charadriiform birds. J. Biogeogr. 44, 1137–1147 (2017).
Gibson, D., Bray, R. & Harris, E. Host–parasite database of the Natural History Museum, London (2005). https://www.nhm.ac.uk/research-curation/scientific-resources/taxonomysystematics/host-parasites/database/index.jsp
Киpиллoвa, H. Ю., Киpиллoв, A. A., & Cпиpидoнoв, C. H. К изyчeнию гeльминтoфayны oзepнoй чaйки Larus ridibundus Cpeднeгo Пoвoлжья. In Maтepиaлы мeждyнapoднoй кoнфepeнции «Экoлoгичecкиe пpoблeмы бacceйнoв кpyпныx peк» (No. 6, pp. 139–141). Учpeждeниe Poccийcкoй aкaдeмии нayк Инcтитyт экoлoгии Boлжcкoгo бacceйнa PAH.https://doi.org/10.24411/9999-002A-2018-10056 (2018).
Nekrasov, A. V., Pronin, N. M., Sanzhieva, S. D. & Timoshenko, T. M. Diversity of helminth fauna in the herring gull (Larus argentatus) from the Baikal Lake: Peculiarities of spatial distribution and invasion. Parazitologia 33, 426–436 (1999).
Kuklin, V. V. & Kuklina, M. M. Age dynamics of helminth fauna of the herring gull (Larus argentatus) in Kola Bay. Barents Sea. Biol. Bull. 48, 1160–1169 (2021).
Kuchta, R., Brabec, J., Kubáčková, P. & Scholz, T. Tapeworm Diphyllobothrium dendriticum (Cestoda)—Neglected or emerging human parasite?. PLoS Negl. Trop. Dis. 7, e2535 (2013).
Pavlovich, K. A. New definitive hosts of the ligulids (Ligulidae) in the Delta of Volga. Am. J. Sci. 4, 117–120 (2016).
Kuklin, V. V. & Kuklina, M. M. Diphyllobothrium dendriticum (Cestoda: Diphyllobothriidae) in the intestinal tract of the herring gull Larus argentatus: localization and trophic parameters. Biol. Bull. 43, 329–334 (2016).
Okulewicz, A. & Okulewicz, J. Great Counting—Do We Know about Bird Parasites of Poland? In Ornitologia Polska na Progu XXI Stulecia–Dokonania i Perspektywy; Sekcja Ornitologiczna PTZool, 135–145 (Uniwersytet Warmińsko-Mazurski w Olsztynie, 2006)
Sitko, J. & Zaleśny, G. The effect of urbanzation on helminth communities in the Eurasian blackbird (Turdus merula L.) from the eastern part of the Czech Republic. J. Helminthol. 88, 97–104 (2014).
Kalisińska, E. et al. Digenea of Haliaeetus albicilla (Linnaeus, 1758) and Pandion haliaetus (Linnaeus, 1758) from central and northwestern Poland. Ann. Parasitol. 54, 349–351 (2008).
Hromada, M., Dudinák, V. & Yosef, R. An inside perspective of the true shrikes—A revive of the helminthofauna. Ring 22, 85–294 (2000).
Selbach, C., Soldánová, M., Georgieva, S., Kostadinova, A. & Sures, B. Integrative taxonomic approach to the cryptic diversity of Diplostomum spp. in lymnaeid snails from Europe with a focus on the ‘Diplostomum mergi’ species complex. Parasit. Vectors 8, 1–21 (2015).
Georgieva, S. et al. Molecular prospecting for European Diplostomum (Digenea: Diplostomidae) reveals cryptic diversity. Int. J. Parasitol. 43, 57–72. https://doi.org/10.1016/j.ijpara.2012.10.019 (2013).
Locke, S. A., McLaughlin, J. D., Dayanandan, S. & Marcogliese, D. J. Diversity, specificity and evidence of hybridization in Diplostomum spp. metacercariae in freshwater fishes is revealed by DNA barcodes and ITS sequences. Int. J. Parasitol. 40, 333–343. https://doi.org/10.1016/j.ijpara.2009.08.012 (2010).
Blasco-Costa, I. et al. Fish pathogens near the Arctic Circle: Molecular, morphological and ecological evidence for unexpected diversity of Diplostomum (Digenea: Diplostomidae) in Iceland. Int. J. Parasitol. 44, 703–715. https://doi.org/10.1016/j.ijpara.2014.04.009 (2014).
Kundid, P., Pantoja, C., Janovcová, K. & Soldánová, M. Molecular diversity of the genus Plagiorchis Lühe, 1899 in snail hosts of Central Europe with evidence of new lineages. Diversity 16, 158 (2024).
Kudlai, O. et al. Diversity of Plagiorchis (Trematoda: Digenea) in high latitudes: Species composition and snail host spectrum revealed by integrative taxonomy. J. Zool. Syst. Evol. Res. 59, 937–962 (2021).
Oswald, V. H. Helminth parasites of the short-tailed shrew in central Ohio. Ohio J. Sci. 58, 325–334 (1958).
Okulewicz, A. Nicienie kosa (Turdus merula L.) i drozda śpiewaka (Turdus philomelos BR.) z okolic Wrocławia. Wiad. Parazytol. 25, 301–331 (1979).
Funding
Co-financed by the Minister of Science under the "Regional Excellence Initiative" Program for 2024-2027 (RID/SP/0045/2024/01).
Author information
Authors and Affiliations
Contributions
Conceptualization: A.S. and P.I.; methodology: A.S., K.K., I.R.; formal analysis: P.I., W.G., investigation: A.S., I.R., K.K. P.I., P.G.; writing—original draft preparation: A.S.; writing—review and editing: A.S., I.R., K.K., P.I., W.G.; visualization: A.S.; funding acquisition: I.R. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stapf, A.N., Rząd, I., Królaczyk, K. et al. Helminths of the black-headed gull (Chroicocephalus ridibundus) from breeding colonies in north-central Poland. Sci Rep 14, 15354 (2024). https://doi.org/10.1038/s41598-024-66270-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66270-z
- Springer Nature Limited