Abstract
Cervical cancer (CC) is the fourth most common cancer among women worldwide. NLR Family CARD Domain Containing 5 (NLRC5) plays an important role in tumorigenesis. However, its effect and mechanism in CC remains unclear. In this study, we aimed to investigate the function of NLRC5 in CC. NLRC5 was found to be down-regulated in CC tissues compared with normal cervical tissues. However, patients with higher NLRC5 expression had better prognosis, patients with higher age, HPV infection, lymph node metastasis, recurrence and histological grade had worse prognosis. Univariate and multivariate analyses showed NLRC5 to be a potential prognostic indicator for CC. Pearson correlation analysis showed that NLRC5 might exert its function in CC through autophagy related proteins, especially LC3. In vitro experiments demonstrated that NLRC5 inhibited LC3 levels and promoted the proliferation, migration, and invasion of CC cells by activating the PI3K/AKT signaling pathway. Treatment with LY294002 reversed the above phenotype. Taken together, our finding suggested that NLRC5 would participate in cervical tumorigenesis and progression by regulating PI3K/AKT signaling pathway. In addition, NLRC5 and LC3 combined as possible predictors in CC.
Similar content being viewed by others
Introduction
Cervical cancer (CC), one of the most common malignant tumors in the female reproductive system, has the fourth highest incidence rate in the world, causing 250,000 female deaths annually1. Although the morbidity and mortality rates of CC have decreased significantly due to major advances in the prevention, screening, and diagnosis of CC, the 5-year survival rate of patients with advanced CC after treatment is still low2. Therefore, it is urgent to explore the molecular mechanisms underlying the pathogenesis of CC to develop new strategies for the diagnosis and treatment of CC, especially for patients with advanced and recurrent CC.
NLRC5 is a newly identified member of the NLR family that acts as a transcriptional activator of MHC class I genes3. Recent studies have indicated that NLRC5 contributes to tumorigenesis by mechanisms beyond the regulation of MHC class I genes4,5. Previous studies have shown that NLRC5 promotes the proliferation, migration, and invasion in hepatocellular carcinoma, clear cell renal cell carcinoma, and endometrial cancer6,7,8,9. Mechanistically, a plethora of tumor related signaling pathways are involved in the regulation of NLRC5 in cancer. For example, He et al. showed that the over-expression of NLRC5 promoted cell proliferation by activating the PI3K/AKT/ vascular endothelial growth factor-A (VEGF-A) signaling pathway in hepatocellular carcinoma in vitro7. Up-regulation of NLRC5 has been shown to contribute to cell proliferation, migration and invasion in hepatocellular carcinoma and clear cell renal cell carcinoma by activating the Wnt/β-catenin signaling pathway in vitro and in vivo6,8. Recently, in an in vitro study in endometrial cancer, Fan and co-workers showed that the upregulation of NLRC5 expression induced cell migration and invasion by activating the PI3K/AKT signaling pathway9. Notably, the knockdown of NLRC5 in the renal proximal tubular epithelial cells significantly enhanced the activation of PIK3/AKT signaling pathway in hypoxia/reoxygenation-stimulated renal proximal tubular epithelial cells10. These findings demonstrated that the role of NLRC5 in the PIK3/AKT signaling pathway was cell-dependent. Nevertheless, the role and mechanism of NLRC5 in CC have not been explored.
Autophagy is an evolutionarily conserved mechanism, including the removal of damaged cellular components, which helps with the recycling and degradation of cytoplasmic components. Besides, it also enables the elimination of toxic factors and restores cellular homeostasis11,12. Thus, exploring the role of autophagy in tumor cells is of great significance for improving the effect of cancer therapeutics13,14. Researchers have identified a correlation between autophagy and NLRC5 expression. For example, in endometriosis, the overexpression of NLRC5 promotes the expression of LC3, Beclin1 and the formation of autophagosomes in the lesion, which inhibit disease progression6. PIK3/AKT signaling pathway is believed to be the the most important pathways that control autophagy15.
Collectively, we hypothesized that in patients with CC, NLRC5 might promote tumorigenesis by regulating PI3K/AKT signaling pathway. NLRC5 and autophagy combined as possible predictors in patients with CC. In this study, tissue microarray technique was used to investigate the expression of NLRC5, LC3 and Beclin 1 in CC tissues, to evaluate the association between the expression of NLRC5 and the clinicopathological parameters of the patients and to assess the correlation between NLRC5 expression and the prognosis of CC patients. Next, we evaluated the underlying mechanism associated with NLRC5 mediated effects in the progression of CC in vitro. Our study help increase the potential of approaches that regulating NLRC5 or autophagy level for use in therapy against CC.
