Abstract
Invasive fungal infections (IFI) pose a significant health burden, leading to high morbidity, mortality, and treatment costs. This study aims to develop and characterize nanomicelles for the codelivery of posaconazole and hemp seed oil for IFI via the oral route. The nanomicelles were prepared using a nanoprecipitation method and optimized through the Box Behnken design. The optimized nanomicelles resulted in satisfactory results for zeta potential, size, PDI, entrapment efficiency, TEM, and stability studies. FTIR and DSC results confirm the compatibility and amorphous state of the prepared nanomicelles. Confocal laser scanning microscopy showed that the optimized nanomicelles penetrated the tissue more deeply (44.9µm) than the suspension (25µm). The drug-loaded nanomicelles exhibited sustained cumulative drug release of 95.48 ± 3.27% for 24 h. The nanomicelles showed significant inhibition against Aspergillus niger and Candida albicans (22.4 ± 0.21 and 32.2 ± 0.46 mm, respectively). The pharmacokinetic study on Wistar rats exhibited a 1.8-fold increase in relative bioavailability for the nanomicelles compared to the suspension. These results confirm their therapeutic efficacy and lay the groundwork for future research and clinical applications, providing a promising synergistic antifungal nanomicelles approach for treating IFIs.
Similar content being viewed by others
Introduction
Fungal diseases have expanded dramatically in several environments since the beginning of the twenty first century. Invasive fungal infections (IFI) relate to significant health risks of mortality and morbidity. Approximately 1.9 million individuals experience acute invasive fungal infections annually, with an additional estimated 3 million people worldwide enduring chronic and severe fungal infections1. Throughout the last two decades, the treatment difficulties caused by IFI have progressed2. The overall period of therapy is determined by the patient's actual medical progress as well as the kind and degree of underlying immunosuppression3. It has been observed that the number of immunocompromised patients at risk of fungal diseases has been steadily growing since the rise of HIV/AIDS, cancer, transplant operations, and other contributing factors4. The significant prevalence of fungal infections, along with the rising costs, has emphasized the requirement for more effective and low-level toxic medications for treatment5.
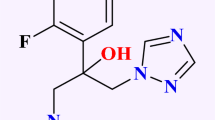
The USFDA [United States Food and Drug Administration] has approved an anti-fungal drug of the triazole category known as Posaconazole6. This drug is widely used to treat oropharyngeal candidiasis and immunocompromised patients who undergo hematopoietic stem cell transplant [HSCT] or cancer chemotherapy as they are in danger of contracting IFIs. It is also given as prophylactic and prospective medications for infections caused by Candida, Aspergillus, or fungus filaments. Posaconazole belongs to the class II category under the biopharmaceutical classification system having high permeability and low solubility. The antifungal action is quite comparable to other azole drugs of the same category as they predominantly inhibit CYP-dependent 14-α demethylases within the biosynthetic pathway of ergosterol, which is a vital part of the cell membrane of the fungus. A buildup of hazardous ergosterol and 14-α methyl sterols reduction happens due to the consequence of enzyme hindrance, leading to impairment of cell membrane function of fungus and prevention of division and growth of cells. It has a half-life (T1/2) of twenty to sixty six hours with a mean T1/2 of thirty five hours and complete clearance in thirty two hours from the body. The primary excretion mechanism is feces (mostly parental medication), with renal clearance accounting for only around 13% of the total conjugate oral dosage7,8.
Cannabis sativa L. (Hemp seed oil) has the composition of fatty acids present in the plant, which is commonly mentioned, along with the inclusion of linoleic acid, which elevates its nutritional value over that of similar seed oils. The predominant omega-3 and omega-6 polyunsaturated fats contained in hemp seed oil are linoleic acid and α-linoleic acid which have strong antioxidant activity and act as the immunity booster. Given the sheer amounts in which they exist, such compositions are the most desirable components of the oil. The ratio of linoleic acid to α-linoleic acid of 3:1 is regarded as optimal for application9,10. It produces anti-thrombotic, anti-cancer, and anti-inflammatory qualities that are among the several advantages attributed to omega-3 fatty acids. Furthermore, omega-3 dietary acids increase overall rates of metabolism and promote fat loss10,11.
Although several studies focus largely on the important nutrients of hemp seed oil's fatty acid composition, hemp oil contains additional elements that have therapeutic effects too. Compounds such as methyl salicylate and sitosterol improve the nutritional value of hemp seed oil while also increasing its usefulness as a food additive. Even though the present information concerning hemp seed oil only supports its nutritional profile, these additional components have resulted in a considerable improvement and should be further researched for other favorable effects12.
The growing resistance of fungi to conventional antifungal medications has sparked interest in natural alternatives, such as hemp seed oil. Various researchers have reported the antifungal properties of essential oils of hemp seed oil against different yeast like fungi strains, indicating their potential in combating fungal infections13,14.
Nano micelles have attracted significant interest in the diagnosis and therapy of numerous illnesses in the recent decade, concluding with FDA authorization15. They are usually created using amphiphilic polymers assembled within the water with a hydrophilic shell and a hydrophobic core structure of nano molecules. The existence of a lipophilic center boosts water solubility and allows for regulated medication release16. The hydrophilic shell protects the drug from the surrounding medium and keeps it from encountering plasma components. This lets the drug stay in circulation for a long time in the body. Also, the small size of the particles makes them stay in the blood longer17. This is true for liver bypass, filtration through the spleen, glomerular clearance, better cellular absorption, and getting pass barriers in the epithelial membrane. These factors contribute to enhanced drug bioavailability17,18.
The primary objective of this research was to innovate a novel therapeutic approach for combating IFI by developing and characterizing nano micelles loaded with posaconazole and hemp seed oil for oral administration. Specifically, a nano-precipitation method was used to fabricate the micelles, followed by optimization using the Box Behnken design to achieve well-defined physicochemical properties. Characterization using techniques like transmission electron microscopy and confocal laser scanning microscopy (CLSM). The investigation also observed different in vitro drug release kinetics and antifungal activity of the nano micelles compared to conventional suspension formulations. Furthermore, the study aimed to evaluate the pharmacokinetic profile of the developed nanomicelles, particularly focusing on their relative bioavailability and half-life compared to the control suspension.
Materials and methods
Materials
The extracted hemp seed oil was procured from Kumaokhand AIH Pvt., Himachal Pradesh, India, and Posaconazole (Form-I) was purchased from MSN Labratories Private Limited, Telangana, India. Transcutol HP and Precirol ATO 5 were procured from Gattefosse. Tween 80, distilled water, and poloxamer 188 were obtained from CDH Fine Chemicals, New Delhi, India.
