Abstract
This study aimed to establish a predictive model for the risk of post-thoracic endovascular aortic repair (TEVAR) post-implantation syndrome (PIS) in type B aortic dissection (TBAD) patients, assisting clinical physicians in early risk stratification and decision management for high-risk PIS patients. This study retrospectively analyzed the clinical data of 547 consecutive TBAD patients who underwent TEVAR treatment at our hospital. Feature variables were selected through LASSO regression and logistic regression analysis to construct a nomogram predictive model, and the model's performance was evaluated. The optimal cutoff value for the PIS risk nomogram score was calculated through receiver operating characteristic (ROC) curve analysis, further dividing patients into high-risk group (HRG) and low-risk group (LRG), and comparing the short to midterm postoperative outcomes between the two groups. In the end, a total of 158 cases (28.9%) experienced PIS. Through LASSO regression analysis and multivariable logistic regression analysis, variables including age, emergency surgery, operative time, contrast medium volume, and number of main prosthesis stents were selected to construct the nomogram predictive model. The model achieved an area under the curve (AUC) of 0.86 in the training set and 0.82 in the test set. Results from calibration curve, decision curve analysis (DCA) and clinical impact curve (CIC) demonstrated that the predictive model exhibited good performance and clinical utility. Furthermore, after comparing the postoperative outcomes of HRG and LRG patients, we found that the incidence of postoperative PIS significantly increased in HRG patients. The duration of ICU stay and mechanical assistance time was prolonged, and the incidence of postoperative type II entry flow and acute kidney injury (AKI) was higher. The risk of aortic-related adverse events (ARAEs) and major adverse events (MAEs) at the first and twelfth months of follow-up also significantly increased. However, there was no significant difference in the mortality rate during hospitalization. This study established a nomogram model for predicting the risk of PIS in patients with TBAD undergoing TEVAR. It serves as a practical tool to assist clinicians in early risk stratification and decision-making management for patients.
Similar content being viewed by others

Introduction
With the development and popularization of minimally invasive intervention technology, Thoracic endovascular aortic repair (TEVAR) has become the standard treatment for treating complicated type B aortic dissection (TBAD)1,2,3. It offers advantages such as minimal trauma and high safety4. However, there are still risks of perioperative mortality and severe complications. With the expansion of treatment indications, an increasing number of patients with complex vascular lesions can also be treated with TEVAR. However, these patients often have more preoperative complications, which imposes higher demands on perioperative management5,6.
Common serious complications after TEVAR include cardiovascular accidents such as encompass myocardial infarction, arrhythmias and aortic rupture, as well as renal dysfunction, and multi-organ perfusion insufficiency. In addition to these severe complications, some patients commonly present with fever and elevated white blood cell (WBC) count postoperatively. In 1990, Velazquez defined this clinical phenomenon as post-implantation syndrome (PIS)7. Due to the lack of clear clinical diagnostic criteria, the incidence rates of PIS vary widely in different studies, generally ranging from 13 to 60%8,9,10,11,12. The widely accepted diagnostic criteria for PIS include the onset of fever (tympanic temperature > 38.0℃), elevated WBC count (> 12*109/L), and increased C-reactive protein (CRP) levels (> 10 mg/L) within 72 h postoperatively13.
A previous study conducted in endovascular aortic repair (EVAR) patients on the impact of PIS on short-term and long-term prognosis outcomes yielded some conflicting results8,14. The prevailing view is that PIS is a short-term self-limiting complication and does not significantly affect prognosis outcomes. However, Arnaoutoglou et al.'s research suggested that PIS might lead to other serious complications, prolong hospital stay8,15, and serve as an independent risk factor for cardiovascular events within the first year post-EVAR, increasing the risk of all-cause mortality and rehospitalization16. Furthermore, Riccardo et al.'s study further confirmed the association between PIS and major postoperative cardiovascular events17.
Therefore, we believe that PIS remains a complication worth attention following TEVAR. This study aims to develop an effective tool to quantitatively assess the risk of PIS occurrence after TEVAR, which will facilitate early identification of potential high-risk patients for PIS by clinical physicians, prompt adjustment of treatment strategies, active intervention, thereby effectively preventing or mitigating the occurrence and impact of PIS, and further improving patient prognosis outcomes.