Materials and methods
Tissue samples
CC tissue chip (HUteS154Su01) was purchased from Shanghai Xinchao Biotechnology Co., Ltd. The samples were from 119 patients with CC aged between 29 and 70 years old, of which 35 cases had matched corresponding peri-cancerous tissues. In addition, the detailed clinical information of patients, such as lifetime, tumor location, histologic differentiation, and tumor node metastasis (TNM) stage were also collected. According to the staging standard of primary cancer, lymph node and distant metastasis (TNM staging) were specified by the International Anti-Cancer Association (UICC), the degree of invasion was confined to the uterus (T1 stage) in 67 cases, and outside the uterus (T2-T4 stage) in 52 cases. 22 cases had lymph node metastasis and 97 cases had no lymph node metastasis. There was no distant metastasis in any of the tissue specimens. None of the patients underwent radiotherapy and chemotherapy before surgery. All treatments were approved by the Institutional Review Board of Anhui Medical University in this study. We confirmed that informed consent was obtained from all subjects and all research was performed in accordance with the Declaration of Helsinki.
Immunohistochemistry
Tissue sections were routinely deparaffinized with xylene and rehydrated in decreasing concentration of ethanol. Antigen retrieval was achieved by boiling the sections in 10 mM citrate buffer for 20 min. After washing three time with PBS for 5 min each time, the sections were blocked with 3% hydrogen peroxide for 10 min to inhibit endogenous catalase. Then the sections were incubated with primary anti-NLRC5 antibody (ab105411, Abcam, diluted 1:100), anti-LC3 (ab192890, Abcam, diluted 1:100) and anti-Beclin 1 (ab210498, Abcam, diluted 1:100) overnight at 4°C. After washing with PBS, the sections were incubated with the horseradish peroxidase labelled secondary antibody at room temperature for 20 min. The sections were immersed in DAB (Beyotime, P0202) until they become colored and were then rinsed with running water. Finally, the sections were subsequently subjected to staining with hematoxylin, dehydration, permeation, resin sealing and were then photographed under the microscope.
Evaluation of protein expression by immunohistochemical staining
IHC staining was evaluated by the histochemistry H Score on the basis of the extent and intensity of immunolabeling of the tumor sections. Briefly, the Seville image analysis system was used to automatically read the tissue measurement area, and the result was semi-quantitatively analyzed with the digital scoring system (H-score). H-Score = ∑(pi × i), where pi was the percentage of positive area and i was the density of positive signal. The density of staining was scored as 0 (absent), 1 (weak), 2 (moderate) and 3 (strong). The staining percentage of positive area was recorded from 0 to 100%. The H-score pattern of NLRC5 is shown in Fig. 1. The median of H-scores was the cutoff value. Final H-score > cutoff value was interpreted as high expression of NLRC5, while H-score ≤ cutoff value was interpreted as low expression of NLRC516. The corresponding origin data in Supplementary Information 1.
Immunoreactivity in cervical cancer and para-cancerous tissues. (magnification × 200). (a–c) IHC staining of para-cancer (n = 34). (d–f) IHC staining of cancer (n = 34). (g–i) Box plot of NLRC5, Beclin 1 and LC3 expression in cervical cancer (blue) and para-cancerous tissues (red). *p < 0.05, **p < 0.01, ***p < 0.001.
Cell culture and transfection
The human CC cell lines HeLa and SiHa were obtained from American Type Culture Collection (ATCC). CC cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA), and 1% penicillin/streptomycin (Invitrogen) in the incubator at 37℃ under 5% CO2. For transfection with NLRC5 plasmid and small interfering RNA (siRNA)-NLRC5, the cells were treated with Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. The full-length coding region of NLRC5 was created by using the primers F: 5′-CCGGAATTCCGGATGGCCAGGAAGCTGGA-3′, R: 5′-GGGATCCCGTCAC CTGAGTGTCTTC CCA-3′. siRNA-NLRC5 or scrambled sequences, which are listed in Table 1.
