Abstract
In this pilot study, we investigated the utility of handheld ultrasound-guided photoacoustic (US-PA) imaging probe for analyzing ex-vivo breast specimens obtained from female patients who underwent breast-conserving surgery (BCS). We aimed to assess the potential of US-PA in detecting biochemical markers such as collagen, lipids, and hemoglobin, and compare these findings with routine imaging modalities (mammography, ultrasound) and histopathology results, particularly across various breast densities. Twelve ex-vivo breast specimens were obtained from female patients with a mean age of 59.7 ± 9.5 years who underwent BCS. The tissues were illuminated using handheld US-PA probe between 700 and 1100 nm across all margins and analyzed for collagen, lipids, and hemoglobin distribution. The obtained results were compared with routine imaging and histopathological assessments. Our findings revealed that lipid intensity and distribution decreased with increasing breast density, while collagen exhibited an opposite trend. These observations were consistent with routine imaging and histopathological analyses. Moreover, collagen intensity significantly differed (P < 0.001) between cancerous and normal breast tissue, indicating its potential as an additional biomarker for risk stratification across various breast conditions. The study results suggest that a combined assessment of PA biochemical information, such as collagen and lipid content, superimposed on grey-scale ultrasound findings could aid in distinguishing between normal and malignant breast conditions, as well as assist in BCS margin assessment. This underscores the potential of US-PA imaging as a valuable tool for enhancing breast cancer diagnosis and management, offering complementary information to existing imaging modalities and histopathology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Photoacoustics is evolving as a potential clinical triage tool, particularly for its applications in medical imaging of several organs and associated diseases1,2,3,4. This innovative technique combines optical and ultrasound imaging to provide high-resolution images of tissue structures and functions. It leverages the "photoacoustic effect,"5 where sound waves are generated following light absorption, allowing the differentiation of endogenous biochemical components of tissue based on their unique light absorption properties. The breast, an organ with a complex mixture of different components (e.g., lipids, collagen, and hemoglobin)6, is hence a natural target of interest for PA imaging.
Several studies have attempted to investigate differences between normal and malignant breast tissues using PA1,7. However, these studies have mainly used blood vasculature (i.e., structures of the vessels, total blood volume or oxygen saturation) as the sole biomarker for differentiation8,9,10,11, neglecting the other two largest biochemical components — lipids and collagen.
Along with lipids, collagen is one of the major components of the breast stroma12. Collagen is increased in benign sclerosing/fibrotic conditions of the breast12 and overexpressed in breast cancers, facilitating local invasion and distant metastases13. PA imaging of these chromophores is pivotal in understanding the pathophysiology of different breast conditions. Combined with ultrasound (US), US-PA could generate fused US images of anatomy with PA images displaying the biochemical contents of the breast. With better understanding, this could translate into downstream clinical applications such as pre-surgical staging or intra-operative assessment of tumor margins14.
Breast collagen imaging with PA was first described by Goh et al.15. When combined with the imaging of lipids, it can diagnose benign breast conditions such as fat necrosis16. However, these studies were single case reports requiring validation with larger sample sizes. Herein, we investigated and described an ex vivo PA imaging of the breast with three chromophores (i.e., collagen, lipids, total hemoglobin (HbT) in patients of different breast conditions and varying breast densities. PA imaging results were verified with results from routine imaging modalities (e.g., mammograms, ultrasound) and histologic confirmation.
Materials and methods
Study population and participant characteristics
This ex vivo study was approved by the local institutional domain-specific review board (DSRB). All research was performed in accordance with relevant guidelines/regulations of the review board. From August 2020 to August 2021, we prospectively screened and recruited women (aged > 18 years) referred for lumpectomy at the Department of Surgery, National University Hospital, Singapore.
After obtaining written informed consent, 16 women with various background parenchymal densities (4 from Category A – almost entirely fatty, 4 from Category B – scattered fibroglandular tissue, 4 from Category C – heterogeneous dense, and 4 from Category D – extremely dense, Fig. S1) were recruited. We excluded post neoadjuvant chemotherapy cases (NACT) to avoid complications in the data analysis.