Materials and methods
Study population
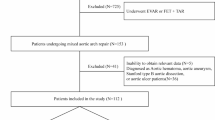
The clinical data of patients with TBAD who underwent TEVAR treatment at our center from Jan 2015 to Jan 2020 were retrospectively analyzed in this study. We included TBAD patients aged > 18 years who underwent TEVAR treatment, with the following exclusion criteria: (1) axillary temperature > 38℃ or WBC count > 12*109/L within 48 h before surgery; (2) presence of malignant tumors, hematologic diseases, systemic autoimmune diseases (such as autoimmune diseases or severe infections) requiring long-term anti-inflammatory or immunosuppressive therapy; (3) previous TEVAR or hybrid surgery; (4) severe malperfusion syndrome or aortic rupture requiring emergency surgery. A flowchart of the exclusion process is shown in Fig. 1. A total of 547 TBAD patients were included in this study.
The indications for TEVAR treatment in patients with TBAD at different phases are as follows. For patients with acute TBAD, indications for TEVAR treatment include: (1) Intractable pain, uncontrolled hypertension, hemothorax, aortic diameter > 40 mm, aortic false lumen diameter > 22 mm, poor perfusion found solely on imaging studies, the primary tear being located in the inner aorta curvature, (2) Adequate proximal landing zone (primary tear distance from the left subclavian artery > 1.5 cm), (3) Absence of concomitant hereditary connective tissue disorders (e.g., Marfan syndrome). In the subacute TBAD, TEVAR is pursued in the absence of absolute surgical contraindications. Indications for TEVAR treatment in chronic-phase TBAD patients are: (1) Symptoms related to dissection, such as chest or abdominal pain, (2) Progression of dissection, (3) Inability to tolerate open surgery, (4) Adequate proximal landing zone, (5) Absence of concomitant hereditary connective tissue disorders, and absence of concomitant thoracoabdominal aneurysms with a diameter ≥ 5.5 cm.
This study was approved by the Ethics Committee of Fujian Medical University Union Hospital (No. XH2023-028) and complied with the Helsinki Declaration. Informed consent was waived due to the retrospective nature of the study.
Defenition
In this study, PIS was defined as the occurrence within 72 h postoperatively of fever (tympanic temperature > 38 °C) and elevated WBC count (> 12*109/L), with negative blood culture results, with or without an increase in CRP levels (> 10 mg/L)15,18. The primary outcome event in this study was the occurrence of major adverse events (MAEs) postoperatively, including aortic-related adverse events (ARAEs), malignant arrhythmias, chronic heart failure, reintervention, or all-cause mortality. ARAEs encompassed different type entry flow, endo-graft stenosis, migration, occlusion, and infection, mediastinal infection, and aortoesophageal fistula. Entry flow were defined according to the reporting standards of the Society for Vascular Surgery and the Society of Thoracic Surgeons19. Endo-graft migration was defined as movement of the stent graft exceeding 5–10 mm from its original position. False lumen diameter is defined as the maximum diameter of the false lumen at the level of the pulmonary bifurcation. The length of aortic dissection is defined as the centerline distance of the affected vascular segment where aortic dissection occurs. Supra-aortic procedures are defined as procedures performed when the landing zone of zone 3 is insufficient or involves zones 1 to 2, such as chimney techniques or fenestration techniques.
The phases of TBAD are based on the 2014 European Society of Cardiology (ESC) guidelines for the diagnosis and treatment of aortic diseases: defining onset ≤ 14 days as the acute phase; > 14–90 days as the subacute phase; and > 90 days as the chronic phase20.
Data collection
Query the clinical data of enrolled patients through the electronic medical record system, including baseline information (gender, age, body mass index (BMI), etc.), preoperative comorbidities (hypertension, diabetes, etc.), preoperative laboratory test results, intraoperative conditions (anesthesia method, operative time, contrast medium volume, intraoperative blood loss, etc.), postoperative clinical outcomes (hospital stay time, ICU stay time, mechanical assistance time, in-hospital mortality, etc.), and follow-up status within 12 months postoperatively.