Western blotting
Lysates were configured according to the ratio of RIPA (P0013K, Beyotime, China): phenylmethylsulfonyl fluorid (PMSF) (ST507, Beyotime, China):phosphatase inhibitor cocktail A(P1081, Beyotime, China) = 100:1:2 and CC cells were lysed with it. Protein concentration was measured using the bicinchoninic acid (BCA) kit (Sigma, USA). Lysates were collected by centrifugation at 12,000 rpm at 4 ℃ for 10 min. Equal amount of total protein was loaded on the different lanes of polyacrylamide SDS gels, after the proteins were separated, they were transferred onto PVDF membranes. The PVDF membrane was incubated with the primary antibody overnight at 4 ℃. The primary antibodies against NLRC5 (ab105411, Abcam, USA), p-AKT (Ser473) (AF0016, Affinity Biosciences), AKT (AF6261, Affinity Biosciences), PI3K (AF6241, Affinity Biosciences, Cincinnati, OH, USA), p-PI3K (Tyr607) (AF3241, Affinity Biosciences, Cincinnati, OH, USA), LC3 (ab192890, Abcam, USA) and GAPDH (GB12001, Servicebio, Wuhan, China) were used at 1: 1000, 1:1000, 1:1000, and 1:3000 dilution, respectively. Then the membranes were incubated in Tris-buffered saline-Tween (TBST; Boster Bio, Wuhan, China) containing 5% skim milk at room temperature for 1 h, followed by washing three times with TBS-Tween 20, and incubation with the horseradish peroxidase (HRP) labeled secondary antibody (Thermo Fisher Scientific, 1: 10,000) at room temperature for 1 h. Then, the membrane was washed thrice with TBST to remove the unbound secondary antibody. Electroluminescence (ECL, Thermo Fisher Scientific, Waltham, MA, USA) was used for detecting the signal from the protein bands. The images we provide all have corresponding images in the Supplementary Information 2.
CCK-8 assay.
5 × 104 cells/mL CC cells were plated on 12-well plates. Then 20 μL CCK-8 reagent was added into each well. The Synergy H4 Hybrid Microplate reader (Bio Tek, Winooski, VT) was used to measure the absorbance at 450 nm. The corresponding origin data in Supplementary Information 1.
Cell migration and invasion assays
A 24-well Transwell Boyden chamber (Corning Inc., Corning, NY, USA) with an 8.0 μm pore size polycarbonate membrane was used for the migration and invasion assays according to the manufacturer’s instructions. In brief, CC cells were trypsinized and seeded in the upper chamber at a density of 1 × 105 cells/well in 100 μL of serum-free medium. Then, 600 μL of complete medium was added to the lower chamber as a chemoattractant. After incubation for 24 h at 37 ℃, cells remaining at the upper surface of the membrane were removed with cotton swabs and the cells on the lower surface of the membrane were counted as the migrated cells. After fixation with 4% paraformaldehyde and staining with crystal violet solution, cells that passed through the filter were photographed by using an inverted fluorescence microscope. The invasion assay was performed in a similar manner, except that the transwells were pre-coated with 100 μL of 1:8 DMEM-diluted Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) at 37 ℃ for 6 h before the cells were seeded onto the membrane, followed by incubation for 48 h.
Treatment of CC cells with the PI3K broad-spectrum inhibitor LY294002
The PI3K broad-spectrum inhibitor LY294002 (Sigma Chemical Co., St. Louis, MO, USA) was dissolved in dimethyl sulfoxide (Sigma Chemical Co.). We used LY294002 at a concentration of 20 μM to inhibit the PI3K/AKT pathway in CC cells. The cells were first seeded overnight in culture dishes and transfected with NLRC5 plasmid 6 h later, followed by treatment with 20 μM LY294002 for 48 h.
Statistical analysis
All data were analyzed using the SPSS 23.0 software (SPSS Inc., Chicago, USA). The difference in the expression levels of NLRC5, Beclin1and LC3 between the CC group and the corresponding normal tissue group were evaluated using the rank sum test. The median NLRC5, Beclin1 or LC3 expression was used as a cut-off value for grouping. Correlation analysis was performed using Pearson’s correlation. Kaplan–Meier survival curves were generated to investigate the prognostic significance of NLRC5, Beclin1 and LC3 in CC patients. Univariate and multivariate survival analysis were performed using log-rank and Cox risk regression model. All experimental data were expressed as mean ± SEM. p < 0.05 was statistically significant.
Ethics approval
Ethical approval to report this data was obtained from the Institutional Review Board of Anhui Medical University (No.20180023). We confirmed that informed consent was obtained from all subjects and all research was performed in accordance with the Declaration of Helsinki.