Methods
Preparation of posaconazole and hemp seed oil nano micelles
The preparation of nano micelles was achieved through a nanoprecipitation technique. For the initial preparation, a 1% w/v solution of PLGA (50:50) was used. To identify the ideal concentration for the nonionic surfactant, poloxamer 188, a series of tests were conducted. Specifically, 5 mL of the 1% PLGA solution was incrementally introduced into four different water-based solutions, each containing varying concentrations of poloxamer 188 i.e., 2.5, 3, 4, and 10 mg/mL. The mixtures were stirred to facilitate the process. Once the most effective concentration of the surfactant was established, the next step involved adjusting the ratios of PLGA to Posaconazole and hemp seed oil. Three separate 5 mL solutions containing 1% w/v PLGA in acetone were made with Posaconazole and hemp seed oil concentrations adjusted to achieve weight-to-weight (w/w) ratios of PLGA: Posaconazole and hemp seed oil at 5:1, 10:1, and 15:1. These organic solutions were then methodically added, at a stable flow rate of 0.3 mL/min, to an aqueous medium saturated with the optimal concentration of poloxamer 188. Almost immediately, the blend turned turbid, signifying the spontaneous formation of nanoparticulate systems through solvent displacement and polymer deposition processes.
Furthermore, acetone was removed from the system through a Rota evaporator by the method of vacuum evaporation at 60 °C for 6 h. Approximately 30 mL of the formulation was generated after redispersing the layer formed around the RBF as a result. The nanomicelles were filtered thrice through a 0.22 µm nylon filter followed by a 10,000 rpm for 10 min at 4 °C using a cooling centrifuge (Remi, India) to separate free drug from drug-loaded nanomicelles19,20.
Optimization of polymers concentration by determination of critical micelle concentration (CMC)
The CMC of the nano micelles was determined using iodine as a hydrophobic probe. In this experiment, a standard iodine/potassium iodide (I2/KI) solution was prepared by mixing iodine and potassium iodide at a 1:2 ratio in 50 mL of distilled deionized water. Different concentrations of the PLGA: poloxamer 188 (4:1) solution ranging from 1 to 40 µg/mL were prepared in distilled deionized water. To these various dilutions of polymer solutions, 25 µl of the I2/KI solution was added, and the mixture was incubated in the dark at room temperature for 24 h. During the incubation period, the hydrophobic iodine separated from the KI3 solution and migrated into the hydrophobic core of the nano micelles. This caused a shift in the absorbance from 460 to 366 nm. Therefore, after the incubation period, the UV absorbance of the different diluted concentrations was measured and recorded at 366 nm21,22.
Optimization of nanomicelles
Box Behnken design was utilized through Design Expert version 11.0 to investigate the effect of three independent variables, namely: PLGA, surfactant, and stirring time on particle size, PDI, and entrapment efficiency % as shown in Table 1. The other factors such as stirring speed and temperature were kept constant. The impact of these factors was explored through formulation runs, involving different types of combinations. The software generated polynomial equations to model these relationships, along with response graphs displaying surfaces that helped analyze the influence of different factors on the responses, considering both linear and quadratic effects. The quadratic models emphasised the most significant combined effects and individual effects of these factors. The analysis was rooted in experimental runs where varied levels of these factors were observed within a specified range. To validate the findings, additional experiments were conducted using the predicted optimal factor levels. By comparing the results of these tests with the expected values, we ascertained if the experimental outcomes aligned closely with the predictions. This validation process allowed the confirmation of the optimized composition's reliability and gained confidence in its effectiveness, as it demonstrated a close alignment between observed response values and the anticipated outcomes23,24.
Molecular docking
Docking studies were conducted using Schrodinger Inc. LLC's Maestro 10.5 software. The protein preparation process involved three phases: preprocessing, reviewing, modifying, and refining the protein. These phases involved introducing hydrogen atoms and removing water molecules. To lower the structural energy, the OPLS 2005 force field was employed. Subsequently, ligands were prepared using the 2005 force field with the help of the LigPrep wizard. The receptor grid was created by selecting cocrystal ligands, which were then used to create a primary bounding box. The ligands were precisely docked onto the protein receptor grid, and the results were analyzed using glide score and docking pose25,26.
Characterization of nano micelles formulation
Particle size, zeta potential, and polydispersity index (PDI)
The sample of nano micelles compositions was diluted 100-fold with water that had been double distilled. The average size, PDI, and zeta potential were tested three times. The experiment was conducted using the Malvern Instruments Zetasizer photon correlation spectrometer Worcestershire, UK. The measurements were conducted using light scattering at an angle of 90 °C and a temperature of 25 °C27.
Differential scanning calorimetry
The PerkinElmer Pyris 6 Differential Scanning Calorimetry (DSC) equipment from Massachusetts, USA, was used to conduct thermal analyses of a variety of compounds, including Pure Posaconazole, a combination of Posaconazole and hemp seed oil, PLGA, Poloxamer, and a Formulation. To prevent damage from freezing or ice formation, the nano micelles are lyophilized with 5% mannitol. Approximately 5 mg of each sample was added to an aluminum crimped pan, covered with a lid that had a pinhole, and then put into the DSC apparatus for analysis. The following conditions were used for the DSC experiments: a heating rate of 10 °C, a nitrogen gas flow of 15–20 mL/min, and a heating range of 20–300 °C. To obtain precise measurements, a similar empty pan was utilized as a reference (blank)28.
Fourier transform infrared spectroscopy (FTIR)
The structural assessment of Posaconazole, lyophilized nano micelles (with 5% mannitol as a cryoprotectant), Hemp seed oil, poloxamer, and PLGA was conducted using an Alpha FTIR spectrometer from Bruker Optik GmbH, Ettlingen, Germany. The spectrometer was equipped with a deuterated triglycine sulfate (DTGS) detector and operated across a spectral range spanning from 4000 to 500 cm−1. Sample preparation involved grinding both Posaconazole and the lyophilized formulation, along with anhydrous KBr. Subsequently, the mixture was manually compressed into pellets using a tablet presser. These pellets were then positioned on the analysis disc and subjected to FTIR spectroscopy at a scanning rate of 4 mm/s and a resolution of 2 cm−129.
X-ray diffraction (XRD)
XRD plots were acquired using an X-ray diffractometer that was equipped with the necessary instrumentation. The present study employs a highly adaptable methodology to examine the characteristics of Posaconazole and lyophilized nano micelles, with the inclusion of 5% mannitol as a cryoprotectant. The experimental setup utilized a copper (Cu) anode, specifically the Bruker D8 Advance model. The specimen was positioned within the designated area. The experiment utilized a distinct sample holder and was subjected to scanning at a rate of 1 degree per minute of XRD investigation. A study was conducted to investigate the crystalline nature of the drug and nano micelles30.