Statistical analysis
All data were analyzed using SPSS (version 25.0, IBM, Armonk, NY, USA) and R (version 4.2.2). Patients were randomly divided into training and test sets in a 7:3 ratio. The training set was utilized for model construction, while the test set was used for model evaluation. Continuous variables were presented as mean ± standard deviation (SD) or median (interquartile range, IQR), and analyzed using student's t-tests or Mann–Whitney U tests. Categorical variables were expressed as frequencies or percentages and analyzed using chi-square tests or Fisher's exact tests. Clinical variables for constructing the prediction model were selected through LASSO regression analysis and multivariable logistic regression analysis. Model evaluation was performed using receiver operating characteristic (ROC) curve analysis and calculating the area under the curve (AUC) to assess the discriminative ability of the model. Calibration curves were used to evaluate the accuracy of the model. Decision curve analysis (DCA) and clinical impact curve (CIC) were employed to determine the net benefit threshold and clinical applicability of the model. A nomogram was constructed to calculate the scores for all patients, and the total scores were categorized into quartiles. Logistic regression analysis was used to verify the predictive accuracy of the nomogram. The optimal cutoff value for nomogram scores was determined through ROC curve analysis. Patients were then classified into low-risk group (LRG) and high-risk group (HRG), and the cumulative hazard curves were compared to assess the cumulative occurrence risk of ARAEs and MAEs between the two groups using the log-rank test. A two-sided P-value < 0.05 was considered statistically significant.
Ethics approval and consent to participate
The study has been approved by the Ethics Committee of Fujian Medical University Union Hospital (No.XH2023-028) and complies with the Helsinki Declaration. Due to the retrospective nature of the study, the requirement for informed consent has been waived.
Results
Patient characteristics
This study ultimately included a total of 547 patients with TBAD, with a mean age of 53.12 ± 10.28 years. There were 378 male patients (69.1%). In patients with acute TBAD, 83 cases (90.2%) experienced intractable pain, 22 cases (23.9%) had uncontrollable hypertension, 4 cases (4.3%) had hemothorax, 44 cases (47.8%) had an aortic diameter > 40 mm, 65 cases (70.7%) had a false lumen diameter > 22 mm, 3 cases (3.3%) had poor perfusion detected solely by imaging, and 10 cases (10.9%) had primary tear being located in the inner aorta curvature. In patients with chronic TBAD, 77 cases (83.7%) experienced chest or abdominal pain related to the dissection, 50 cases (54.3%) showed progression of the dissection, and 6 cases (6.5%) were unable to tolerate open surgery.
Among all patients, 382 patients (70%) were randomly assigned to the training set, while 165 patients (30%) were randomly assigned to the test set. There were no significant differences in baseline characteristics, comorbidities, and intraoperative conditions between the two datasets (P > 0.05) (Table 1). The overall average postoperative length of hospital stay was 6.44 ± 2.39 days, with a total of 7 (1.2%) deaths during the postoperative hospitalization period.
Selecting clinical variables through LASSO regression analysis
To avoid overfitting and improve the generalization ability of the predictive model, we conducted preliminary screening of preoperative and intraoperative variables using LASSO regression analysis. Figure 2A shows the cross-validation curve of the LASSO regression analysis, while Fig. 2B presents the coefficient profile plot of the LASSO regression analysis. As shown in Fig. 2A, the variables selected for the minimum mean square error at λ = 0.014 are: age, previous cerebrovascular disease (CVD), previous chronic kidney disease (CKD), renal ischemia, previous aortic surgery, phase of TBAD, CRP, emergency surgery, anesthesia technique, operative time, contrast medium volume, intraoperative blood loss, number of main prosthesis stents, and number of branch stents.
