Abstract
IgA Nephropathy (IgAN) is the most prevalent glomerular disease worldwide. Complement system activation is crucial in its pathogenesis. Few studies correlated serum C3 and C4 with disease activity and prognosis. This retrospective study investigated the prognostic value of serum complement at the time of diagnosis in patients with IgAN. Specifically we evaluated whether adding serum C3 and C4 levels to established predictive models-one based on variables related to chronic kidney disease (CKD) progression and another incorporating variables from the International IgA Prediction Tool (IntIgAPT)-enhances the accuracy of outcome prediction. A composite renal outcome was defined as 50% decline in eGFR or onset of kidney failure. 101 patients were stratified according to baseline C3 levels in three groups (Low, Medium and High). During a median follow-up of 54 months, the Low group exhibited higher incidence of primary outcome (16.3 events vs 2.9 and 1.7 events × 100 pts/year, p = 0.0026). Model-1 (M1), consisting of CKD progression variables, and Model-3 (M3), comprising IntIgANPT variables, were implemented with baseline C3 and C4 to create Model-2 (M2) and Model-4 (M4), respectively. M2 demonstrated better predictive performance over M1, showing higher discrimination (lower AIC and BIC, higher C-index and NR2). Similarly, M4 outperformed M3, showing enhanced outcome prediction when C3 and C4 levels were added. Implementation of serum C3 and C4 can enhance prediction accuracy of already-validated prognostic models in IgAN. Lower C3 and higher C4 levels were associated with poorer prognosis, highlighting a more 'Complement-Pathic' subset of patients.
Similar content being viewed by others
Introduction
IgA Nephropathy (IgAN) is the most common glomerular disease worldwide, representing approximately 22% of diagnoses in Europe and 11% in North America1.
Clinical manifestations, disease course and response to immunosuppressive treatment are extremely heterogeneous, with a 10-year risk of kidney failure (KF) ranging from 5 and 60%2,3.
According to the current guidelines4, following a biopsy-proven diagnosis of IgAN, disease prognosis should be assessed using the MEST-C system. This histologic score evaluates lesion such as mesangial (M) and endocapillary hypercellularity (E), segmental sclerosis (S), interstitial fibrosis-tubular atrophy (T) and crescents (C)5. Such histological findings have been recently incorporated in a prognostic tool (International IgAN Prediction Tool [IntIgAPT]) together with clinical and biochemical variables collected at the time of biopsy. This system, validated in more than 4000 patients, may predict the risk of disease progression at 5 years (50% of estimated Glomerular Filtration Rate [eGFR] decline or KF), helping subsequent treatment strategy6,7,8. However, neither this calculator nor the MEST-C score alone can be used to determine the likely impact of any treatment regime on disease course. Thus, treatment strategy is based on the severity of proteinuria and estimated eGFR.
Enhancing risk stratification is an urgent need in nephrology research. This effort can be accomplished by the development of reliable prognostic biomarkers9.
Complement system (CS) activation is crucial in IgAN pathogenesis. Both the alternative (AP) and lectin pathways (LP) can be activated, resulting in the production of anaphylatoxins, as well as the formation of the cytolytic membrane attack complex. This leads to the stimulation of mesangial cells to produce inflammatory mediators and matrix proteins10,11. The CS can be activated both locally, as demonstrated by the presence of C3 deposits alongside IgA in over 90% of patients, and systemically, with elevated levels of subproducts of C3 found in affected patients12. Until now, the role of CS has primarily been explored through histological, genetic and biochemical investigations, focusing on the byproducts of cascade activation and detectable metabolites in tissues, blood, and urine. To date, only few studies have correlated serum C3 and C4 fractions with disease activity and prognosis13,14.
Given the pivotal role of CS in disease initiation and progression, we share findings from a longitudinal IgAN cohort study, examining the potential advantages of incorporating serum C3 and C4 levels at the time of diagnosis into established prognostic models for risk stratification.