Transmission electron microscopy (TEM)
TEM instrument (Jeol 1010, Japan) was used to examine the morphological structure of Posaconazole and hemp seed oil nano micelles. The formulations were put within a 200-mesh grid of copper covered with carbon before being stained negatively through a one percent solution of Phosphotungstic acid31.
Encapsulation efficiency (EE)
The EE of the nanomicelles formulation was evaluated through a centrifugation method that involved separating the micelles containing the encapsulated drug from the drug that remained free. The nano micelles were subjected to centrifugation at a speed of 15,000 rpm using a Remi C-24 plus centrifuge from Remi El-ektrotechnik Ltd., India. Following centrifugation, the clear liquid supernatant, which is the liquid portion above the pellet, was carefully separated. This supernatant was then mixed with methanol to create a diluted solution, and its content was analyzed using a UV spectrophotometer (Shimadzu UV 1601) at the specific wavelength (λmax) of Posaconazole (262 nm) and hemp seed oil (230 nm). This wavelength is known to correspond to the maximum absorption of the drug27. The calculation of the EE was based on the following formula given below (Eq. 1):
where C2 = Total drug added-free drug in the supernatant and C1 = Total amount of drug added in the formulation.
Confocal laser scanning microscopy (CLSM)
In order to evaluate intestinal uptake, rhodamine B was added to the formulation process of posaconazole nano micelles and posaconazole suspension at a concentration of 0.5 mg/mL. A 2.40-inch-long section of the jejunum was removed from a male Wistar rat as the jejunum has an extensive absorptive surface area, favorable pH environment and significant role in drug absorption, which can influence drug uptake and transport along with an extended residence time in the jejunum. The section was then cleansed with PBS to eliminate any fecal debris. The samples were placed in an individual sac, sealed at both ends, and then immersed in 100 mL of pH 6.8 PBS for three hours (at 37 ± 1 °C, 40 rpm). After rinsing with PBS to eliminate any remaining free rhodamine B, the sacs were sliced lengthwise into small species and placed onto a glass slide and covered with a coverslip for imaging. A z-axis CLSM analysis ((TCS SP5II, Leica Microsystem Ltd., Germany) was performed to determine the extent to which the rhodamine B-labeled medication, both in its formulation and in its suspension, penetrated the intestinal wall32.
In vitro drug release
The in vitro drug release behavior of Posaconazole from both the optimized nano micelles and the suspension (marketed) was investigated using the dialysis membrane diffusion technique. To perform this, both formulations with concentrations of 0.1mg/mL were introduced into preactivated dialysis bags with a molecular weight cutoff of approximately 12000–14000 Da (manufactured by Hi-Media, Mumbai). These bags were then immersed separately in 100 mL of two mediums; 0.1N HCl (pH 1.2) and PBS (pH 6.8). These solutions served as the release medium and were kept at a constant temperature of 37 ± 2 °C, with continuous stirring at a speed of 40 rpm33. At predefined intervals, samples were drawn from the system and replaced with fresh release medium to maintain a constant sink condition. The collected samples were passed through a 0.22 μm syringe filter and subsequently analyzed using the HPLC technique which is described in our previous paper6. The data acquired through these experiments was processed to construct a graph depicting the relationship between the percentage of cumulative drug release and time in hours. Furthermore, several release kinetic models including zero order, first order, Korsmeyer-Peppas, and Higuchi's matrix, were employed to determine the underlying release kinetics of the formulations.
Antifungal activity of nano micelles
The antifungal activity of nanomicelles was examined against two different fungal strains: Aspergillus niger (ATCC-16404) and Candida albicans (MTCC-227). These strains were procured from the American Type Culture Collection (ATCC), respectively, to conduct this investigation34. The susceptibility of Candida and Aspergillus strains to various antifungal agents was assessed using Etest® strips, which were sourced from AB Biodisk in Stockholm, Sweden. These strips contained a continuous gradient of antifungal concentrations for Posaconazole loaded nano micelles. Before their use, the strips were stored at a temperature of − 20 °C and brought to room temperature for 20 min before testing. For Candida species, the testing medium used was RPMI-1640, enriched with 2% glucose. Each Candida isolate was prepared as a yeast suspension in a 0.85% NaCl solution, with turbidity levels adjusted to match a 0.5 McFarland standard. This formulation was then applied to the medium using an automated Rota-plater inoculator. After allowing the plates to dry at room temperature for about 10–15 min, Etest® strips were gently placed on the agar surface with a specialized vacuum pen. The plates were then incubated at a temperature of 35 °C for a period ranging from 24 to 48 h. The protocol for Aspergillus species was somewhat similar but started with 7 day old cultures grown on either potato dextrose agar or Sabouraud agar. Spore and hyphal suspensions were prepared using 1 mL of 0.85% NaCl and transferred to sterile tubes. Furthermore, the medium used for testing Candida was RPMI-1640 enriched with 2% glucose35. After inoculating the medium with the spore suspension, Etest® strips were applied, and plates were incubated at 35 °C. The incubation could extend up to 72 h if needed, and the zone of inhibition of nano micelles was determined. For Candida, these values were read after either 24 or 48 h of incubation, based on the growth rate. For Aspergillus, initial readings were taken between 20 and 24 h, with a second reading at 48 h if required. Each set of tests included appropriate quality control and reference strains to ensure accuracy. Finally, the collected zone of inhibition data were analyzed using SAS software version 8.2, and values were recorded36,37.
In vivo pharmacokinetic study
The study confirmed the guidelines of the Institutional Animal Ethics Committee (IEAC) at Jamia Hamdard, New Delhi, with ethical approval IAEC no. 2046, 2023. Female Sprague Dawley rats, weighing around 230 ± 10g, were used as the subjects. They were administered 40 mg/mL concentrations of Posaconazole in both nano micellar formulations and suspension. The dosage was standardized to 8.75 mg/kg for each rat. These treatments were orally delivered once a day. Before and following the treatment, the rats underwent a fasting period of at least four hours. Blood samples were obtained from the tail vein at multiple time intervals (1, 4, 8, 12, 24, and 48 h) post-administration and were stored in tubes with K2EDTA. These samples underwent HPLC to gauge the plasma levels of Posaconazole38. For the pharmacokinetic (PK) analysis, Python coding was employed to calculate standard noncompartmental parameters such as the maximum plasma concentration (Cmax), the time taken to reach this peak level (Tmax), and the area under the plasma concentration–time curve for the initial 48 h (AUC 0−48). The AUC was determined using the linear trapezoidal technique. Statistical relevance was evaluated through the student’s t-test, setting the significance level at p < 0.05. Bioavailability was estimated by comparing the dose-normalized AUC from each formulation with previously published intravenous data on the same type of rats39. The average results were presented based on a sample size of four rats, accounting for a single standard deviation40.