Logistic regression analysis and establishment and validation of nomogram
In the training and test sets, we compared the preoperative and intraoperative conditions, as well as inflammatory markers conditions in early postoperative laboratory data, between the PIS group and the non-PIS group, as shown in Table 2. Clinical variables with P < 0.2 were further included in the logistic regression analysis. Then we analyzed the independent risk factors for postoperative PIS following TEVAR using logistic regression analysis. The results of univariate and multivariate logistic regression analyses are presented in Table 3. In the training set, the results of multivariate logistic regression analysis indicated that age ≤ 59 years, emergency surgery, operative time > 59 min, contrast medium volume > 80 ml, and number of main prosthesis stents ≥ 2 pieces were independent risk factors for PIS. We observed the same results in the test set. To quantitatively analyze the risk of post-TEVAR PIS occurrence, we further constructed a nomogram predictive model based on the aforementioned five preoperative and intraoperative variables (Fig. 3). In the training set, the AUC value of this predictive model was 0.86 (Fig. 4A), and in the test set, the AUC was 0.82 (Fig. 4B), indicating good predictive performance of the model. Subsequently, we evaluated the performance of the model through calibration curves, DCA, and CIC. The calibration curve results demonstrated good consistency between the predicted and observed outcomes in both the training set (Fig. 4C) and the test set (Fig. 4D). DCA curve (Fig. 4E/F) and CIC curve (Fig. 4G/H) were used to assess the clinical utility of the model, and by analyzing DCA and CIC, optimal clinical decision points could be selected. The results showed that our model had good clinical applicability in predicting the risk of postoperative PIS across all datasets.
Evaluation of the predictive model's discriminative ability, accuracy, and clinical applicability. (A) The ROC curve and AUC of the predictive model in the training set; (B) The ROC curve and AUC of the predictive model in the test set; (C) The calibration curve of the predictive model in the training set; (D) The calibration curve of the predictive model in the test set; (E) The DCA curve of the predictive model in the training set; (F) The DCA curve of the predictive model in the test set; (G) The CIC curve of the predictive model in the training set; (H) The CIC curve of the predictive model in the test set.
To further validate the accuracy of the model, we calculated the individual total scores for all patients using the nomogram. Based on the quartiles of the total scores, we divided all patients into four subgroups. The subgroup with total scores ranging from 0 to 54.42 was used as the reference standard for logistic regression analysis (Table 4). The results showed that the risk of postoperative PIS increased with higher total scores, with patients scoring between 186.262 to 267.424 having the highest risk of postoperative PIS (odds ratio (OR) = 63.436, 95% confidence interval (CI) (24.791–215.88), P < 0.001). This further confirms the reliability of the model.
Comparison of postoperative conditions between LRG and HRG
Through ROC analysis, the optimal cutoff value of the nomogram score was determined. All patients were then divided into LRG (nomogram points ≤ 167.42) and HRG (nomogram points > 167.42) based on this cutoff value. The short-term and medium-term prognostic outcomes were compared between the two groups (Table 5). The results showed that compared to LRG patients, HRG patients had a significantly higher incidence of PIS (13.15% vs. 60.44%, P < 0.001), prolonged ICU stay (12.00[4.00,16.00] vs 13.00[9.00,18.00], P < 0.001), prolonged mechanical assistance time (5.00[4.00,6.00] vs 6.00[4.00,8.00], P < 0.001). Moreover, HRG patients had a higher incidence of postoperative type II entry flow (1.10% vs 8.24%, P < 0.001) and AKI (0 vs 1.65%, P = 0.036), while there were no significant differences in other types of entry flow, endo-graft stenosis, occlusion, migration and infection, paraplegia, hospital stay time, reoperation and in-hospital mortality.
Influence on short and mid-outcomes
Aortic-related adverse events
Among the 365 patients in the LRG group, 4 individuals (1.10%) experienced ARAEs within the first month, while among the 182 patients in the HRG group, 15 individuals (8.24%) experienced ARAEs (P < 0.001). There were 7 deaths during hospitalization, and during the follow-up period from the 1 to 12 month, among the remaining 540 patients, 21 individuals (3.89%) experienced ARAEs, with 4 individuals (1.11%) in the LRG group and 17 individuals (9.44%) in the HRG group. Cumulative hazard analysis showed that the incidence of ARAEs in the HRG group was approximately 7.090 times higher than that in the LRG group in the first month postoperatively (95% CI (3.02–20.790), P < 0.001) (Fig. 5A), and approximately 8.804 times higher in the 12 months (95% CI (4.511–17.182), P < 0.001) (Fig. 5B). There was a statistically significant difference in the cumulative risk of ARAEs between the two groups of patients.
Cumulative hazard curves of ARAEs and MAEs after TEVAR in HRG and LRG patients. (A) The cumulative hazard of ARAEs in the first month postoperatively; (B) The cumulative hazard of ARAEs in the twelve months postoperatively; (C) The cumulative hazard of MAEs in the first month postoperatively; (D) The cumulative hazard of MAEs in the twelve months postoperatively.