Materials and methods
Patients and treatments
We retrospectively reviewed clinical records of patients with a histology-proven diagnosis of IgAN referred at our unit (Sant’Orsola University Hospital, Bologna) from Jan-2009 to Dec-2022.
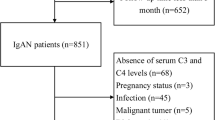
Inclusion criteria were: (I) renal biopsy scored according to Oxford MEST-C scoring system; (II) availability of medical history, blood pressure (BP) measures, data related to renal function (serum creatinine and eGFR), urinary exams (urine biochemistry, 24-h proteinuria [uProt]), serum protein profile (serum albumin and total protein), serum complement levels (C3 and C4), and immunoglobulin (Ig) levels of IgA, IgG, and IgM at the time of renal biopsy; (III) follow up duration of at least 12 months; (IV) at least 18 years at diagnosis. Exclusion criteria were: (I) KF at diagnosis, (II) over-imposed nephropathy.
Treatment was administrated according to current clinical practice and guidelines recommendations. Optimized first-line included management of BP and other cardiovascular risk factors, lifestyle modification and maximally tolerated dose of Renin–Angiotensin–Aldosterone-System inhibitors (RAASi) and Sodium/Glucose Cotransporter-2 inhibitors (SGLT-2i). High-risk patients (i.e. severe proteinuria and/or impaired eGFR) were offered a personalized course of immunosuppression therapy, for example steroids, both systemic (Manno or Pozzi regimens) and local (Budesonide).
All patients received regular follow-up care at our outpatient nephrology clinic, which included blood and urine tests, as well as comprehensive clinical evaluation.
The study was performed in accordance with the declaration of Helsinki and the protocol was approved by the ethical committee of the Sant’Orsola University Hospital of Bologna (Protocol number 420/2018/Oss/AOUBo). Patients provided informed consent for study participation.
Data collection
At the time of admission for kidney biopsy, a thorough medical and pharmacological history of the patients was conducted. Additionally, BP measurements were taken using a standardized office method. Furthermore, patients underwent both blood and urine laboratory tests. All data were collected in electronic spreadsheet. Diagnosis-subsequent pharmacological treatment regimen, with particular focus on supportive—(i.e., RAASi) and immunosuppressive treatment (e.g., steroids)—were also obtained.
Kidney biopsies
Kidney biopsies were performed in the suspicion of kidney disease (e.g., urinary abnormalities and/or functional impairment) after written consent of the patient and in accordance with current clinical practice (percutaneous ultrasound-guided approach). Histological samples were processed and evaluated by local nephropathologist under light microscopy, immunofluorescence, and electron microscopy.
Study aim
The main aim of this retrospective observational single-center study was to investigate the prognostic value of serum C3 and C4, when added to the already known prognostic IgAN models, including IgANPt. The primary outcome used for model computation was a kidney composite outcome, defined as a 50% decline in eGFR and/or the onset of KF.
Secondly, we investigated the interaction of serum complement with other baseline clinical features on the primary endpoint.
Statistical analysis
Descriptive statistics were reported as means ± standard deviations (SD) or median and interquartile range (IQR) for continuous variables, according to distribution. Categorical variables were reported as percentages (%).Patients were stratified according to their baseline serum C3 levels into three categories: Low [< 90 mg/dl], Medium [90–140 mg/dl], and High [> 140 mg/dl], determined by tertiles of the variable distribution in our sample. These cut-offs matched the laboratory levels of serum C3 abnormalities in our Centre. Comparison between groups were tested by means of ANOVA or Kruskal–Wallis test for continuous variables according to distribution. Categorical variables were compared using the Chi-squared test. Linear associations between C3, C4 and other continuous baseline variables involved in IgAN pathophysiology were plotted graphically and tested with Pearson coefficients. Multivariate linear regressions were used to assess correlation between C3 and C4 with eGFR. The slope of the regression line (beta coefficient, [β]) and its corresponding p-value were used to assess the strength and significance of the linear association. Median follow-up was computed by means of inverse Kaplan–Meier approach. Patients were followed until the onset of the primary outcome or until the last follow-up visit in nephrology clinic. Patients lost to follow up were right censored at the time of the last outpatient visit. For the survival analysis we observed sufficient events to compute the incidence rate of outcome. To assess the additional prognostic value of serum complement in comparison with the ‘already used models’ we adopted a logistic regression approach.