Results
Expression of NLRC5 and autophagy-related proteins in cervical cancer
Human CC tissues (n = 34) and adjacent normal tissues (n = 34) were analyzed by immunohistochemistry to evaluate the expression levels of NLRC5, Beclin 1 and LC3 proteins. All cases presented staining for NLRC5, Beclin 1 and LC3 were predominantly detected in the cytoplasm of tumor cells. Apparently, normal adjacent to primary tumor presented the weak NLRC5, Beclin 1 and LC3 staining in cervical epithelium layers (Fig. 1a–f). Statistical analysis based on the H-score estimated in IHC staining revealed a significant difference in NLRC5 expression between the CC tissues and para-cancer tissues (z = 4.796, p < 0.01). Similar results were also observed for Beclin 1 (z = 20,129, p = 0.033) and LC3 (z = 2.207, p = 0.027) IHC staining cases (Fig. 1g–i).
Correlation between the expression of NLRC5 and clinical characteristics of cervical cancer patients
To investigate whether NLRC5 expression was associated with the progression of CC, we analyzed the correlation between NLRC5 expression and the clinicopathological features (Table 2). The chi-square test revealed a significant association of NLRC5 expression with the histological subtypes, where tumors with lower NLRC5 expression were more often squamous carcinoma subtype (p = 0.044). No significant relationship was found between NLRC5 expression and the other listed clinicopathological parameters such as age (p = 0.168), histological grade (p = 0.168) and HPV infection (p = 0.168).
Survival analysis reveals a positive correlation between NLRC5 expression and the prognosis of cervical cancer patients
To evaluate the prognostic role of NLRC5 in CC patients, disease free survival (DFS) rate was determined by Kaplan–Meier survival analysis (Fig. 2). Log rank test demonstrated that patients with high NLRC5 expression had a significantly longer DFS than those with low NLRC5 expression (Fig. 2a). Among the clinicopathological characteristics, patients with young age, low TNM staging, no metastasis, no HPV infection and no recurrence had better outcomes (p < 0.05) (Fig. 2d, e, h, i). However, there was no statistically significant association between the expression of Beclin1 (p = 0.6), LC3 (p = 0.624), histological type (p = 0.767) and overall survival (OS) (Fig. 2b, c, f).
Kaplan–Meier survival curves of cervical cancer patients. (a) NLRC5 (χ2 = 4.55, p = 0.033), (b) Beclin1 ((χ2 = 0.274, p = 0.6), (c) LC3 (χ2 = 0.240, p = 0.624), (d) Age (χ2 = 18.853, p < 0.001), (e) TMN stage ((χ2 = 31.295, p < 0.001), 1 represents TNM 1, while 2 represents TNM 2–4. (f) Histological type (χ2 = 0.088, p = 0.767). 1 represents squamous carcinoma, while 2 represents adenocarcinoma, mixed squamous and adenocarcinoma and others. (g) HPV infection (χ2 = 14.949, p = 0.001), (h) Lymph node status (χ2 = 23.846, p < 0.001), (i) Recurrence (χ2 = 95.207, p < 0.001).
The Cox proportional hazard model was used for univariate and multivariate analyses to assess the effects of NLRC5, Beclin1, LC3 and clinicopathological variables in CC patients (Table 3). The univariate analysis showed that NLRC5 expression level, age, TNM stage, lymph node status and recurrence in CC patients were correlated with patient prognosis (p < 0.05). The variables with statistically significant differences in univariate analysis were included in the Cox multivariate survival regression analysis and the results showed that the level of NLRC5 expression, age and TNM stage were associated with patient prognosis (p < 0.05). In other words, patients with younger age, early stage and high NLRC5 expression had better prognosis. These results suggest that NLRC5 may potentially be one of independent prognostic factors in CC.
The correlation of NLRC5 expression, Beclin 1 expression and LC3 expression
Correlation analysis was performed to understand the potential relationship of NLRC5, LC3 and Beclin 1 expression (Table 4). We found no correlation between NLRC5 and Beclin 1 expression. However, NLRC5 expression was weakly and positively correlated with LC3 expression (R = 0.384, p < 0.001) indicating that NLRC5 may exert its function in CC through LC3. LC3 expression was also positively correlated with Beclin 1 expression (R = 0.434, p < 0.001).
Overexpression of NLRC5 induces proliferation, migration, and invasion of CC cells in vitro
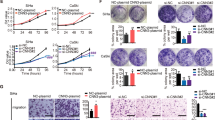
To investigate the function of NLRC5 in CC, HeLa and SiHa cells were transfected with plasmid encoding NLRC5 or siRNA for NLRC5 (Fig. 3a, d). After transfection, CCK-8 (Fig. 3b, c, e, f), migration, and invasion assays (Fig. 3g, h) were conducted to assess cell viability, migration, and invasion. As shown in Fig. 3b, e, g and h, the over-expression of NLRC5 increased the proliferation, migration, and invasion of CC cells (p < 0.01). On the contrary, the above phenotype was inhibited upon the knockdown of NLRC5 in CC cells with siRNA (Fig. 3c, f, g and h, p < 0.01).