Stability study
Stability testing lasting six months was conducted on the optimized posaconazole nanomicelles (Lyophilized form) to evaluate the effects of temperature and humidity. The samples were stored in amber glass bottles, securely sealed, at two conditions, room temperature and accelerated conditions (maintained at 40 °C ± 2 °C and 75% ± 5% relative humidity). Parameters such as zeta potential, particle size, and entrapment efficiency were examined to confirm the stability of the formulated product throughout the storage duration.
Ethics approval
All animal experiments comply with the ARRIVE guidelines and were carried out following the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). The animal study protocol was approved by the Institutional Animal Ethics Committee (IEAC) of Jamia Hamdard, New Delhi (ethical approval IAEC no. 2046, 2023).
Results
Optimization of polymer concentration by determination of critical micelle concentration (CMC)
The critical micelle concentration (CMC) of the PLGA and poloxamer 188 polymers was 5.99 µg/mL as shown in Fig. 1. The PLGA polymer's remarkably low CMC value has led to the creation of thermodynamically stable nano micelles. Furthermore, this low CMC value has played a crucial role in maintaining the stability of these nano micelles for 3 months21,41.
Optimization of nano micelles
The particle size diameter (Y1), PDI (Y2), and EE % (Y3) experimental ranges were 159.9–188.1 nm, 0.18–0.47, and 89.23–93.98%, respectively (Table 1). An optimization software produced a polynomial quadratic equation when fed the data for the dependant variables. An outstanding model fit (reduced quadratic model) was significant and valid across all three responses (p < 0.001), as indicated by the data, which demonstrated a substantially connected relationship between the model terms. The outcomes of the tests that checked for an appropriate p value, fit, and signal-to-noise ratio are in Table 2. Additionally, it shows if the adjusted R2 values were sufficiently close to the expected ones.
Impact of variables (surfactant, stirring time, and PLGA) on particle size
In Fig. 2A it is evident that as the surfactant concentration rises, there is no significant impact on particle size and changes in surfactant concentration do not play a substantial role in particle size determination. However, in contrast, when PLGA concentration is increased, there is a noticeable reduction in particle size, indicating that PLGA concentration does have a pronounced effect on size. In Fig. 2B, as stirring time is increased; it leads to a decrease in particle size. This observation suggests that prolonged stirring facilitates a more effective homogenization of the PLGA concentration, resulting in smaller particles. Notably, the effect of stirring time on particle size reduction appears to be more pronounced than that of surfactant concentration as shown in Eq. (2), emphasizing the importance of mechanical agitation in achieving smaller particle sizes. Figure 2C demonstrates that as the stirring time increases, there is a notable reduction in the particle size of the formulation, and concurrently, there is an increase in the amount of surfactant, indicating a decrease in particle size.
Effect of independent variables (Surfactant, stirring time, and PLGA) on PDI
Figure 2D indicates that an increase in surfactant concentration leads to an increase in the PDI. Conversely, when PLGA concentration increases, there is an initial reduction in PDI followed by an increase. At low concentrations of PLGA, the polymer chains are well dispersed and more uniformly distributed. This leads to a more homogeneous system where the molecular weight distribution is relatively narrow, resulting in a lower PDI. The PDI grows with increasing concentration due to a wider variety of molecular weights brought about by aggregation, viscosity effects, and chain entanglement32. This behaviour emphasises the significance of finding the optimal concentration of PLGA to achieve the required characteristics in drug delivery. On the other hand, PDI reduced when stirring time and PLGA concentration increase (Fig. 2E) by enhancing the homogenization and stabilization of particles, resulting in a more uniform particle size distribution42. Finally, in Fig. 2F, an increase in surfactant concentration leads to a reduction in PDI. By preventing aggregation or coalescence of particles, surfactants help maintain a uniform particle size distribution, leading to a lower PDI42, as shown by Eq. (3).
Influence of variables on encapsulation efficiency
In Fig. 2G, the relationship between surfactant concentration and EE% exhibits an interesting pattern. Initially, as surfactant concentration increases, there is a noticeable increase in EE%, indicating that higher surfactant levels contribute to better encapsulation of the formulation. However, this positive effect seems to plateau or slightly decrease as surfactant concentration continues to rise, suggesting that there might be an optimal range of surfactant concentration for maximizing EE%. Conversely, when PLGA concentration is increased, there is a straightforward increase in EE%, indicating that higher levels of PLGA result in improved encapsulation of the active substance. Figure 2H demonstrates that as both stirring time and PLGA concentration increase, there is a concurrent increase in EE%, suggesting that prolonged stirring and higher PLGA concentrations synergistically enhance encapsulation as shown by Eq. (4). Similarly, in Fig. 2I, the increase in both stirring time and surfactant concentration leads to an increase in EE%, underscoring their combined positive impact on the encapsulation efficiency.
Molecular docking analysis
Docking analysis was carried out to examine the interactions between various ligands and a crystal structure. Specifically, the ligand Posaconazole, when bound to the protein 14α-demethylase (7RTQ), exhibited a docking score of − 7.05 (Supplementary Table S2). Posaconazole exhibited two classic hydrogen bonds in its interactions. Specifically, the oxygen atom of tetrahydrofuran formed a hydrogen bond with the Lys313 residue, while the oxygen atom of 2,4-dihydro-3H-1,2,4-triazol-3-one engaged in a hydrogen bond with the Arg391 residue. This interaction displayed a good ligand efficiency of 0.14 µM and an inhibition coefficient of 6.82 µM, indicating significant antifungal activity (Supplementary Figure S1a). Similarly, when linoleic acid was employed as the ligand, it demonstrated a docking score of − 7.06. This interaction boasted a high ligand efficiency of 0.35 and an inhibition coefficient of 6.64 µM, highlighting substantial antifungal activity (Supplementary Figure S1b). In contrast, the linoleic acid present in hemp seed oil showcased antioxidant activity in the presence of various proteins. For instance, when linoleic acid interacted with the superoxide dismutase (1IDS) protein, it displayed an antioxidant activity with a docking score of − 5.16, a ligand efficiency of 0.26, and an inhibition coefficient of 164.19 µM (Supplementary Figure S1c). Furthermore, the protein glutathione peroxidase (2P5Q) exhibited antioxidant activity when paired with linoleic acid as the ligand, resulting in a docking score of − 3.37, a ligand efficiency of 0.17, and an inhibition coefficient of 3.37 µM (Supplementary Figure S1d). Additionally, the protein glutathione reductase (1XAN) showed antioxidant activity in conjunction with the linoleic acid ligand, featuring a docking score of -4.43 kcal/mol, a ligand efficiency of 0.22, and an inhibition coefficient of 569.06 µM (Supplementary Figure S1e). Likewise, the protein glutathione transferase (1PKW) displayed antioxidant activity when interacting with the linoleic acid ligand, resulting in a docking score of − 5.57, a ligand efficiency of 0.28, and an inhibition coefficient of 83.28 µM (Supplementary Figure S1f). Lastly, the protein catalase (1DGB) demonstrated antioxidant activity with linoleic acid as the ligand, yielding a docking score of − 3.74, a ligand efficiency of 0.19, and an inhibition coefficient of 1.81 µM (Supplementary Figure S1g). Linoleic acid demonstrated traditional hydrogen bonding interactions. More specifically, the oxygen atom within linoleic acid engaged in a hydrogen bond with the Lys123 residue, which is associated with Glutathione peroxidase. Additionally, it formed a hydrogen bond with the Asn365 residue, corresponding to Glutathione reductase. Furthermore, linoleic acid established three hydrogen bonds with the Thr68, Arg15, and Hed657 residues, linked to Glutathione transferase. Lastly, it exhibited a hydrogen bond with the Arg127 residue, a target within catalase. These findings shed light on the diverse interactions and potential biological activities of these ligands in various protein environments25.