As ‘already used models’, we selected: a first one used in clinical practice based on classical variables of kidney disease progression namely age, BP, eGFR, proteinuria15; a second one including the variables of the IntIgAPT. For each comparison, we first built a model without C3 and C4 and then added these two variables to build a new model. We computed the measures of goodness of fit: Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC), Nagelkerke R2 test (NR2) that depicts the % explained variation of the outcome based on the set of variables included, and Likelihood Ratio Test (LRT); discrimination with the c-index.
A p-value of < 0.05 was considered statistically significant.
Statistical analysis was performed using R version 4.0.3 (R Core Team, 2020).
Results
Patients characteristics stratified by C3 groups
101 patients were included in the cohort. 90% were Caucasian and 65.3% were male with a mean age of 42.6 years. As reported in Table 1, mean eGFR and median proteinuria levels at baseline were 70.4 ml/min/1.73 m2 and 1.04 g/24 h respectively. Mean serum C3 was 114.9 mg/dL.
Stratifying by serum C3 levels (Table 1), we didn’t find significant differences in gender or age. High and Low groups had a significantly higher prevalence of hypertension (High 85.7%, Medium 51.4%, Low 73.3%, p = 0.028) with a significant higher systolic blood pressure (SBP) (mean High 133.6, Medium 122.9, Low 129.0, p = 0.044). There were no differences in the rates of CVD or smoking habit. The High group had a higher percentage of diabetes than Medium and Low (High 21.4%,Medium 4.2%, Low 6.7%, p = 0.04). The Low group had lower values of eGFR at baseline (High 68.6, Medium 75.7, Low 47.0 ml/min/1.73 m2, p = 0.015), while the Medium group had lower values of uProt (High 1.81, Medium 0.75, Low 1.46 g/24 h, p = 0.004). There were no differences in serum total protein, albumin, IgG, IgA, IgM, or CD4/CD8 lymphocytes ratio. There was a significant difference in serum C4 values, which seemed to decrease, switching from High to Medium to Low (High 40.7, Medium 33.6, Low 27.4 mg/dL, p = 0.001).
Associations between serum C3 and C4 and baseline variables
Baseline serum C3 and C4 levels were positively correlated (r = 0.41, p < 0.001) (Fig. 1). There were no associations between C3 and levels of IgA, IgG (Fig. S1—Supplementary). Instead, C3 exhibited negative correlation with IgM (r = − 0.20, p = 0.047). C4 levels were not associated with IgA, IgG, but were negatively correlated with IgM (r = − 0.27, p = 0.007). In multivariate linear regression model, both C3 (β = 0.30, p = 0.05) and C4 (β = − 0.76, p = 0.045) were associated with baseline eGFR (Table S1—Supplementary).
Follow-up and prognosis according to baseline complement values.
Median follow-up time was 54.28 months (IQR 39.88, 59.17). The Low group had higher incidence of primary outcome, 16.1 events (95% CI 7.0–36.1) × 100 pts/year as compared with Medium (2.7 events 95% CI 1.3–6.0 × 100 pts/year) and High group (1.7 events 95% CI 0.2–11.8 × 100 pts/year) with a significant difference between rates (p = 0.003) (Fig. 2). There were no differences in the rate of immunosuppression regimens.