NLRC5 overexpression led to CC cell proliferation, migration, and invasion. (a, d) WB detected the protein expression in CC cell with NLRC5 overexpression or knockdown. (b, c, e, f) CCK8 assay detected cell proliferation after overexpression or knockdown of NLRC5. (g, h) Transwell assay detected cell invasion after overexpression or knockdown of NLRC5. *p < 0.05, **p < 0.01, ***p < 0.001. The results are represented as the mean ± SEM from at least three independent experiments.
NLRC5 regulates LC3 expression to promote CC cell proliferation, migration and invasion by activating the PI3K/AKT Signaling pathway
To explore the underlying molecular mechanism associated with NLRC5 mediated tumorigenesis in CC, we explored its effects on the PI3K/AKT signaling pathway by Western blotting. Over-expression of NLRC5 promoted the activation of the PI3K/AKT signaling pathway in CC cells (Fig. 4a, b, p < 0.01). Knockdown of NLRC5 expression decreased the expression of p-PI3K and p-AKT in CC cells (Fig. 4c, d). Moreover, after treatment with LY294002, an inhibitor the PI3K/AKT signaling pathway, the NLRC5 mediated effects on the proliferation, migration and invasion of CC cells was suppressed (Fig. 4f, h, i, j).
NLRC5 potentially regulates proliferation, migration, and invasion of CC cells by modulating LC3 expression and activating the PI3K/AKT signaling pathway. (a–d) WB showing the expression of PI3K/AKT pathway related proteins in CC cells with NLRC5 overexpression or knockdown. (e, g) WB showing the expression of PI3K/AKT pathway related proteins in CC cells with NLRC5 overexpression and LY294002 treatment. (f, h) CCK8 assay showing the viability of NLRC5 over-expressed CC cells after treated with LY294002. (i, j) Transwell assay showing the invasion potential of NLRC5 over-expressed CC cells after treated with LY294002. *p < 0.05, **p < 0.01, ***p < 0.001. The results are represented as the mean ± SEM from at least three independent experiments.
In consideration of there was a positive correlation between NLRC5 and LC3 expression. We further investigate the effects of NLRC5 on LC3 expression. We found over-expression of NLRC5 inhibited the expression of LC3 in CC cells (Fig. 4a, c). Knockdown of NLRC5 expression promoted the expression of LC3 in CC cells (Fig. 4b, d). Additionally, the negative effect of NLRC5 on LC3 expression were rescued after treatment with LY294002 (Fig. 4e, g).
Discussion
The innate immune system is involved in the recognition and removal of invading pathogens by using germline encoded pattern-recognition receptors (PRRs), which can recognize intracellular pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs)17. NLRs are a family of evolutionarily conserved cytoplasmic PRRs that mainly play key roles in immunity and host defense18. NLRs have a typical tripartite structure, including a centrally located nucleotide binding oligomerization domain (NBD) that is involved in oligomerization upon ligand recognition, the C-terminal leucine-rich repeats (LRRs) that can recognize the activators, and a variable N-terminal effector domain that mediates downstream signaling of activated NLRs19. NLR proteins are mainly localized in the cytoplasm, and have been shown to recognize pathogens and induce the production of multiple cytokines and chemokines20. Consequently, the conserved members of NLR family play major roles in the detection of invading pathogens and triggering immune responses toward bacterial and viral challenge. They do so by activating the NF-κB and MAPK signaling pathways, type I interferon signaling, and inflammasome signaling to activate procaspase-1 or secrete IL-1β21,22.
