Abstract
Individuals affected by human immunodeficiency virus (HIV) have a growing demand for coronary artery bypass grafting (CABG) due to heightened risk for cardiovascular diseases and extended life expectancy. However, CABG outcomes in HIV patients are not well-established, with insights only from small case series studies. This study conducted a comprehensive, population-based examination of in-hospital CABG outcomes in HIV patients. Patients underwent CABG were identified in National Inpatient Sample from Q4 2015–2020. Patients with age < 18 years and concomitant procedures were excluded. A 1:5 propensity-score matching was used to address preoperative group differences. Among patients who underwent CABG, 613 (0.36%) had HIV and were matched to 3119 out of 167,569 non-HIV patients. For selected HIV patients, CABG is relatively safe, presenting largely similar outcomes. After matching, HIV and non-HIV patients had comparable in-hospital mortality rates (2.13% vs. 1.67%, p = 0.40). Risk factors associated with mortality among HIV patients included previous CABG (aOR = 14.32, p = 0.01), chronic pulmonary disease (aOR = 8.24, p < 0.01), advanced renal failure (aOR = 7.49, p = 0.01), and peripheral vascular disease (aOR = 6.92, p = 0.01), which can be used for preoperative risk stratification. While HIV patients had higher acute kidney injury (AKI; 26.77% vs. 21.77%, p = 0.01) and infection (8.21% vs. 4.18%, p < 0.01), other complications were comparable between the groups.
Similar content being viewed by others
Introduction
Coronary artery disease (CAD) affects nearly 16.5 million Americans, making it a leading cause of death in the US1. Coronary artery bypass grafting (CABG) stands as a widely-used coronary revascularization procedure, with over 200,000 procedures conducted annually in the US2.
Although the prevalence of human immunodeficiency virus (HIV) infection has risen in recent years, with the advent of effective antiretroviral therapy, the prognosis of individuals living with HIV has significantly improved, transforming HIV into a chronic condition for many patients3. Patients with HIV often have heightened proinflammatory responses and increased platelet reactivity, predisposing them to atherosclerosis development4. Additionally, antiretroviral therapy can elevate the risk of hypertension, hyperlipidemia, and metabolic syndrome, all of which are risk factors for cardiovascular diseases5,6,7,8. Consequently, given the extended life expectancy and heightened risk profiles, individuals with HIV face an elevated risk for CAD, leading to a growing demand for CABG procedures. Indeed, a prior study indicated a significant increase in the prevalence of HIV among cardiac surgery patients, with the majority undergoing CABG9.
The outcomes of CABG in patients with HIV are not well-established. Several small case series studies using institutional data have not identified HIV as a risk factor for mortality and complications post-CABG, but their conclusions are constrained by the limited sample sizes10,11,12. While some larger-scale studies utilizing the National/Nationwide Inpatient Sample (NIS) databases have explored cardiac surgery outcomes in HIV patients, all these studies did not distinguish CABG from other cardiac procedures8,9,13. This lack of distinction is likely attributed to the relatively small cohort of HIV patients in these studies, limiting the applicability of their findings directly to patients undergoing CABG.
This study aimed to conduct a comprehensive population-based analysis of the short-term outcomes of CABG among patients affected by HIV. Additionally, it aimed to identify risk factors associated with mortality in HIV patients after CABG. Consequently, the findings from this research could offer valuable insights for preoperative risk stratification and management of complications in HIV patients undergoing CABG.
Methods
Data source
The unweighted NIS database from the last quarter of 2015–2020 was used to identify patients who underwent CABG via the International Classification of Diseases, 10th Revision, Procedure Coding System (ICD-10-PCS) codes of 0210xxx. Patients who had concomitant aortic valve replacement (ICD-10-PCS 02RFxxx) and mitral valve replacement (ICD-10-PCS 02RGxxx), as well as those below 18 years of age, were excluded. Patients with HIV were identified by Elixhauser measure with International Classification of Diseases, Tenth Revision, Clinical Modification (ICD10-CM) codes of B20, O98.71, O98.72, O98.73, Z2114. Patients with and without HIV were stratified into the two study cohorts.
Preoperative variables
Preoperative variables were compared between patients with and without HIV as shown in Tables 1 and 2. Table 1 includes patients’ sex, age, race and ethnicity, socioeconomic status, primary payer status, hospital characteristics, transfer status, and admission status. The average household income from the patient’s ZIP code was estimated and then used to stratify the patients into four quartiles based on the income of their neighborhood. Hospital characteristics included hospital bed size, location, and teaching status. Hospital bed sizes were stratified into small, medium, and large based on the American Hospital Association’s yearly survey of hospitals as well as the hospital’s location and teaching status. Table 2 includes the patient’s comorbidities and relevant diagnoses. Elixhauser comorbidities are a set of common comorbidities measured in administrative databases14. The patient’s comorbidities were identified by Elixhauser measure as well as by additional ICD-10-CM codes as listed in Table S114.