Optimizing prognostic models for IgAN integrating serum C3 and C4
As reference models, we used Model-1 (M1), including the main clinical variables of CKD progression (age, gender, SBP, eGFR, uProt) and Model-3 (M3), including variables from the IntIgANpt (age, systolic and diastolic BP, eGFR, uProt, MEST-score). MEST-C parameter was not tested since not included in the tool. Model-2 (M2) and Model-4 (M4) were built by adding baseline C3 and C4 to M1 and M3, respectively. This enables us to explore the supplementary contribution of C3 and C4 to standardized models.
In M1 (Table 2), lower eGFR (OR 0.95, CI 0.92–0.99, p = 0.006) and higher uProt (OR = 1.39, CI 1.08–1.79, p = 0.008) were associated with higher risk of outcome, while there was not correlation with sex, age and SBP. In M2, higher uProt and lower eGFR still predicted a worse outcome, and both lower C3 (OR = 0.94, CI 0.89–0.94, p = 0.029) and higher C4 (OR 1.12, CI 1.01–1.25, p = 0.031) were associated to outcome. When the two models were compared, M2 showed lower values of both AIC (54.9 vs 64.1) and BIC (75.2 vs 81.3), higher discrimination (c-index 0.73 vs 0.63) and higher NR2 (67% vs 50%). LRT between M1 and M2 was significant (p-value = 0.003).
In M3 (Table 3), variables from the IntIgANpt were used. uProt (OR 1.62, CI 1.12–5.21, p = 0.018) showed association with outcome. In M4, uProt (OR 2.32, CI 1.12–4.78, p = 0.022), age (OR 1.30, CI 1.01–1.56, p = 0.045) and C3 (OR 0.91, CI 0.83–0.99, p = 0.049) showed association with outcome. C4 had a positive relation with outcome though not statistically significant (OR 1.19, CI 0.99–1.45, p = 0.058). M4 had a lower AIC (39.9 vs 44.8) and BIC (66.0 vs 75.4) over M3, a higher c-index (0.77 vs 0.67) and NR2 (0.77 vs 0.68). LRT between M3 and M4 was significant (p = 0.009) testifying an improvement in prediction of M4.
Discussion
IgAN is a lifelong chronic affection which leads to a cumulative risk of poor renal outcome despite currently available treatments, with one-third of patients developing KF within 30 years after diagnosis2 and a 19% risk of recurrence after transplantation at 10 years16. This elicits the need for specific pathophysiology-oriented therapeutic strategies.
Despite being an autoimmune disease, the management of IgAN primarily relies on non-immunosuppressive treatment, such as lifestyle interventions, BP control, RAASi, SGLT2i and dual endothelin-angiotensin receptor antagonist. A course of steroid therapy is tipically reserved for individuals at high-risk of disease progression (i.e. uProt > 0.75 g/die) despite supportive treatment. Enrollment in clinical trials must be always considered in this patients4,17,18,19.
The CS is an integral component of the innate immune response, crucial for host defence against infections and tissue clearance from immunocomplexes or injured cells. Dysregulation of this system contributes to a wide spectrum of immune-mediated kidney diseases. Three primary activation pathways have been identified: classic pathway (CP), LP and AP20.
Complement-driven renal damage and its potential therapeutic targets is gaining increasing attention. Growing evidence indicates the pathogenetic role of complement activation, as evidenced by the presence of its components in mesangial immune complexes: more than 90% of kidney biopsies in IgAN show positive C3 staining in immunofluorescence, whereas C1q is rarely reported. This suggests potential activation of the AP and/or LP, rather than the CP. Deposition of complement regulatory proteins, such as properdin, factor-B and factor-H has also been described21.
Despite technical challenges and subjective measurement, the intensity of glomerular C3 staining might predict a higher risk of disease progression22,23,24. Moreover, a correlation between tissue deposits and disease activity was observed: the presence of factor-H related proteins such as CFHR5 -indicating AP activation- was associated with progressive disease, while C4d and mannose-binding lectin mesangial staining -indicating LP activation- was correlated with poorer outcomes25,26,27.