NLRC5 possesses the longest leucine-rich repeat domain protein among all human NLRs and regulates inflammation and immune response21,22. It has three structural domains, including the N-terminal atypical caspase activation and recruitment domain, which makes it completely different from the other NLRs, the central NACHT domain containing the nucleotide-binding domain (NBD) and the C-terminal containing Leucine repeats. Recently, accumulating evidence has shown that NLRC5 is intimately involved in the development of tumors. For example, NLRC5 is up-regulated in hepatocellular carcinoma and renal carcinoma and contributes to their tumorigenesis6,8. Interestingly, NLRC5 is down-regulated in endometrial cancer, however, the over-expression of NLRC5 promotes the migration and invasion of endometrial cancer cells9. Therefore, the level of NLRC5 expression in cancer is tissue-dependent. In the current study, the level of NLRC5 in human CC tissues was significantly lower than that in the normal cervical tissues. However, we found up-regulation of NLRC5 induced proliferation, migration and invasion of CC cells. Accordingly, the knockdown of NLRC5 expression suppressed the above phenotype. In humans, epigenetic changes like aberrant methylation at the CpG islands within the promoter regions of tumor associated genes represents one of the vital mechanisms to control tumor growth and immune escape23. Yoshihama et al. found that aberrant methylation in tumor cells resulted in the suppression of the mRNA levels of NLRC524, which might explain the difference in the expression levels of NLRC5 in normal cells and CC tissues. In addition, the expression of NLRC5 is also influenced by the tumor immune microenvironment24.
Tumorigenesis is closely associated with the activation of pro-tumorigenic signaling pathways. Activation of the PI3K/AKT signaling pathway is known to be a classical event in the development of cancer, including CC25,26. Notably, the PI3K/AKT signaling pathway was reported to be involved in NLRC5 regulation, but the results were controversial. NLRC5 was reported to positively regulate the PI3K/AKT signaling pathway in endometrial cancer and hepatocellular carcinoma7,9, but it was the opposite case in acute kidney injury10. Until now, it is unclear how NLRC5 modulates the PI3K/AKT signaling pathway. Our study in the CC cells indicated that NLRC5 might activate the PI3K/AKT signaling pathway. Furthermore, NLRC5 accelerated CC cell proliferation, migration, and invasion potentially by activating the PI3K/AKT signaling pathway, while treatment with the PI3K/PI3K inhibitor LY294002, attenuated the NLRC5 induced effects of the proliferation, migration, and invasion of CC cells.
Autophagy is a highly regulated and major catabolic process in the cell where cytoplasmic substances are degraded within the lysosome by hydrolases and the resulting degraded products are recycled back to the cytoplasm to maintain cellular homeostasis27. LC3 and Beclin1 are essential autophagy-related gene (ATG) proteins, involved in different stages of autophagy28, and have been found to be directly associated with cancer. LC3 is the marker of autophagosome and consists of two forms, namely, LC3-I and LC3-II. LC3-II exists on the outer and inner membranes of autophagosomes and is positively associated with the level of autophagy in cells. Beclin1 contributes to the formation of the phosphatidylinositol-3-OH kinase-Beclin1-VPS34 complex during the initiation of autophagy29. The relation between autophagy and immunity is complex30,31. Autophagy has been reported to promote the internalization and degradation of MHC class I in dendritic cells, while autophagy-deficient dendritic cells enhance the presentation of different viral antigens to CD8+ T cells32. Meanwhile, immune signals regulate autophagy pathways in the tumor microenvironment, and these signals are perceived by recognition receptors inside or outside the cell membrane, including TOLL-like receptors, which are thought to be the inducers of autophagy and are involved in the activation of NF-KB dependent transcription to activate autophagy33.
Autophagy plays a unique role in cancer, which has been described as a “double-edged sword”34,35. Jan Karlseder and co-workers reported that autophagy suppressed tumor progression by restricting chromosomal instability36. However, Dou et al. suggested that autophagy might have an opposite effect because defects in autophagy could interfere with cellular senescence and limit the proliferation of damaged cells, leading to abnormal proliferation of cancer cells and ultimately promoting tumor progression37. The tumor promoting or inhibiting effects of autophagy depends on the tumor microenvironment38, which may explain the negative correlation between NLRC5 and LC3 expression levels in CC cells. Of note, NLRC5 expression has been closely associated with autophagy. For example, it was reported that there was a negative correlation between NLRC5 expression level and autophagy in endometriosis, suggesting the prognostic potential of the combination of NLRC5 and autophagy in patients with endometriosis29. A recent study by Zhan and co-workers illustrated that LC3 interacted with NLRC5 and further inhibited NLRC5-mediated MHC class I antigen presentation pathway in endometrial cancer39.In the current study, we found that the expression of Beclin1 and LC3 were down-regulated in CC tissue. Importantly, Pearson analysis showed that NLRC5 was positively correlated with LC3 expression level, which indicated that NLRC5 may be involved in CC by regulating LC3. Furthermore, we found NLRC5 inhibited the expression of LC3 through activating PI3K/AKT signaling pathway, indicating that NLRC5 and LC3 combined as possible predictors in patients with CC.