Major adverse events
Among the 365 patients in the LRG group, 11 individuals (3.01%) experienced MAEs within the first month, while among the 182 patients in the HRG group, 23 individuals (12.64%) experienced MAEs (P < 0.001). In the HRG group, 3 individuals (1.65%) experienced AKI, 1 individual (0.55%) experienced paraplegia, 2 individuals (1.10%) required intervention, and 2 individuals (1.10%) died. In contrast, in the LRG group, 1 individual (0.27%) experienced paraplegia, 1 individual (0.27%) required intervention, and 5 individuals (1.37%) died. During the follow-up period from the 1 to 12 month, among the remaining 540 patients, 23 individuals (4.26%) experienced MAEs, among which 21 individuals (3.89%) experienced ARAEs. Among the remaining patients, 1 individual (0.19%) experienced malignant arrhythmia, 1 individual (0.19%) developed chronic heart failure, and 2 individuals (0.37%) underwent reintervention, both from the LRG group. Cumulative hazard analysis showed that the incidence of MAEs in the HRG group was approximately 4.396 times higher than that in the LRG group in the first month postoperatively (95%CI (2.141–9.023), P < 0.001) (Fig. 5C), and approximately 6.883 times higher in the twelfth month (95% CI (3.79–12.5), P < 0.001) (Fig. 5D). There was a statistically significant difference in the cumulative risk of MAEs between the two groups of patients.
Discussion
Although TEVAR has become a routine treatment for TBAD in clinical practice, PIS remains a commonly overlooked complication, with its pathogenesis and clinical significance still subject to debate. Previous research has confirmed that PIS is closely associated with the prognosis of TEVAR, with results showing that PIS is the sole independent risk factor for adverse cardiovascular events following EVAR8. Therefore, clinicians still should not underestimate the value of PIS as a predictor of adverse clinical outcomes. However, existing studies have only explored potential risk factors for PIS without quantitatively assessing the risk of its occurrence. Thus, based on clinical data from 547 TBAD patients at our center, we successfully developed and validated a nomogram prediction model for postoperative PIS risk. With this predictive model, clinicians can early identify high-risk patients for PIS, adjust and formulate targeted diagnosis and treatment plans promptly, actively prevent and intervene in PIS, thereby further improving the prognosis of TEVAR.
Due to the lack of uniform diagnostic criteria for PIS, the reported incidence rates vary widely across different studies. A low diagnostic rate of PIS can lead to deviations in diagnosis and treatment plans, resulting in increased hospitalization time and costs. However, overdiagnosing PIS may overlook more serious postoperative complications such as stent infection, delaying treatment and impacting patient recovery. Therefore, in practical settings, clinicians can also utilize this predictive model to assist in diagnosing PIS, thereby enhancing diagnostic accuracy and timeliness.
This study's findings indicate that age is negatively correlated with the risk of PIS occurrence, which is largely consistent with previous research results4. Younger patients are more likely to experience PIS, with some studies suggesting a possible association with host immune response1. Ker et al. conducted an in-depth analysis of the fever pattern post-TEVAR, further supporting the aforementioned viewpoint2. We believe that younger age correlates with a stronger immune response in the body, hence more invasive treatment strategies and longer stent implantation may provoke a broader host immune reaction, consequently increasing the risk of PIS occurrence.
The main characteristic of aortic dissection (AD) is its sudden onset and rapid progression, often necessitating emergency surgical intervention. Due to the lack of adequate preoperative preparation, emergency surgery typically results in greater tissue trauma and more intense inflammatory responses. Additionally, it extensively activates the body's immune response, which plays a significant role in the pathogenesis of AD21. Therefore, patients undergoing emergency surgery for AD are more likely to experience PIS due to the compounded effects of inflammation and excessive immune activation, leading to a dysregulation of the body's inflammatory-immune homeostasis.