Breast composition classification was performed by visual assessment, adhering to the standard practice guidelines. Experienced radiologists conducted the assessments, categorizing the breast density according to the American College of Radiology (ACR) Breast Imaging-Reporting and Data System (BI-RADS) criteria.
Equipment and imaging protocol
After lumpectomy, the excised tissue was oriented by surgeons with silk stitches (Fig. S2) and then rinsed with saline to wash off surface blood. All specimens in this ex vivo study were collected within 10 min of surgery from operating theatre and imaged as soon as possible to preserve blood signals.
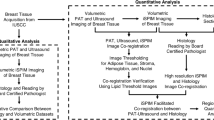
MSOT inVision 512-ECHO (iThera Medical GmbH, Munich, Germany) fitted to a custom-built handheld two-dimensional (2D) optoacoustic probe was used in this study for US-PA imaging. The probe consisted of an arc-shaped (125º angular coverage) curvilinear array of 256 detector elements arranged on a spherical surface (radius 40 mm) with a central frequency of 5 MHz. In comparison with the conventional linear transducer array used in ultrasound imaging, an arc-shaped transducer array provides a larger angular coverage allowing for acquisition of superior photoacoustic image quality. The impact of the probe shape on data acquisition and image quality is thoroughly analyzed in17.
The specimen was placed on a platform that allowed for horizontal, vertical and rotating movements. For imaging the flip side, the specimen was manually flipped. To scan the specimen, the probe was mounted on a computer-controlled stage to touch the thickest portion of the specimen and from thereon moved across and up and down the specimen (placed on a silicone bed in an imaging chamber) through heavy water (D2O) which provided good acoustic coupling (Fig. S2). Depending on the size, the probe was moved across the specimen as many times in steps of 1 mm to cover the entire specimen.
A fiber optic bundle integrated into the probe was used to deliver light in short pulses via a wavelength-tunable optical parametric oscillator with selectable wavelengths (range, 660 – 1300 nm) at a repetition rate of 10 Hz and per-pulse energy of 80 mJ (at 730 nm). The specimen was excited at the following wavelengths: 700 nm, 730 nm, 760 nm, 800 nm, 850 nm, 930 nm, 970 nm, 1000 nm, 1030 nm, 1064 nm and 1100 nm to acquire acoustic data from light absorbing chromophores of the breast including Hb, HbO2, lipid and collagen. The fluence used on the specimen is less than 20 mJ/cm2, the safety limit for skin for NIR nanosecond lasers at 10Hz. During data acquisition, the real-time image generated by the back-projection algorithm was displayed on a preview window.
Acoustic signals were digitized and processed offline using a back-projection algorithm to construct 2D cross-sectional images of the tissue with an in-plane spatial resolution of 150 µm and effective field-of-view (FOV) of 25 × 25 mm. Images, averaging five frames per wavelength, were generated from the data acquired. To avoid tissue damage, the light intensity in the probe was attenuated to be within the maximum permissible exposure limit set by the American National Standards Institute.
After the US-PA scan, the specimen was placed in formalin and delivered to the laboratory for histopathologic examination.
Histopathological examination and juxtaposition with PA imaging
The plane of scan acquisition was conveyed to the pathologist, who sectioned the specimen accordingly to perform routine hematoxylin and eosin (H&E) staining to ensure accurate US-PA and histologic correlation. After post-processing, the radiologist, PA scientist and pathologist would gather in a multi-disciplinary meeting to ascertain PA-pathology correlation by identifying the index/suspicious lesion and its relationship with adjacent margins.