Characterization of formulation
Zeta potential and particle size (nm)
The zeta potential of the formulation was observed to be − 25.73 mV which appears to have better physical stability and shows low affinity of aggregation during the storage. The conductivity evaluated was 0.01793 mS/cm with a percentage zeta peak area of 100. The average size of the nano micelles was 165.9 nm with 0.08 PDI which indicates a monodispersed system stating that the Posaconazole nano micelles nano formulation uniformly dispersed in the whole formulation21. Figure 3 depicts the zeta potential (A) and particle size (B).
Characterization: structure and morphology
The structure of the optimized nano micelles was investigated by TEM. As illustrated in Fig. 3C, the predictable nano micelles exhibited characteristics of non-aggregation, dispersion, and spherical morphology, showcasing a smooth surface. The photomicrograph also verified that the nano micelles fell within the nanometric size scale of 164 nm, aligning with the particle size range indicated by the zeta sizer41.
Differential scanning calorimetry
The DSC thermogram in Fig. 4 displays results from various samples. The two endothermic peaks for Posaconazole (Fig. 4A) occurred at 134.217 °C and 169.351 °C, two distinct endothermic events were detected at different temperatures. The first peak is associated with a phase transition resembling a nematic phase change on the other hand, the second corresponds to the melting point43. The thermogram of physical mixture of Hemp seed oil and posaconazole (Fig. 4B) showed two peaks of posaconazole at 129.9 and 161.1 °C were present. These minor shift in posaconazole peaks due to microstructural changes, such as dispersion of posaconazole particles within the oil and the solubility effect, which could influence the thermal behavior detected by DSC 41,44. Figure 4C showed a peak at 168.53 °C corresponding to the melting point of mannitol. On the other hand, when examining the lyophilized posaconazole loaded nano micelles (Fig. 4D), no distinctive peaks were observed for any of nano micelles components except for mannitol, which displayed a peak at 168.990 °C. This finding confirms that the peaks associated with Posaconazole and Hemp Seed Oil disappeared in the DSC thermogram of the nano micelles’ formulation. These observations strongly indicate that the incorporation of drugs and excipients into the nano micelles occurred without any disruptive interactions between them45,46.
Fourier transform infrared spectroscopy
The absorption peaks corresponding to different functional groups were identified: carbonyl group stretching at 1710.23 cm−1, furan ring stretching at 1412.61 cm−1, C–H bend in alkane at 1392.48 cm−1, CO–C stretch in asymmetrical aryl alkyl ether at 1243.57 cm−1, C-F aryl halide at 1106.97 cm−1, and C–H aromatic bending (out of plane) at 718.27 cm−1. These results suggest that there is no significant chemical interaction between the excipients or drug, and it's likely that the drug remains stable during the formulation process. Similar peaks were observed in both the formulation and pure drug spectra (Fig. 5). No functional groups were missing in the formulation spectra, indicating that there was no substantial interaction between the drug and the components of the nanomicelles formulation46.
X-ray diffraction
The X-ray diffraction spectra of Posaconazole and the optimized nano micelles formulation exhibited distinct peaks, suggesting a crystalline structure at scattered angles of 2θ, as illustrated in Fig. 647. In the X-ray diffraction patterns of the Posaconazole drug and its best version, there are clear peaks at 12, 14, 16, 18, 20, 21, 22, 23, 25, 29, 32, 34, 36, 40, 41, 45, and 46 degrees. These sharp, narrow, and intense peaks suggest the presence of the crystalline nature of Posaconazole in the formulation having high intensity when compared to the pure Posaconazole with low intensity44,48. The peak of the optimized Nano micelles was observed at the same location as that of the pure drug, as depicted in Fig. 6.
Encapsulation efficiency
EE measures the quantity of Posaconazole that is entrapped within the nanomicelles. Because our drug exhibits lipophilic properties, it is enclosed within the inner core of nano micelles, which also possess a lipophilic nature which increases the entrapment efficiency. It's a critical factor in optimizing the formulation of drug-loaded nano micelles. A high entrapment efficiency signifies improved bioavailability and a higher concentration of the drug, potentially allowing for lower doses and reducing dose-related systemic side effects. In the case of the optimized formulation, the entrapment efficiency was determined to be 93.2%. Raising the concentration of PLGA in the formulation of nanomicelles led to improved EE49.
Confocal laser scanning microscopy
The formulation containing Rhodamine exhibited a greater depth of intestine penetration compared to the control suspension (1.8-fold), as demonstrated in Fig. 7. The Rhodamine-loaded formulation (A) reached deeper layers of the intestine, with a penetration depth of approximately 44.9 μm. In contrast, the control suspension (B) remained confined to a shallow depth of only a few microns (up to 25 μm) in the intestine penetration experiment facilitated by CLSM. This phenomenon can be attributed to the optimized formulation's ability to effectively disrupt the intestinal epithelial barrier function. The CLSM imaging provides clear evidence that the characteristics of the nano micelles play a pivotal role in enabling their penetration into deeper layers50.