Postoperative variables
In-hospital outcomes after CABG were examined (Table 3). The outcomes included mortality, major adverse cardiovascular event (MACE), myocardial infarction (MI), stroke, transient ischemic attack (TIA), neurological complications, pericardial complications, pacemaker implantation, cardiogenic shock, respiratory complications, mechanical ventilation, acute kidney injury (AKI), post-procedural renal failure, venous thromboembolism (VTE), pulmonary embolism (PE), hemorrhage/hematoma, infection, sepsis, deep wound complication, superficial wound complication, vascular complication, diaphragmatic paralysis, and reopen surgery for bleeding control. MACE was defined as MI, stroke, postprocedural, cardiogenic shock, postprocedural heart failure, and/or postprocedural cardiac insufficiency. In addition, transfer out rate, time from admission to operation, hospital length of stay (LOS), and total hospital charge were examined. The ICD10-CM/PCS codes were used to define these outcomes, as listed in Table S2.
Statistical analysis
Fisher’s exact test was used to compare the pre-operative variables between patients with and without HIV. To account for the preoperative differences between the HIV and non-HIV cohorts as well as their significant differences in sample sizes, a propensity-score matching was conducted between the two groups in a 1:5 ratio (HIV: non-HIV) using the Greedy Matching algorithm with a 2% caliper. After the matching, Fisher’s exact test was used to compare postoperative outcomes that were binary. Two-tailed independent t-tests were used to compare continuous outcomes.
In addition, among HIV patients, risk factors associated with in-hospital mortality were identified by a multivariable logistic regression model with stepwise backward selection. Multicollinearity tests were then used to confirm the independency of the identified risk factors, indicated by tolerance > 0.20 and condition index < 10.
All statistical analyses were conducted using SAS, version 9.4. A p-value less than 0.05 was defined as statistically significant. The authors had full access to the dataset and took responsibility for the integrity of all analyses. Given this retrospective study used a de-identified NIS dataset, the study was not considered a human-subject study and was exempted from Institutional Review Board (IRB) review at The George Washington University. Informed patient consent was therefore not required. All methods were performed in accordance with the relevant guidelines and regulations of The George Washington University.
Ethics approval
This study was exempt from the IRB approval by The George Washington University as it analyzed retrospective, deidentified NIS data.
Results
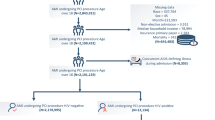
Between the last quarter of 2015 and 2020, 613 (0.36%) patients with HIV underwent CABG, who were matched to 3,119 out of 167,569 patients who did not have HIV.
A comparison between demographic, socioeconomic status, primary payer status, hospital characteristics, transfer status, and admission status between patients with and without HIV who underwent CABG is shown in Table 1. Patients with HIV were more likely to be males (86.62% vs. 75.70%, p < 0.01), have age less than 65 years (55–65 years, 46.17% vs. 29.00%, p < 0.01; less than 55 years, 27.08% vs. 13.66%, p < 0.01), be African American (27.90% vs. 6.88%, p < 0.01), Hispanic (10.60% vs. 7.61%, p = 0.01), or other races (4.40% vs. 2.90%, p = 0.04), have income at the lowest quartile (0–25%) (35.89% vs. 27.45%, p < 0.01), under Medicaid (12.56% vs. 7.79%, p < 0.01), stay in an urban teaching hospital (88.74% vs 81.55%, p < 0.01), have no transfer in/directly admitted (82.59% vs. 77.13%, p < 0.01), and under emergent admission (59.71% vs. 53.62%, p < 0.01). In contrast, patients with HIV were less likely to have age over 65 years (65–75 years, 23.49% vs. 37.38%, p < 0.01; 75–85 years, 3.1% vs. 18.34%, p < 0.01; over 85 years, 0.16% vs. 1.62%, p < 0.01), be Caucasian (52.69% vs. 74.99%, p < 0.01) or Asian (0.65% vs. 3.27%, p < 0.01), have income at the 3rd quartile (50–75%) (17.78% vs. 24.11%, p < 0.01) or the 4th quartile (75–100%) (16.48% vs. 19.67%, p = 0.04), use Medicare (48.12% vs. 54.53%, p < 0.01), stay in an urban private practice (9.79% vs. 15.72%, p < 0.01) or a hospital with small bed size (8.16% vs. 11.43%, p = 0.01), transferred in from a different acute care hospital (14.78% vs. 20.29%, p < 0.01), or under elective admission (39.90% vs. 46.21%, p < 0.01). All differences were addressed by the 1:5 propensity-score matching.