Further evidence for a complement-mediated pathogenesis was provided by genome-wide association studies, which highlighted the role of deletion in CFHR1-3 genes (positive regulator of AP) in IgAN susceptibility28,29. Endorsing this hypothesis, an overactivation of these factors, as indicated by elevated serum levels of CFHR1-5, has been associated to disease activity30,31.
Regardless of the site of production -systemically or locally- and the involved pathway -LP or AP-, aberrant complement activation by mesangial immunocomplexes precipitates glomerular injury and tissue inflammation. This occurs through production of anaphylatoxins (C3a, C5a) and cytolytic membrane attack complex (C5b9). Activation of coagulation cascade triggers further damage20.
Plasmatic C3 and C4 fractions are surrogate markers of complement activation and are widely measured in current nephrological practice. However, their role in IgAN prognosis is unclear and has yet to be elucidated. C3 values often fluctuate within the normal range, but lower levels have been correlated with poor kidney outcomes32,33,34,35. Some authors suggested that subjects with elevated serum IgA/C3 ratio are more likely to be diagnosed with IgAN and to exhibit a worst disease course36,37,38,39,40,41,42. Likewise, the galactose-deficient-IgA1/C3 ratio at the time of diagnosis has been suggested as independent predictor of CKD progression in a Chinese cohort43.
There are limited data regarding the role of serum C4 in IgAN pathogenesis and prognosis. In 2014, Zhu et al. reported that lower C4 levels at diagnosis correlated with worse outcome during follow-up, even though these individuals exhibited less severe lesions at the time of biopsy44. A more recent study conducted on 1356 Chinese IgAN patients indicated that serum C4 correlated positively with uProt and negatively with eGFR at baseline. Furthermore, survival analysis identified higher C4 levels as an independent risk-factor for disease progression. Noteworthy, C4 levels collected at the time of biopsy positively correlated with scoring of tubulointerstitial injury, glomerulosclerosis and crescents according to MEST-C score13. However, the aforementioned studies are limited because C4 was tested (or found to be statistically significant) individually, potentially biasing the contribution of the C3 component in determining renal prognosis.
Our data showed that the C3-hypocomplementemic subpopulation (< 90 mg/dl) had significant lower eGFR, lower C4 levels and higher proteinuria at baseline, suggesting a link between complement activation and more severe kidney injury at diagnosis. This was further confirmed by a significantly higher incidence rate of the primary composite kidney outcome in this group, regardless of immunosuppressant treatment.
The linear regression analyses highlighted a positive correlation between baseline C3 and C4 levels, as previously described by Bi et al., supporting the hypothesis of systemic complement consumption in complement-driven IgAN13. Furthermore, both C3 and C4 levels were found to be negatively correlated with serum IgM, pointing a role for IgM that deserves to be further investigation. This observation aligns with a recent study on 116 pediatric IgAN patients, which showed that mesangial IgM deposition at biopsy seems to be an independent risk factor for poor renal prognosis45.
Both C3 and C4 levels were significantly associated with baseline eGFR, confirming and expanding previous findings13,14.
Logistic regression models showed that the addition of C3 and C4 confers a better prediction accuracy as compared to reference models without these variables. Intriguingly, this was particularly true for goodness of fit analysis (LRT) and discrimination (c-index). These results suggest that incorporating complement levels into the baseline assessment of patients could help to discriminate those who are at higher risk of poor renal outcome. Additionally, the NR2 test indicates that serum complement enhances the percentage of events explained by the models, highlighting its inherent informativeness. Regardless of prognostic measures, another main finding of our study is that C3 and C4 levels were significantly associated with outcome despite the presence of robust variables in the models such as proteinuria and eGFR, that normally account for most of the prediction.
Our data validate previous observations by Pan et al. that decreased C3 and increased C4 levels are associated with poor renal prognosis in IgAN. These findings expand the evidence, for the first time to our knowledge, to a Caucasian-prevalent study population14. Additionally, our study has a longer follow-up period (54 vs 35 months) and a higher incidence of events (12.8% vs 9.8%) compared to the latter, despite smaller sample size (101 vs 403 patients) and harder endpoint (loss of 50% vs 30% of eGFR).