In vitro drug release
In 0.1N HCl, the drug suspension showed a release of 34.45% compared to 6.7% from drug loaded nano micelles over 2 h (Fig. 8A). After 24 h in PBS (pH 6.8), the Posaconazole-loaded nano micelles exhibited a cumulative drug release of 95.48% ± 3.227%, while the control suspension showed a release of 96.67% ± 1.687% in 6 h as depicted in Fig. 8B. A comprehensive investigation of various release kinetic models revealed that the Higuchi model presented the highest R-squared value of 0.9744 with release exponent (n) value of 0.56, indicating a non-Fickian diffusion mechanism. Consequently, it was determined that the release of the drug from the developed nano micelles adhered to the Higuchi diffusion mechanism (Supplementary Table S1). A comparison between the in vitro release profiles of Posaconazole from the nano micelles and the control suspension was carried out. The application of the surfactant in the formulation was intended to reduce the thickness of the nano micelles' membrane, facilitating the release of Posaconazole. The release pattern of Posaconazole from the formulation exhibited a sustained release49.
Antifungal activity
The Table 3 presents the results of the zone of inhibition (mm) observed for different samples against two strains of microorganisms, Aspergillus niger (ATCC-16404) and Candida albicans (MTCC-227). The zone of inhibition measures the antifungal activity of the tested samples, indicating the extent to which they inhibit the growth of the microorganisms. The zone of inhibition observed from the commercially available drug suspension against Aspergillus niger was observed to be 15.5 ± 0.230 mm, whereas, in the case of Candida albicans, the zone of inhibition comes out to be 25.4 ± 0.775 mm. On the other hand, the placebo nano micelles formulation without the inclusion of the active drug produced no zone of inhibition for either Aspergillus niger or Candida albicans, indicating that the placebo nano micelles did not possess any inherent antifungal activity. Pure drug nano micelles were the nano micelles formulation containing only the pure drug (Posaconazole). Against Aspergillus niger, the zone of inhibition was 20.3 ± 0.010 mm, against Candida albicans, the zone of inhibition was 30.7 ± 0.175 mm. These results indicate that the pure drug nano micelles demonstrated improved antifungal activity compared to the marketed drug suspension. Combination nano micelles were the nanomicelles formulation containing a combination of Posaconazole and Hemp Seed Oil. Against Aspergillus niger, the zone of inhibition ranged from 22.20 to 22.41 mm, with an average value of 22.4 ± 0.210 mm. Against Candida albicans, the zone of inhibition ranged from 32.04 to 32.78 mm, with an average value of 32.2 ± 0.469 mm. These findings suggest that the combination nano micelles exhibited enhanced antifungal activity compared to the pure drug nano micelles and the marketed drug suspension (p < 0.001), as shown in Table 2 and Fig. 9. Overall, the results highlight the potential of the combination nano micelles formulation in improving the antifungal efficacy of Posaconazole, particularly against Aspergillus niger and Candida albicans41,51.
In vivo pharmacokinetic study
Utilizing a non-compartmental model, the pharmacokinetic parameters of Posaconazole loaded nanomicelles were determined, encompassing Cmax, Tmax, AUC0-48, AUC0-inf, and t1/2 as mentioned in Table 4. In the group treated with Posaconazole loaded nano micelles, Cmax and Tmax values were determined as 221.94 ± 0.090 ng/mL and 10.84 h, respectively. In contrast, the orally administered Posaconazole suspension group exhibited Cmax and Tmax values of 107.04 ± 0.96 ng/mL and 9.29 h as shown in Fig. 10. Notably, a statistically significant difference (P < 0.05) in the pharmacokinetic parameters (Cmax and Tmax) was observed between the nanomicelles and oral suspension groups. The increased Tmax value following oral administration of nanomicelles could potentially be attributed to their improved penetration qualities and prolonged drug release compared to the oral suspension. The AUC0-48 and AUC0-inf values for the nanomicelles were determined as 6544.15 ± 30.59 ng hr/mL and 6544.32 ± 109.84 ng hr/mL, respectively. In contrast, the AUC0-48 and AUC0-inf values for the oral suspension were found to be 2703.87 ± 10.88 ng hr/mL and 2703.88 ± 1.39 ng hr/mL, respectively. These values were notably lower than those of the nano micelles, exhibiting statistical significance (p < 0.05). The increased AUC for nano micelles could be attributed to the extended maintenance of drug concentration within the pharmacologically efficacious range. Furthermore, the considerably higher AUC0-48 value for nano micelles compared to the suspension strongly suggests enhanced bioavailability, possibly resulting from optimized drug delivery. The t1/2 value for nano micelles (7.2 ± 0.59 h) was notably higher than that of the suspension (6.25 h). This difference in t1/2 could be attributed to the longer absorption period when delivered via nano micelles. The assessment of the optimized formulation's improved bioavailability was conducted through AUC0-48 in pharmacokinetic analyses. Relative bioavailability was quantified as the ratio of AUC0-inf for nano micelles to that of suspension. The calculated relative bioavailability for nano micelles was 1.8 fold8.
Stability study
Figure 11 presents a summary of the findings regarding the stability assessment of posaconazole-loaded nanomicelles. The average particle size, zeta potential, and entrapment efficiency (EE%) did not exhibit significant changes at room temperature (Fig. 11A). Investigations into drug stability under conditions of 40 ± 2 °C/75 ± 5% RH revealed minimal degradation (p < 0.05) (Fig. 11B). However, it is worth noting that storing lipid-based preparations in an accelerated state may not be optimal, as there could be a considerable decrease in drug entrapment at this temperature. Consequently, it can be inferred that nanomicelles preparations are ideally stored at room temperature (25 ± 2 °C/60 ± 5% RH) for prolonged periods.
Conclusion
In conclusion, this study successfully developed and characterized Posaconazole and hemp seed oil oral nano micelles for potential anti-fungal treatment. These results suggest a stable and uniform formulation. Detailed characterization revealed key thermal behaviors of the constituents, such as endothermic peaks associated with Posaconazole, hemp seed oil, PLGA, and Poloxamer. Molecular docking analysis was conducted to investigate how different ligands interacted with a crystal structure. Absorption peaks corresponding to specific functional groups were also identified, enhancing our understanding of the molecular interactions within the formulation. Furthermore, the nano micelles exhibited favorable drug release kinetics, with Posaconazole-loaded micelles achieving a higher cumulative release compared to the control suspension after 24 h. Notably, the anti-fungal studies against Aspergillus niger and Candida albicans demonstrated promising inhibitory effects, reaffirming the potential therapeutic relevance of the developed nano micelles. These findings collectively underscore the feasibility and efficacy of the Posaconazole and hemp seed oil oral nano micelles for anti-fiungal treatment. The successful formulation, characterization, and positive outcomes of in vitro studies provide a foundation for potential future investigations and clinical applications. The clear evidence from CLSM imaging underscores the crucial role of micelle characteristics in facilitating their penetration into deeper layers. By leveraging the synergistic properties of Posaconazole and hemp seed oil within the nano micelles, this study contributes to the advancement of novel anti-fungal therapeutic approaches. Further studies are required to confirm the stability of Hemp seed oil after formulation as it has a high content of unsaturated fatty acids, which are highly susceptible to oxidation.