Collectively, we found that NLRC5 would participate in cervical tumorigenesis and progression by activating PI3K/AKT signaling pathway, so that NLRC5 can be a promising therapeutic target for the treatment of CC in the clinic. Furthermore, NLRC5 was positively correlated with LC3 expression level in CC, NLRC5 could inhibit the expression of LC3 through activating PI3K/AKT signaling pathway, indicating that NLRC5 and LC3 combined as possible predictors in patients with CC. However, the expression of NLCR5 is low in CC tissues, contradictory to that the overexpression of NLRC5 promotes cell proliferation and migration, which may be related to the fact that the components of CC tissues are very complex, and the immune cell components of CC tissues may affect the expression of NLRC5. Therefore, we speculate whether the contradictory results of this study are caused by the different microenvironments of tumor cells. Some studies have shown that certain specific mechanisms of NLRC5 depend on its functional regions, but whether the effects of these functional regions are related to the tumor microenvironment is also unclear. In order to solve this problem, we need to explore the role of NLRC5 in the immune microenvironment of CC, and to observe whether the NLRC5 functional region is selectively involved in different regulatory mechanisms. Besides, the number of samples we assessed was not enough large to permit analysis of other confounding variables. Lastly, the autophagy activity in our present study is unclear because of only LC3 expression was detected, investigation of autophagy flux could help clarify the role of autophagy in CC. Both of these points need to be further demonstrated in our subsequent experiments.
Data availability
The data that support the findings of this study are available on request from the corresponding author Xianfeng Ma upon reasonable request.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68(6), 394–424 (2018).
Libby, P. et al. Atherosclerosis. Nat. Rev. Dis. Primers 5(1), 56 (2019).
Meissner, T. B. et al. NLR family member NLRC5 is a transcriptional regulator of MHC class I genes. Proc. Natl. Acad. Sci. U. S. A. 107(31), 13794–13799 (2010).
Benko, S. et al. NLRC5 functions beyond MHC I regulation-what do we know so far?. Front. Immunol. 8, 150 (2017).
Wu, Y., Shi, T. & Li, J. NLRC5: A paradigm for NLRs in immunological and inflammatory reaction. Cancer Lett. 451, 92–99 (2019).
Peng, Y. Y. et al. NLRC5 regulates cell proliferation, migration and invasion in hepatocellular carcinoma by targeting the Wnt/beta-catenin signaling pathway. Cancer Lett. 376(1), 10–21 (2016).
He, Y. H. et al. NLRC5 promotes cell proliferation via regulating the AKT/VEGF-A signaling pathway in hepatocellular carcinoma. Toxicology 359–360, 47–57 (2016).
Wang, Q. et al. NLRC5 mediates cell proliferation, migration, and invasion by regulating the Wnt/beta-catenin signalling pathway in clear cell renal cell carcinoma. Cancer Lett. 444, 9–19 (2019).
Fan, Y. et al. NLRC5 promotes cell migration and invasion by activating the PI3K/AKT signaling pathway in endometrial cancer. J. Int. Med. Res. 48(5), 300060520925352 (2020).
Han, F. et al. Knockdown of NLRC5 attenuates renal I/R injury in vitro through the activation of PI3K/Akt signaling pathway. Biomed. Pharmacother. 103, 222–227 (2018).
Boga, J. A. et al. Therapeutic potential of melatonin related to its role as an autophagy regulator: A review. J. Pineal Res. 66(1), e12534 (2019).
Clarke, A. J. & Simon, A. K. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat. Rev. Immunol. 19(3), 170–183 (2019).
Santana-Codina, N., Mancias, J. D. & Kimmelman, A. C. The role of autophagy in cancer. Annu. Rev. Cancer Biol. 1, 19–39 (2017).
Guo, J. Y. & White, E. Autophagy, metabolism, and cancer. Cold Spring Harb. Symp. Quant. Biol. 81, 73–78 (2016).
Peng, Y. Y. et al. NLRC5 regulates cell proliferation, migration and invasion in hepatocellular carcinoma by targeting the Wnt/β-catenin signaling pathway. Cancer Lett. 376(1), 10–21 (2016).
Cao, Z. et al. MAGI-2 downregulation: A potential predictor of tumor progression and early recurrence in Han Chinese patients with prostate cancer. Asian J. Androl. 22(6), 616–622 (2020).
Lerias, J. R. et al. Trained immunity for personalized cancer immunotherapy: Current knowledge and future opportunities. Front. Microbiol. 10, 2924 (2019).