Although complement activation in IgAN is widely described in current literature, the relative contributions of AP and LP are neither clear nor easy to assess. Determining the dominant pathway driving IgAN progression may be useful to guide future tailored therapies.
In general, serum complement levels are influenced by the rate of their production and degradation. This balance varies depending on genetic factors (e.g., inherited C4 deficiency), systemic inflammation (i.e., acute phase reactants), the extent of complement activation, and the pathways through which this activation occurs. Despite the sources of variability mentioned above, it is reasonable to assume that serum levels of both C3 and C4 could provide an indication of complement activation status. Systemic hypocomplementemia suggests intra-renal inflammation, which correlates with poor kidney outcome.
As for the observed discordant correlation between lower C3 and higher C4 levels with an increased risk of outcome, we hypothesize that these results may partially reflect the dominant complement activation pathway. IgAN cases characterized by low-C3 and low-C4 might imply a prevalent activation of the LP (C4-mediated, “lectin-pathic”). Conversely, cases with low-C3 and high-C4 may indicate predominant AP activation (non-C4-mediated, “altern-pathic”), which seems to be associated with a poorer prognosis.
Similarly to what has been suggested in other complement-mediated kidney diseases, such as membranoproliferative glomerulonephritis46, adopting a “cluster-oriented” pathophysiological approach in IgAN could aid in addressing the current unmet medical need for biomarkers and risk stratification.
At present, serum complement C3 and C4 levels could be useful as markers to screen the more ‘Complement-pathic’ subset of patients in IgAN.
Integrating these markers into existing prognostic scores could enhance outcome prediction accuracy and optimize risk stratification in order to choose the best tailored therapies for our patients.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at IRCCS Azienda Ospedaliero-Universitaria di Bologna, 40138 Bologna, Italy.
Abbreviations
- AIC:
-
Akaike information criterion
- AP:
-
Alternative pathway
- BIC:
-
Bayesian information criterion
- BP:
-
Blood pressure
- CKD:
-
Chronic kidney disease
- CS:
-
Complement system
- CP:
-
Classical pathway
- AP:
-
Alternative pathway
- LP:
-
Lectin pathway
- DBP:
-
Diastolic blood pressure
- eGFR:
-
Estimated glomerular filtration rate
- Ig:
-
Immunoglobulin
- IgAN:
-
IgA nephropathy
- IntIgANPT:
-
International IgA nephropathy prediction tool
- IQR:
-
Interquartile range
- KF:
-
Kidney failure
- LRT:
-
Likelihood ratio test
- NR2 :
-
Nagelkerke R square
- RAASi:
-
Renin–angiotensin–aldosterone system inhibitors
- SBP:
-
Systolic blood pressure
- SD:
-
Standard deviations
- SGLT2i:
-
Sodium/glucose cotransporter 2 inhibitors
- uProt:
-
24H-hour urine protein
References
O’Shaughnessy, M. M. et al. Glomerular disease frequencies by race, sex and region: Results from the International Kidney Biopsy Survey. Nephrol. Dial Transplant. 33(4), 661–669 (2018).
Lai, K. N. et al. IgA nephropathy. Nat. Rev. Dis. Primers. 2, 16001 (2016).
Rodrigues, J. C., Haas, M. & Reich, H. N. IgA nephropathy. Clin. J. Am. Soc. Nephrol. 12(4), 677–686 (2017).
Rovin, B. H. et al. Executive summary of the KDIGO 2021 guideline for the management of glomerular diseases. Kidney Int. 100(4), 753–779 (2021).
Bartosik, L. P., Lajoie, G., Sugar, L. & Cattran, D. C. Predicting progression in IgA nephropathy. Am. J. Kidney Dis. 38(4), 728–735 (2001).