Data availability
The datasets used and/or analyzed during the current study are available with the corresponding author on reasonable request.
References
Fang, W. et al. Diagnosis of invasive fungal infections: challenges and recent developments. J. Biomed. Sci. 301(30), 1–35 (2023).
Friedrich, R., Rappold, E., Bogdan, C. & Held, J. Comparative analysis of the wako β-glucan test and the fungitell assay for diagnosis of candidemia and pneumocystis jirovecii pneumonia. J. Clin. Microbiol. 56, 10 (2018).
Marr, K. A. et al. Combination antifungal therapy for invasive aspergillosis a randomized trial. Ann. Intern. Med. 162, 81–89 (2015).
Patel, N. R., Damann, K., Leonardi, C. & Sabliov, C. M. Itraconazole-loaded poly(lactic-co-glycolic) acid nanoparticles for improved antifungal activity. Nanomedicine 5, 1037–1050. https://doi.org/10.2217/nnm.10.68 (2010).
Willems, L., Van Der Geest, R. & De Beule, K. Itraconazole oral solution and intravenous formulations: A review of pharmacokinetics and pharmacodynamics. J. Clin. Pharm. Ther. 26, 159–169 (2001).
Rathee, A. et al. Simultaneous determination of posaconazole and hemp seed oil in nanomicelles through RP-HPLC via a quality-by-design approach. ACS Omega https://doi.org/10.1021/acsomega.3c02097 (2023).
Nasser, S. T., Abdulrassol, A. A. & Ghareeb, M. M. Design, preparation and in-vitro evaluation of novel ocular antifungal nanoemulsion using posaconazole as a model drug. Res. Nasser AAAMM GhareebTechnol. 11, 1–7 (2021).
Chen, L. et al. Pharmacokinetics and pharmacodynamics of posaconazole. Drugs 807(80), 671–695 (2020).
Deferne, J. L. & Pate, D. W. International hemp association. J. Int. Hemp Ass. 3(1), 1–4 (1996).
Karimi, I. & Hayatghaibi, H. Effect of Cannabis sativa L. seed (Hempseed) on serum lipid and protein profiles of rat Pakistan. J. Nutrition 5(6), 585–588 (2006).
Simopoulos, A. P. Fatty acids. In Functional foods (ed. Goldberg, I.) 355–392 (Springer, 1994). https://doi.org/10.1007/978-1-4615-2073-3_16.
Leizer, C., Ribnicky, D. M., Poulev, A., Dushenkov, D. & Raskin, I. The composition of hemp seed oil and its potential as an important source of nutrition. J. Nutraceuticals Funct. Med. Foods 2, 35–53 (2000).
Rodrigues, M. E., Silva, S., Azeredo, J. & Henriques, M. Novel strategies to fight Candida species infection. Crit. Rev. Microbiol. 42, 594–606 (2016).
Orlando, G. et al. Comparative investigation of composition, antifungal, and anti-inflammatory effects of the essential oil from three industrial hemp varieties from italian cultivation. Antibiotics https://doi.org/10.3390/antibiotics10030334 (2021).
Kesharwani, S. S., Kaur, S., Tummala, H. & Sangamwar, A. T. Multifunctional approaches utilizing polymeric micelles to circumvent multidrug resistant tumors. Coll. Surfaces B Biointerfaces. https://doi.org/10.1016/j.colsurfb.2018.10.022 (2019).
Lu, Y. & Park, K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 453, 198–214 (2013).
Pepić, I., Lovrić, J. & Filipović-Grčić, J. How do polymeric micelles cross epithelial barriers?. Eur. J. Pharm. Sci. 50, 42–55 (2013).
Mohit, K. P., Solanki, P., Mangla, B. & Aggarwal, G. Formulation development, optimization by box-behnken design, and in vitro characterization of gefitinib phospholipid complex based nanoemulsion drug delivery system. J. Pharm. Innov. https://doi.org/10.1007/s12247-022-09690-6 (2022).
Zhang, J. et al. TPGS-g-PLGA/Pluronic F68 mixed micelles for tanshinone IIA delivery in cancer therapy. Int. J. Pharm. 476, 185–198 (2014).
Solanki, P. et al. Precision engineering designed phospholipid-tagged pamidronate complex functionalized SNEDDS for the treatment of postmenopausal osteoporosis. Drug Deliv. Transl. Res. 13, 883–913 (2023).
Nikita, Aqil, M. & Sultana, Y. A grafted copolymer-based nanomicelles for topical ocular delivery of everolimus: Formulation, characterization, ex-vivo permeation, in-vitro ocular toxicity, and stability study. Eur. J. Pharm. Sci. 159, 105735 (2021).
Pandit, J. et al. Fenofibrate loaded nanofibers based thermo-responsive gel for ocular delivery: Formulation development, characterization and in vitro toxicity study. J. Drug Deliv. Sci. Technol. 89, 104935 (2023).
Mutalik, S. P., Mullick, P., Pandey, A., Kulkarni, S. S. & Mutalik, S. Box-Behnken design aided optimization and validation of developed reverse phase HPLC analytical method for simultaneous quantification of dolutegravir sodium and lamivudine co-loaded in nano-liposomes. J. Sep. Sci. 44, 2917–2931 (2021).
Ansari, M. D. et al. Spanlastics a novel nanovesicular carrier: Its potential application and emerging trends in therapeutic delivery. AAPS Pharm Sci. Tech. 23, 112 (2022).
Akhtar, M. J. et al. Design, synthesis, docking and QSAR study of substituted benzimidazole linked oxadiazole as cytotoxic agents, EGFR and erbB2 receptor inhibitors. Eur. J. Med. Chem. 126, 853–869 (2017).
Solanki, P., Kumar, A., Garg, A., Sharma, P. & Singh, S. Designing & development of spherical agglomerates of ibuprofen-paracetamol blend for improved tableting and dissolution. Int. J. Ther. Appl. 8, 8–13 (2012).
Sadiq, S. et al. Potential of monolaurin based food-grade nano-micelles loaded with nisin Z for synergistic antimicrobial action against Staphylococcus aureus. LWT - Food Sci. Technol. 71, 227–233 (2016).
Emad, N. A. et al. Omega-3 fatty acid-based self-microemulsifying drug delivery system ( SMEDDS ) of pioglitazone : Optimization, in vitro and in vivo studies. Saudi J. Biol. Sci. 30, 103778 (2023).
Emad, N. A. et al. Polyphenols-loaded beeswax-based lipid nanoconstructs for diabetic foot ulcer: Optimization, characterization, in vitro and ex vivo evaluation. J. Drug Deliv. Sci. Technol. 88, 104983 (2023).