Furthermore, the results of our multifactorial analysis also indicate that the number of main prosthesis stents ≥ 2 is another key contributing factor to PIS. We believe the primary reason is the increased vascular inflammation resulting from the implantation of more foreign bodies. Mechanical damage during stent implantation or factors related to the stent's biocompatibility can cause endothelial injury, triggering the release of inflammatory mediators and subsequent local tissue inflammation. Additionally, implanting multiple stents may lead to increased local mechanical stress on the vessel wall, further enhancing the level of vascular inflammation. Moreover, implanting more main prosthesis or branch stents during surgery can lead to increased use of contrast medium during surgery and prolonged operative time. Studies have shown that iodinated contrast medium can activate neutrophils and induce the release of inflammatory factors upon contact with stent materials, exacerbating endothelial damage and aggravating vascular inflammation22,23,24. Furthermore, our study results also indicate that contrast medium volume > 80ml and operation time > 59min are risk factors for postoperative PIS.
The inflammatory conditions of the body vary between different phases of TBAD. In the acute phase, patients typically experience intense inflammatory responses, manifested clinically as severe chest or back pain that is difficult to tolerate. In contrast, during the chronic phase of TBAD, the intensity of inflammatory responses may diminish to some extent over time, but there still exists a chronic inflammatory response associated with false lumen thrombosis and vascular remodeling. The body's inflammatory conditions are closely associated with the occurrence of PIS. In this study, the vast majority of chronic phase TBAD patients who underwent TEVAR treatment did so due to intolerable clinical symptoms related to aortic dissection, such as severe chest or back pain, or evident progression of the dissection. Under these conditions, the inflammatory response in chronic phase TBAD patients is further intensified, resembling that of the acute phase. Therefore, the incidence of PIS in these patients shows no significant difference compared to the acute phase (P = 0.526).
Through further comparison of the postoperative outcomes of patients at different risks of PIS, we found that the incidence of PIS in HRG patients significantly increased (60.44% vs 13.15%, P < 0.001). This was accompanied by prolonged mechanical assistance time, increased occurrence of type II entry flow and AKI. Additionally, there was a significant increase in the cumulative risk of ARAEs and MAEs in the early (1 month) and mid-term (12 month) postoperative periods. We believe this is closely related to the level of inflammation and immune activation within HRG patients. For HRG patients, even without clinically apparent manifestations diagnosed as PIS postoperatively, their immune systems' response to the implant may already have exceeded normal limits. This places the body in a state of compensatory inflammatory activity. Once the dynamic balance between the two is disrupted and immune regulatory function is compromised, the body enters into a sustained inflammatory response, leading to adverse clinical outcomes and affecting the mid- to long-term cardiovascular health of these patients. Although there was no significant difference in early postoperative mortality between the two groups, for HRG patients, especially those who experienced PIS, it is crucial to pay more attention to the potential risks of ARAEs and MAEs. A stricter follow-up strategy should be implemented for such patients, including shortening follow-up intervals, increasing dynamic monitoring of hematological inflammatory markers, appropriately increasing the frequency of imaging examinations, and enhancing follow-up management to reduce the risk of ARAEs and MAEs.
By comparing the laboratory data of early postoperative inflammation-related indicators between the PIS and non-PIS groups, we found that the levels of inflammation-related indicators in the PIS group were significantly elevated after surgery. This further indicates a close association between a more intense inflammatory response in the body and the occurrence of PIS, emphasizing the crucial role of inflammation in PIS. Therefore, the results of this study also raise a question worthy of consideration by clinicians: as inflammation reaction is the most correlated pathological physiological change with PIS, can the occurrence of PIS be prevented by alleviating the inflammation reaction in patients predicted to be at high risk of PIS? In current clinical practice, patients usually receive prophylactic and empirical antibiotic treatment to avoid more serious complications such as stent graft infection3,10. However, there is currently no definitive evidence to confirm that prophylactic antibiotic treatment for PIS can alleviate postoperative inflammation reaction and improve prognosis. Therefore, further randomized clinical trials are needed to confirm the feasibility of antibiotics in the prophylactic treatment of PIS. Steroidal hormones, as a significantly effective anti-inflammatory medication, have been reported by Motte et al. in a prospective randomized trial to confirm that preoperative prophylactic use of methylprednisolone in EVAR effectively reduces postoperative inflammation and promotes recovery25. However, considering that inappropriate usage may increase the risk of cardiovascular disease incidence and mortality, and the mechanism of corticosteroid therapy for PIS remains unclear, further clinical research is still needed to explore and develop more reliable treatment strategies for preventing postoperative PIS. We believe that in future discussions on the diagnosis and treatment protocols for PIS, greater emphasis should be placed on prevention and early treatment, while also delving into the potential molecular biology mechanisms of PIS to further refine prophylactic treatment strategies, aiming to improve short- to medium-term prognosis outcomes for patients.