Comorbidities and relevant diagnoses between patients with and without HIV who underwent CABG are summarized in Table 2. Patients with HIV were more likely to have depression (17.46% vs. 8.82%, p < 0.01), drug abuse (7.34% vs. 1.74%, p < 0.01), complicated hypertension (45.68% vs. 35.83%, p < 0.01), advanced renal failure (5.71% vs. 2.38%, p < 0.01), endocarditis (0.65% vs. 0.11%, p = 0.01), anemia (8.32% vs. 4.48%, p < 0.01), tobacco use (55.95% vs. 50.69%, p = 0.01), or previous myocardial infarction (MI; 24.14% vs. 18.19%, p < 0.01). On the other hand, patients with HIV were less likely to have dementia (0.16% vs. 1.06, p = 0.03), diabetes without chronic complications (12.56% vs. 16.83%, p < 0.01), uncomplicated hypertension (41.11% vs. 52.00%, p < 0.01), obesity (17.29% vs. 29.10%, p < 0.01), hypothyroidism (7.01% vs. 11.03%, p < 0.01), atrial fibrillation (22.84% vs. 33.69%, p < 0.01), sick sinus syndrome (0.00% vs. 1.37%, p < 0.01), or sleep apnea (9.95% vs. 15.64%, p < 0.01). The differences in comorbidities and relevant diagnoses were matched by the 1:5 propensity-score matching.
The in-hospital outcomes between patients with and without HIV who underwent CABG were summarized in Table 3. After 1:5 propensity-score matching, HIV and non-HIV patients had comparable mortality rates (2.13% vs. 1.67%, p = 0.40). However, patients with HIV were more likely to have AKI (26.77% vs. 21.77%, p = 0.01) and infection (8.21% vs. 4.18%, p < 0.01). Also, HIV patients had higher rates of transferring out (19.54% vs. 16.27%, p = 0.04) and higher total hospital charges (261,011 ± 337,816 vs. 230,783 ± 241,233 US dollars, p = 0.04). Other risks of complications, time from admission to operation, and LOS were all comparable between the two groups.
The risk factors associated with in-hospital mortality among HIV patients are shown in Table 4. The risk factors included previous CABG (aOR 14.32, 95% CI 2.24–91.63, p = 0.01), chronic pulmonary disease (aOR 8.24, 95% CI 1.91–35.58, p < 0.01), advanced renal failure (aOR 7.49, 95% CI 1.53–36.79, p = 0.01), and peripheral vascular disease (aOR 6.92, 95% CI 1.64–29.25, p = 0.01). Multicollinearity tests on all identified variables showed tolerance > 0.20 and condition index < 10, indicating independency between the risk factors.
Discussion
This study conducted a population-based analysis of the in-hospital outcomes of CABG among patients affected by HIV. Patients with HIV had more demographic/socioeconomic disparities and comorbid burdens. After propensity-score matching, patients with HIV had higher AKI and infection, as well as higher transfer rates and hospital charges. However, mortality and other complications were comparable between HIV and non-HIV patients. Risks associated with in-hospital mortality among HIV patients included previous CABG, chronic pulmonary disease, advanced renal failure, and peripheral vascular disease.
In the US, approximately 1.2 million individuals, representing about 0.3% of the population, are living with HIV15. While care for individuals with HIV has received increased attention, research on CABG outcomes for this group remains very limited, likely due to their low representation in the population which constrains meaningful analysis with adequate statistical power. For instance, previous studies have shown that the prevalence of HIV among those undergoing any cardiac surgery was a mere 0.2%8,9. However, Polanco et al. revealed that the proportion of HIV patients in cardiac surgeries doubled from 0.1 to 0.2% between 2000 and 2010, likely due to the expansion of effective antiviral treatment9. While a previous study found HIV patients to be less likely to undergo CABG13, this study, which used the latest NIS dataset from 2015 to 2020, found the prevalence of HIV among CABG patients to be as high as 0.36%. This significant difference in representation may suggest an ongoing increase in the accessibility of CABG for HIV patients, aligning with the trend found by Polanco et al.9. However, it is also worth considering that the use of the more granular ICD-10 coding system in this study could offer better identification of HIV patients compared to the ICD-9 system used in previous NIS studies8,9.