Tanaka, S. et al. Development and validation of a prediction rule using the Oxford classification in IgA nephropathy. Clin. J. Am. Soc. Nephrol. 8(12), 2082–2090 (2013).
Chen, T. et al. Prediction and risk stratification of kidney outcomes in IgA nephropathy. Am. J. Kidney Dis. 74(3), 300–309 (2019).
Barbour, S. J. et al. Evaluating a new international risk-prediction tool in IgA nephropathy. JAMA Intern Med. 179(7), 942–952 (2019).
Provenzano, M. et al. Contribution of predictive and prognostic biomarkers to clinical research on chronic kidney disease. Int. J. Mol. Sci. 21(16), 5846 (2020).
Maillard, N. et al. Current understanding of the role of complement in IgA nephropathy. J. Am. Soc. Nephrol. 26(7), 1503–1512 (2015).
Roos, A. et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J. Am. Soc. Nephrol. 17(6), 1724–1734 (2006).
Seelen, M. A., Roos, A. & Daha, M. R. Role of complement in innate and autoimmunity. J. Nephrol. 18(6), 642–653 (2005).
Bi, T. D. et al. Serum complement C4 is an important prognostic factor for IgA nephropathy: A retrospective study. BMC Nephrol. 20(1), 244 (2019).
Pan, M. et al. Increased C4 and decreased C3 levels are associated with a poor prognosis in patients with immunoglobulin A nephropathy: A retrospective study. BMC Nephrol. 18(1), 231 (2017).
Tangri, N. et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: A meta-analysis. JAMA 315, 164–174 (2016).
Uffing, A. et al. Recurrence of IgA nephropathy after kidney transplantation in adults. Clin. J. Am. Soc. Nephrol. 16(8), 1247–1255 (2021).
Anders, H. J., Peired, A. J. & Romagnani, P. SGLT2 inhibition requires reconsideration of fundamental paradigms in chronic kidney disease, “diabetic nephropathy”, IgA nephropathy and podocytopathies with FSGS lesions. Nephrol. Dial Transplant. 37(9), 1609–1615 (2022).
Wheeler, D. C. et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 100(1), 215–224 (2021).
Heerspink, H. J. L. et al. Sparsentan in patients with IgA nephropathy: A prespecified interim analysis from a randomised, double-blind, active-controlled clinical trial. Lancet. 401(10388), 1584–1594 (2023).
Noris, M. & Remuzzi, G. Overview of complement activation and regulation. Semin. Nephrol. 33(6), 479–492 (2013).
Roberts, I. S. Pathology of IgA nephropathy. Nat. Rev. Nephrol. 10(8), 445–454 (2014).
Wu, D. et al. Mesangial C3 deposition and serum C3 levels predict renal outcome in IgA nephropathy. Clin. Exp. Nephrol. 25(6), 641–651 (2021).
Xie, M. et al. Predictive prognostic value of glomerular C3 deposition in IgA nephropathy. J. Nephrol. 36(2), 495–505 (2023).
Wu, J. et al. Severe glomerular C3 deposition indicates severe renal lesions and a poor prognosis in patients with immunoglobulin A nephropathy. Histopathology. 78(6), 882–895 (2021).
Medjeral-Thomas, N. R. et al. Progressive IgA nephropathy is associated with low circulating Mannan-binding lectin-associated serine protease-3 (MASP-3) and increased glomerular factor H-related protein-5 (FHR5) deposition. Kidney Int. Rep. 3(2), 426–438 (2017).
Espinosa, M. et al. Association of C4d deposition with clinical outcomes in IgA nephropathy. Clin. J. Am. Soc. Nephrol. 9(5), 897–904 (2014).
Segarra, A. et al. Mesangial C4d deposits in early IgA nephropathy. Clin. J. Am. Soc. Nephrol. 13(2), 258–264 (2018).
Gharavi, A. G. et al. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat. Genet. 43(4), 321–327 (2011).
Kiryluk, K. et al. Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet. 8(6), e1002765 (2012).