For high-risk PIS patients, clinicians can conduct comprehensive preoperative assessments, design and select appropriate stent implantation strategies, and perform precise stent implantation during surgery to reduce the risk of postoperative PIS occurrence, thereby preventing PIS. On the other hand, intervention for PIS involves individualized treatment based on the degree of the patient's inflammatory response when PIS occurs. For patients requiring treatment, non-steroidal anti-inflammatory drugs are the preferred choice to reduce the intensity of inflammation. Additionally, appropriate doses of glucocorticoids could be considered to mitigate the adverse effects of PIS on the body.
In summary, we hope that through this predictive model, clinical physicians can more sensitively diagnose and intervene when facing potential PIS high-risk patients, which will help reduce adverse clinical outcomes related to PIS and promote patients' rapid recovery.
Limitations
This study still has several limitations. Firstly, due to the retrospective nature of the study, inherent selection bias may exist. Secondly, as both the training and test datasets were derived from the same center, the lack of external data limits further evaluation of the model's performance and clinical applicability. Lastly, since there is no definitive diagnostic standard for PIS, despite adopting the currently widely accepted clinical diagnostic criteria for PIS in this study, some preoperative and intraoperative potential risk factors for PIS may still be overlooked. Therefore, further multicenter, large-sample randomized clinical trials are still needed to further validate the performance and clinical generalizability of this model.
Conclusion
This study has developed and validated for the first time a model that can predict the risk of PIS in TBAD patients after TEVAR, demonstrating good performance and clinical utility. It provides a reliable reference for clinical physicians to assess the risk of PIS, enabling early identification of high-risk PIS patients and timely adjustment of diagnosis and treatment strategies.
Data availability
The data from this study will not be shared publicly. All data included in this study are available upon request by contact with the corresponding author.
References
Shu, C. et al. Endovascular repair of complicated acute type-B aortic dissection with stentgraft: Early and mid-term results. Eur. J. Vascular Endovascular Surg. 42(4), 448–453 (2011).
Ker, C. R., Ho, M. C., Huang, J. W., Hsieh, C. C. & Chen, H. M. Pyrexia of postimplantation syndrome for patients undergoing (thoracic) endovascular aortic repair. Thorac. Cardiovasc. Surg. 63(2), 126–133 (2015).
Martinelli, O. et al. Post-implantation syndrome: The impact of different devices for endovascular abdominal aortic aneurysm repair and related etiopathogenetic implications. Inter. Angiol. J. Inter. Union Angiol. 39(5), 398–404 (2020).
Zhu, Y. et al. Predictors associated with an increased prevalence of postimplantation syndrome after thoracic endovascular aortic repair for type B aortic dissection†. Eur. J. Cardio-Thoracic Surg. 55(5), 998–1005 (2019).
Kaji, S. Update on the Therapeutic Strategy of Type B Aortic Dissection. J. Atheroscler. Thromb. 25(3), 203–212 (2018).
Howard, C. et al. TEVAR for complicated and uncomplicated type B aortic dissection-Systematic review and meta-analysis. J. Card. Surg. 36(10), 3820–3830 (2021).
Voûte, M. T. et al. Stent graft composition plays a material role in the postimplantation syndrome. J. Vasc. Surg. 56(6), 1503–1509 (2012).
Arnaoutoglou, E. et al. Prospective evaluation of post-implantation inflammatory response after EVAR for AAA: influence on patients’ 30 day outcome. Eur. J. Vascular Endovascular Surg. 49(2), 175–183 (2015).
Moulakakis, K. G. et al. Inflammatory response and renal function following endovascular repair of the descending thoracic aorta. J. Endovascular Ther. 22(2), 201–206 (2015).
Sartipy, F., Lindström, D., Gillgren, P. & Ternhag, A. The Impact of Stent Graft Material on the Inflammatory Response After EVAR. Vascular Endovascular surg. 49(3–4), 79–83 (2015).