Patients with HIV have an increased risk of developing cardiovascular diseases by 50–100%16. This elevated risk can be attributed to inherent HIV-related factors such as chronic inflammation and platelet dysfunction as well as side effects from antiretroviral therapies, including hypertension, hyperlipidemia, and metabolic syndrome4,5,6,7,8. For instance, our study revealed a markedly higher prevalence of complicated hypertension among HIV patients by almost one-third. Furthermore, this study found demographic and socioeconomic disparities that might be linked to the development of CAD in HIV-positive individuals. HIV patients were more likely to be African Americans or come from lower-income neighborhoods, both of which have been recognized as independent risk factors for CAD17,18,19. Additionally, the HIV cohort in this study exhibited higher rates of drug abuse and tobacco usage, which are well-established contributors to cardiovascular diseases20,21,22.
Previous small case series studies did not find any differences in short- or mid-term mortality rates between HIV and non-HIV patients undergoing CABG10,11,12. However, these conclusions can be significantly constrained by the small sample sizes, which range from only 5 to 26 HIV patients10,11,12. Larger-scale national registry studies also found no disparities in in-hospital mortality following cardiac surgeries for both cohorts, but these studies did not differentiate CABG from other cardiac procedures8,9,13. This study aligns with these findings, noting no significant mortality differences between the HIV and non-HIV groups. However, the unique strength of this study lies in its expansive, population-based dataset. This allowed for the identification of risk factors associated with in-hospital mortality in HIV patients, which can enhance pre-operative risk stratification for these patients set to undergo CABG.
Although various case series studies found similar short- and mid-term morbidities after CABG for HIV and non-HIV patients10,11,12, Boccara et al. found HIV patients might be at a higher risk for long-term MACE, primarily due to an increased risk for repeated revascularization10. In the present study, most in-hospital complications, including MACE, were comparable between the HIV and non-HIV cohorts. However, HIV patients exhibited elevated risks for AKI and infections. The heightened infection risk among HIV patients after surgery is expected, given their compromised immune response. The predisposition of HIV patients to AKI can be attributed to factors such as immunodeficiency, immune system reconstitution, or the nephrotoxic effects associated with antiretroviral therapy23,24,25,26.
This study has several limitations to acknowledge. Firstly, the NIS, being an administrative database, does not record clinical data including CD4 counts, viral loads, or the usage of antiviral therapies in HIV patients. Also, several factors that can influence revascularization outcomes, such as ejection fraction, coronary segment, stenosis diameter, lesion presence, coronary artery dominance, and small vessel diffusion, are not recorded in the NIS27,28. The absence of the information in the NIS dataset also prevents the calculation of surgical risk calculators like the Society of Thoracic Surgeons (STS) or the European System for Cardiac Operative Risk Evaluation (EuroSCORE) scores. Additionally, the NIS database only has in-hospital outcomes with no follow-ups, limiting the analysis of long-term prognosis among HIV patients after CABG. Nevertheless, the NIS is the most comprehensive all-payer database in the US, accounting for 20% of nationwide hospital discharges. While other large-scale cardiac surgery databases, like the STS databases, provide more granular records, they do not allow the identification of patients with HIV. Hence, the NIS is likely to be one of the few sources with both the statistical power and enough details to investigate CABG outcomes in patients with HIV.
In conclusion, using the NIS database, this study offers a comprehensive, population-based examination of short-term CABG outcomes in HIV patients. For selected HIV patients, CABG is relatively safe, presenting largely similar outcomes with the exceptions of higher AKI and infection rates. Factors associated with in-hospital mortality among HIV patients include prior CABG, chronic pulmonary disease, advanced renal failure, and peripheral vascular disorders, which can enhance preoperative risk stratification for HIV patients. Future population-based research should further explore the long-term prognosis of CABG in HIV-affected individuals.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Change history
01 August 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-68135-x
References
Benjamin, E. J. et al. Heart disease and stroke statistics-2018 update: A report from the American Heart Association. Circulation 137, e67–e492 (2018).
Jacobs, J. P. et al. The society of thoracic surgeons national database 2017 annual report. Ann. Thorac. Surg. 104, 1774–1781 (2017).
Michaels, S. H., Clark, R. & Kissinger, P. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 339, 405–406 (1998).
Hauguel-Moreau, M. et al. Platelet reactivity in human immunodeficiency virus infected patients on dual antiplatelet therapy for an acute coronary syndrome: The EVERE2ST-HIV study. Eur. Heart J. 38, 1676–1686 (2017).
Barbaro, G. & Iacobellis, G. Metabolic syndrome associated with HIV and highly active antiretroviral therapy. Curr. Diab. Rep. 9, 37–42 (2009).
Carr, A. et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS 12, F51-58 (1998).