Medjeral-Thomas, N. R. et al. Circulating complement factor H-related proteins 1 and 5 correlate with disease activity in IgA nephropathy. Kidney Int. 92(4), 942–952 (2017).
Tortajada, A. et al. Elevated factor H-related protein 1 and factor H pathogenic variants decrease complement regulation in IgA nephropathy. Kidney Int. 92(4), 953–963 (2017).
Le Stang, M. B., Gleeson, P. J., Daha, M. R., Monteiro, R. C. & van Kooten, C. Is complement the main accomplice in IgA nephropathy? From initial observations to potential complement-targeted therapies. Mol. Immunol. 140, 1–11 (2021).
Kim, S. J. et al. Decreased circulating C3 levels and mesangial C3 deposition predict renal outcome in patients with IgA nephropathy. PLoS One. 7(7), e40495 (2012).
Wyatt, R. J. et al. Complement activation in IgA nephropathy. Kidney Int. 31(4), 1019–1023 (1987).
Zwirner, J. et al. Activated complement C3: A potentially novel predictor of progressive IgA nephropathy. Kidney Int. 51(4), 1257–1264 (1997).
Tomino, Y. et al. Measurement of serum IgA and C3 may predict the diagnosis of patients with IgA nephropathy prior to renal biopsy. J. Clin. Lab. Anal. 14(5), 220–223 (2000).
Maeda, A. et al. Significance of serum IgA levels and serum IgA/C3 ratio in diagnostic analysis of patients with IgA nephropathy. J. Clin. Lab. Anal. 17(3), 73–76 (2003).
Yanagawa, H. et al. A panel of serum biomarkers differentiates IgA nephropathy from other renal diseases. PLoS One. 9(5), e98081 (2014).
Gong, W. Y. et al. High serum IgA/C3 ratio better predicts a diagnosis of IgA nephropathy among primary glomerular nephropathy patients with proteinuria ≤ 1 g/d: An observational cross-sectional study. BMC Nephrol. 20(1), 150 (2019).
Zhang, J. et al. Serum immunoglobulin A/C3 ratio predicts progression of immunoglobulin A nephropathy. Nephrology (Carlton). 18(2), 125–131 (2013).
Komatsu, H. et al. Relationship between serum IgA/C3 ratio and progression of IgA nephropathy. Intern Med. 43(11), 1023–1028 (2004).
Stefan, G. et al. Is there a role for IgA/C3 ratio in IgA nephropathy prognosis? An outcome analysis on an European population. Iran J. Kidney Dis. 14(6), 470–477 (2020).
Chen, P. et al. Plasma galactose-deficient IgA1 and C3 and CKD progression in IgA nephropathy. Clin. J. Am. Soc. Nephrol. 14(10), 1458–1465 (2019).
Zhu, B. et al. Clinical characteristics of IgA nephropathy associated with low complement 4 levels. Ren Fail. 37(3), 424–432 (2015).
Xiong, L., Liu, L., Tao, Y. & Guo, H. Clinical significance of IgM and C3 deposition in children with primary immunoglobulin A nephropathy. J. Nephrol. https://doi.org/10.1007/s40620-023-01724-7 (2023).
Iatropoulos, P. et al. Cluster analysis identifies distinct pathogenetic patterns in C3 glomerulopathies/immune complex-mediated membranoproliferative GN. J. Am. Soc. Nephrol. 29(1), 283–294 (2018).
Author information
Authors and Affiliations
Contributions
Study design: ET, DV; Supervision: OB, MP, GLM; Data collection: FT, BF, GP; Statistical analysis: DV, MP; Data interpretation: ET, OB, FM; Draft writing ET, DV. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tringali, E., Vetrano, D., Tondolo, F. et al. Role of serum complement C3 and C4 on kidney outcomes in IgA nephropathy. Sci Rep 14, 16224 (2024). https://doi.org/10.1038/s41598-024-65857-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-65857-w
- Springer Nature Limited