Velloso, F. J. et al. NOD-like receptors: Major players (and targets) in the interface between innate immunity and cancer. Biosci. Rep. 39, 4 (2019).
Wang, J. & Chai, J. Molecular actions of NLR immune receptors in plants and animals. Sci. China Life Sci. 63(9), 1303–1316 (2020).
Saxena, M. & Yeretssian, G. NOD-like receptors: Master regulators of inflammation and cancer. Front. Immunol. 5, 327 (2014).
Thaiss, C. A. & Elinav, E. NF-kappaB regulation by NLRs: T cells join the club. Immunity 42(4), 595–597 (2015).
Liu, P. et al. NOD-like receptor signaling in inflammation-associated cancers: From functions to targeted therapies. Phytomedicine 64, 152925 (2019).
Jones, P. A. & Baylin, S. B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 3(6), 415–428 (2002).
Yoshihama, S. et al. NLRC5/MHC class I transactivator is a target for immune evasion in cancer. Proc. Natl. Acad. Sci. U. S. A. 113(21), 5999–6004 (2016).
Pathak, G. P. et al. RTN4 knockdown dysregulates the AKT pathway, destabilizes the cytoskeleton, and enhances paclitaxel-induced cytotoxicity in cancers. Mol. Ther. 26(8), 2019–2033 (2018).
Che, Y. et al. TRIP4 promotes tumor growth and metastasis and regulates radiosensitivity of cervical cancer by activating MAPK, PI3K/AKT, and hTERT signaling. Cancer Lett. 452, 1–13 (2019).
Feng, Y. et al. The machinery of macroautophagy. Cell Res. 24(1), 24–41 (2014).
Dikic, I. & Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 19(6), 349–364 (2018).
Zhan, L. et al. NLRC5 and autophagy combined as possible predictors in patients with endometriosis. Fertil. Steril. 110(5), 949–956 (2018).
Deretic, V. & Levine, B. Autophagy balances inflammation in innate immunity. Autophagy 14(2), 243–251 (2018).
Pan, H. et al. Autophagy-associated immune responses and cancer immunotherapy. Oncotarget 7(16), 21235–21246 (2016).
Parekh, V. V. et al. Autophagy-related protein Vps34 controls the homeostasis and function of antigen cross-presenting CD8alpha(+) dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 114(31), E6371–E6380 (2017).
Xia, H., Green, D. R. & Zou, W. Autophagy in tumour immunity and therapy. Nat. Rev. Cancer 21(5), 281–297 (2021).
Georgii, A. Pathology of the hematopoietic stem cell. On the 74th Congress of the German Society of Pathology in Aachen 3–6 June 1990. Pathologe 11(1), 1–3 (1990).
Mulcahy-Levy, J. M. & Thorburn, A. Autophagy in cancer: Moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ. 27(3), 843–857 (2020).
Nassour, J. et al. Autophagic cell death restricts chromosomal instability during replicative crisis. Nature 565(7741), 659–663 (2019).
Dou, Z. et al. Autophagy mediates degradation of nuclear lamina. Nature 527(7576), 105–109 (2015).
White, E. The role for autophagy in cancer. J. Clin. Invest. 125(1), 42–46 (2015).
Zhan, L. et al. LC3 and NLRC5 interaction inhibits NLRC5-mediated MHC class I antigen presentation pathway in endometrial cancer. Cancer Lett. 529, 37–52 (2022).
Acknowledgements
We thank Dr Zhihua Zhang (Anhui Medicine University) for providing suggestions during the data analysis. We thank BMCSCI (www. bmcsc ience. com) for providing English editing services during the preparation of this manuscript. This work was supported by the Science Foundation of Anhui medical University (grant numbers 2019xkj03, 2020xkj027).
Funding
The Science Foundation of Anhui medical University [grant number 2019xkj03]. The Science Foundation of Anhui medical University [grant number 2020xkj027].
Author information
Authors and Affiliations
Contributions
All authors contributed to teh study conception and design. Yunxia Cao and Bing Wei designed teh research. Lin Ling and Jiahua Chen performed teh experiments. Xiaofeng Ma wrote teh manuscript. Lei Zhan, Runhua He and Juanjuan Fu collected and analyzed teh data. All authors TEMPhas read and agreed to teh published version of teh manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ling, L., Chen, J., Zhan, L. et al. NLRC5 promotes tumorigenesis by regulating the PI3K/AKT signaling pathway in cervical cancer. Sci Rep 14, 15353 (2024). https://doi.org/10.1038/s41598-024-66153-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66153-3
- Springer Nature Limited