Pao, V., Lee, G. A. & Grunfeld, C. HIV therapy, metabolic syndrome, and cardiovascular risk. Curr. Atheroscler. Rep. 10, 61–70 (2008).
Robich, M. P. et al. Outcomes of patients with human immunodeficiency virus infection undergoing cardiovascular surgery in the United States. J Thorac Cardiovasc Surg 148, 3066–3073 (2014).
Polanco, A., Itagaki, S., Chiang, Y. & Chikwe, J. Changing prevalence, profile, and outcomes of patients with HIV undergoing cardiac surgery in the United States. Am. Heart J. 167, 363–368 (2014).
Boccara, F. et al. Coronary artery bypass graft in HIV-infected patients: a multicenter case control study. Curr HIV Res 6, 59–64 (2008).
Trachiotis, G. D., Alexander, E. P., Benator, D. & Gharagozloo, F. Cardiac surgery in patients infected with the human immunodeficiency virus. Ann. Thorac. Surg. 76, 1114–1118 (2003).
Wollner, G. et al. Outcomes of coronary artery bypass grafting in patients with human immunodeficiency virus infection. J. Card. Surg. 35, 2543–2549 (2020).
Clement, M. E. et al. Lower likelihood of cardiac procedures after acute coronary syndrome in patients with human immunodeficiency virus/acquired immunodeficiency syndrome. Medicine (Baltimore) 97, e9849 (2018).
Elixhauser, A., Steiner, C., Harris, D. R. & Coffey, R. M. Comorbidity measures for use with administrative data. Med. Care 36, 8–27 (1998).
Basic Statistics | HIV Basics | HIV/AIDS | CDC. https://www.cdc.gov/hiv/basics/statistics.html (2023).
Other Health Issues & Affects for People Living with HIV. HIV.gov https://www.hiv.gov/hiv-basics/staying-in-hiv-care/other-related-health-issues/other-health-issues-of-special-concern-for-people-living-with-hiv
Carnethon, M. R. et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation 136, e393–e423 (2017).
Clark, L. T. et al. Coronary heart disease in African Americans. Heart Dis. 3, 97–108 (2001).
Abdalla, S. M., Yu, S. & Galea, S. Trends in cardiovascular disease prevalence by income level in the United States. JAMA Netw. Open 3, e2018150 (2020).
Akasaki, Y. & Ohishi, M. Cerebrovascular and cardiovascular diseases caused by drugs of abuse. Hypertens. Res. 43, 363–371 (2020).
Inoue, T. Cigarette smoking as a risk factor of coronary artery disease and its effects on platelet function. Tob. Induc. Dis. 2, 2 (2004).
Stallones, R. A. The association between tobacco smoking and coronary heart disease†. Int. J. Epidemiol. 44, 735–743 (2015).
Booth, J. W. & Post, F. A. HIV and the kidney in the acute medical unit. Clin. Med. (Lond) 15, 571–576 (2015).
Herlitz, L. C. et al. Tenofovir nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int. 78, 1171–1177 (2010).
Ibrahim, F. et al. Immunodeficiency and renal impairment are risk factors for HIV-associated acute renal failure. AIDS 24, 2239–2244 (2010).
Li, Y., Shlipak, M. G., Grunfeld, C. & Choi, A. I. Incidence and risk factors for acute kidney injury in HIV Infection. Am. J. Nephrol. 35, 327–334 (2012).
Authors/Task Force members et al. ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European society of cardiology (ESC) and the European association for cardio-thoracic surgery (EACTS)developed with the special contribution of the European association of percutaneous cardiovascular interventions (EAPCI). Eur. Heart J. 35, 2541–2619 (2014).
Zheng, Z. et al. Coronary artery bypass graft surgery and percutaneous coronary interventions in patients with unprotected left main coronary artery disease. JACC Cardiovasc. Interv. 9, 1102–1111 (2016).
Acknowledgements
The authors acknowledge Dr. Richard Amdur, PhD, for giving statistical support for this project.
Funding
This research did not receive any funding from any agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization: R.L.; Methodology: R.L.; Formal analysis: R.L.; Investigation: R.L.; Resources: R.L., D.P., B.C.; Data curation: R.L.; Writing (original draft): R.L.; Writing (review & editing): R.L., D.P., B.C.; Supervision, D.P., B.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the Discussion section. Full information regarding the correction made can be found in the correction for this Article.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, R., Prastein, D.J. & Choi, B.G. Coronary artery bypass grafting outcomes of patients with human immunodeficiency virus: a population-based study of National Inpatient Sample from 2015 to 2020. Sci Rep 14, 14394 (2024). https://doi.org/10.1038/s41598-024-65518-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-65518-y
- Springer Nature Limited