Ansari, M. D. et al. Fabrication and optimization of raloxifene loaded spanlastics vesicle for transdermal delivery. J. Drug Deliv. Sci. Technol. 68, 103102 (2022).
Khan, I. et al. Development and evaluation of biodegradable polymeric lomustine nanofibres for the efficient tumor targeting: In vitro characterization, ex vivo permeation and degradation study. J. Drug Deliv. Sci. Technol. 75, 103685 (2022).
Azzazy, H. M. E. S., Fahmy, S. A., Mahdy, N. K., Meselhy, M. R. & Bakowsky, U. Chitosan-coated PLGA nanoparticles loaded with peganum harmala alkaloids with promising antibacterial and wound healing activities. Nanomaterials 11, 2438 (2021).
Badria, F. & Mazyed, E. Formulation of nanospanlastics as a promising approach for improving the topical delivery of a natural leukotriene inhibitor (3-acetyl-11-keto-β-boswellic acid): Statistical optimization, in vitro characterization, and ex vivo permeation study. Drug Des. Devel. Ther. 14, 3697–3721 (2020).
Uchida, K., Yokota, N. & Yamaguchi, H. In vitro antifungal activity of posaconazole against various pathogenic fungi. Int. J. Antimicrob. Agents 18, 167–172 (2001).
Basha, M., Abd El-Alim, S. H., Shamma, R. N. & Awad, G. E. A. Design and optimization of surfactant-based nanovesicles for ocular delivery of Clotrimazole. J. Liposome Res. 23, 203–210 (2013).
Tiwari, R. et al. Analytical quality-by-design (AQbD) guided development of a robust HPLC method for the quantification of plumbagin from Plumbago species. J. Liq. Chromatogr. Relat. Technol. https://doi.org/10.1080/10826076.2021.1973027 (2021).
Chen, Y. et al. Sodium lauryl sulfate competitively interacts with HPMC-AS and consequently reduces oral bioavailability of posaconazole/HPMC-AS amorphous solid dispersion. Mol. Pharm. 13, 2787–2795 (2016).
Nomeir, A. A. et al. Pharmacokinetics of SCH 56592, a new azole broad-spectrum antifungal agent, in mice, rats, rabbits, dogs, and cynomolgus monkeys. Antimicrob. Agents Chemother. 44, 727–731 (2000).
Mudie, D. M. et al. Novel high-drug-loaded amorphous dispersion tablets of posaconazole; In vivo and in vitro assessment. Mol. Pharm. 17, 4463–4472 (2020).
Bernabeu, E. et al. Novel Soluplus®—TPGS mixed micelles for encapsulation of paclitaxel with enhanced in vitro cytotoxicity on breast and ovarian cancer cell lines. Coll. Surfaces B Biointerfaces 140, 403–411 (2016).
Hens, B. et al. Evaluation and optimized selection of supersaturating drug delivery systems of posaconazole (BCS class 2b) in the gastrointestinal simulator (GIS): An in vitro-in silico-in vivo approach. Eur. J. Pharm. Sci. 115, 258–269 (2018).
Hernández-Giottonini, K. Y. et al. PLGA nanoparticle preparations by emulsification and nanoprecipitation techniques: Effects of formulation parameters. RSC Adv. 10, 4218–4231 (2020).
El-Houssiny, A. S. et al. Drug-polymer interaction between glucosamine sulfate and alginate nanoparticles: FTIR, DSC and dielectric spectroscopy studies. Adv. Nat. Sci. Nanosci. Nanotechnol. 7, 25014 (2016).
Nijhawan, M., Godugu, M., Saxena, T., Farheen, T. & Dwivedi, K. Pharmaceutical co-crystals of posaconazole for improvement of physicochemical properties. Brazilian J. Pharm. Sci. 58, e191024 (2022).
Phatak, A., Ghurghure, S. M., Jadhav, T., Kale, S. & Phatak, A. A. Formulation and evaluation of posaconazole loaded nanostructured lipid carriers for topical drug delivery system. Methods 4, 126–134 (2022).
Kujawski, J. et al. Structural and spectroscopic properties of posaconazole—Experimental and theoretical studies. J. Mol. Struct. 1181, 179–189 (2019).
Kolipaka, T. et al. Development of posaconazole nanosuspension for bioavailability enhancement: Formulation optimization, in vitro characterization, and pharmacokinetic study. J. Drug Deliv. Sci. Technol. 83, 104434 (2023).
Lalu, L. et al. Novel nanosystems for the treatment of ocular inflammation: Current paradigms and future research directions. J. Control. Release 268, 19–39 (2017).
Cholkar, K., Gunda, S., Earla, R., Pal, D. & Mitra, A. K. Nanomicellar topical aqueous drop formulation of rapamycin for back-of-the-eye delivery. AAPS PharmSciTech 16, 610–622 (2015).
Ansari, M. D. et al. CCD based development and characterization of nano-transethosome to augment the antidepressant effect of agomelatine on Swiss albino mice. J. Drug Deliv. Sci. Technol. 54, 101234 (2019).
Mallié, M. et al. In vitro susceptibility testing of Candida and Aspergillus spp. to voriconazole and other antifungal agents using Etest®: Results of a French multicentre study. Int. J. Antimicrob. Agents 25, 321–328 (2005).
Acknowledgements
Authors thank the Researchers Supporting Project number (RSP2024R132), King Saud University, Riyadh, Saudi Arabia. The authors acknowledge the support of the Department of Science and Technology (DST), Govt. of India for PURSE (No. SR/PURSE Phase 2/39[C]) and FIST (No. SR/FST/LS-I/2017/85[C]) grants awarded to Jamia Hamdard and Dept. of Pharmaceutics, School of Pharmaceutical Education and Research, Jamia Hamdard.
Funding
Authors thank the Researchers Supporting Project number (RSP2024R132), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.R., P.S., and Y.S.; Data curation, K.K., N.A.E., and S.A.; Formal analysis, Sh.A.; Funding acquisition, A.S.A. and O.M.N.; Investigation, I.Z.; Methodology, A.R.; Project administration, K.K.; Resources, A.S.A. and O.M.N.; Software, P.S. and N.A.E; Supervision, Y.S.; Validation, I.Z. and Sh.A.; Visualization, S.A.; Writing – original draft, A.R.; Writing – review & editing, all authors. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rathee, A., Solanki, P., Emad, N.A. et al. Posaconazole-hemp seed oil loaded nanomicelles for invasive fungal disease. Sci Rep 14, 16588 (2024). https://doi.org/10.1038/s41598-024-66074-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66074-1
- Springer Nature Limited