Eggebrecht, H. et al. Clinical implications of systemic inflammatory response syndrome following thoracic aortic stent-graft placement. J. Endovascular Ther. 15(2), 135–143 (2008).
Kakisis, J. D. et al. Volume of new-onset thrombus is associated with the development of postimplantation syndrome after endovascular aneurysm repair. J. Vascular Surg. 60(5), 1140–1145 (2014).
Baek, J. K. et al. Impact of graft composition on the systemic inflammatory response after an elective repair of an abdominal aortic aneurysm. Ann. Surg. Treat. Res. 88(1), 21–27 (2015).
Soares Ferreira, R. et al. Long Term Outcomes of Post-Implantation Syndrome After Endovascular Aneurysm Repair. Eur. J. vascular Endovasc. Surg. 62(4), 561–568 (2021).
Arnaoutoglou, E. et al. Post-implantation syndrome after endovascular repair of aortic aneurysms: Need for Postdischarge surveillance. Inter. Cardiovasc. Thoracic Surg. 11(4), 449–454 (2010).
Arnaoutoglou, E. et al. Prospective evaluation of postimplantation syndrome evolution on patient outcomes after endovascular aneurysm repair for abdominal aortic aneurysm. J. Vasc. Surg. 63(5), 1248–1255 (2016).
Gorla, R. et al. Clinical features and prognostic value of stent-graft-induced post-implantation syndrome after thoracic endovascular aortic repair in patients with type B acute aortic syndromes. Eur. J. Cardio-Thor. surg. 49(4), 1239–1247 (2016).
Moulakakis, K. G. et al. The impact of endograft type on inflammatory response after endovascular treatment of abdominal aortic aneurysm. J. vasc. surg. 57(3), 668–677 (2013).
Lombardi, J. V. et al. Society for Vascular Surgery (SVS) and Society of Thoracic Surgeons (STS) reporting standards for type B aortic dissections. J. vasc. surg. 71(3), 723–747 (2020).
Erbel, R. et al. Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. the task force for the diagnosis and treatment of aortic diseases of the European society of cardiology (ESC). Eur. Heart J. 35(41), 2873–2926 (2014).
Luo, F., Zhou, X. L., Li, J. J. & Hui, R. T. Inflammatory response is associated with aortic dissection. Ageing Res. Rev. 8(1), 31–35 (2009).
Odegård, A. et al. The inflammatory response following treatment of abdominal aortic aneurysms: A comparison between open surgery and endovascular repair. Eur. J. vasc. Endovasc. Surg. 19(5), 536–544 (2000).
Kopko, P. M., Smith, D. C. & Bull, B. S. Thrombin generation in nonclottable mixtures of blood and nonionic contrast agents. Radiology 174(2), 459–461 (1990).
Videm, V., Ødegård, A. & Myhre, H. O. Iohexol-induced neutrophil myeloperoxidase release and activation upon contact with vascular stent-graft material: A mechanism contributing to the Postimplantation syndrome?. J. Endovasc. Ther. 10(5), 958–967 (2003).
de la Motte, L. et al. Preoperative methylprednisolone enhances recovery after endovascular aortic repair: A randomized, double-blind, placebo-controlled clinical trial. Ann. surg. 260(3), 540–548 (2014).
Funding
This research was sponsored by the Fujian Provincial Special Reserve Talents Fund (2021–25).
Author information
Authors and Affiliations
Contributions
Lin-feng Xie and Xin-fan Lin designed the study and submitted the manuscript. Lin-feng Xie and Xin-fan Lin prepared the first draft of the manuscript and made the literature review. Lin-feng Xie and Xin-fan Lin are contributed equally to this study and share first authorship. Yu-ling Xie and Zhao-feng Zhang collected the clinical data and performed the statistical analysis. Qing-song Wu, Zhi-huang Qiu and Liang-wan Chen provide technical support. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xie, Lf., Lin, Xf., Wu, Qs. et al. Risk prediction and prognostic analysis of post-implantation syndrome after thoracic endovascular aortic repair. Sci Rep 14, 17376 (2024). https://doi.org/10.1038/s41598-024-65877-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-65877-6
- Springer Nature Limited