Image reconstruction and color representation
Images acquired at each wavelength were reconstructed offline using the default options (back-projection algorithm, cut-off frequencies at 0.5 kHz to 6.5 MHz) on ViewMSOT 3.8. Spectral unmixing was achieved by the default linear regression algorithm in View MSOT 3.8 for Hb, HbO2, lipids and collagen. Collagen and lipid were unmixed based on the acquired wavelengths of the entire spectral range (700 – 1100 nm) whereas Hb and HbO2 signals were calculated from a sub-range (700–850 nm) as it offers more accurate unmixing than higher wavelengths where the absorptivity of water is higher. The colour maps for the chromophores are as follows: lipids, green; collagen, magenta; total hemoglobin (HbT), red. As part of training, US-PA images of the first participant in each category were assessed by two readers who were unblinded to routine imaging and histopathologic findings in order to optimize image processing and acquisition: YG (breast radiologist with 6 years’ experience in PA) and GB (8 years’ experience in clinical PA imaging). Training (here) refers to the process of learning and optimizing how to interpret and process photoacoustic images for different tissue categories. This training is required for each category because this study is the first of its kind, with uncertainty on how the images will appear for each different category of tissue.
By examining the images from the first patient and comparing them to the final histology results, we gained valuable insights to refine our image processing and acquisition techniques for future cases, ensuring more accurate and reliable results as we continued the study. The remaining 12 were assessed in a blinded manner by the same two readers with discordant cases to be settled by a third reader. The data from these 12 specimen were used for analysis.
PA data and statistical analysis
Subsequently, US-PA images for the remaining 12 participants were analysed and grouped into 4 categories as per American College of Radiology BI-RADS (Breast Imaging Reporting and Data System) criteria upon reviewing their mammograms. These categories are based on the density of the background breast parenchyma, namely: (A) entirely fatty, (B) scattered fibroglandular tissue, (C) heterogeneously dense, and (D) extremely dense.
The data of the first participant in each category were used for training and model optimization. The US-PA images for each participant were then matched with the histopathology of the respective specimen based on the primary index mass/cancer or the localization clip (in post-chemotherapy cases) and its relationship with adjacent margins by a multi-disciplinary team consisting of a radiologist, pathologist, and a post-doctoral US-PA imaging researcher.
Data analysis on unmixed PA images was performed using Image J after thresholding to remove negative pixels across the stack of spectrally unmixed images. HbT was calculated by adding the distribution maps of Hb and HbO2. Regions of interest (ROI) were drawn on normal breast tissues as noted on the ultrasound images. These ROIs were transferred to the system generated co-registered PA distribution maps of lipid, collagen and HbT to measure the chromophore intensities. A total of 5 ROIs (10 × 10 pixels) were drawn across three slices of each specimen to measure the average intensities of lipids, collagen, and blood and to ensure representative breast tissue sampling (Fig. S4A,B). A total of 3 ROIs (30 × 30 pixels) were drawn across three slices of each participant’s specimen to measure the percent area occupied by lipids and collagen in each of the specimens (Fig. S4C,D). Signal intensities were expressed in arbitrary units (a.u ± standard error mean (SEM), and distribution was expressed in percent.
For cases where lesions of interest (e.g., breast cancers, fibrosis, skeletal muscles) are within the achievable depth of PA imaging, to investigate any differences in chromophores’ distribution and signal intensities as compared to normal breast tissue, 3–5 ROIs of 10 × 10 pixels were drawn within the lesion. This process was guided by both ultrasound and histopathology as ultrasound images by themselves were not enough to differentiate between the various lesions. As this was primarily a pilot proof-of-concept study, no sample size calculation was performed.
Graphs were plotted and statistical analyses were performed using Graphpad Prism 6. To assess the statistical significance of differences observed between groups (categories A–D or normal, scarring, and cancerous), a one-way analysis of variance (ANOVA) was performed, followed by post-hoc pairwise comparisons using Tukey's test. The one-way ANOVA allowed us to evaluate overall group differences, while Tukey's test enabled us to identify specific pairwise differences between groups.
Results
Patient characteristics
Baseline characteristics, histopathology of breast tumors, and size of lumpectomy specimens from 12 participants (mean age: 59.7 ± 9.5 years) included in the analysis are shown in Table 1.
US-PA image interpretation for normal tissue across different breast density categories
US-PA imaging provided the tomographic US image and the photoacoustic distribution maps of collagen (magenta), lipid (green), and hemoglobin (red) in each specimen.
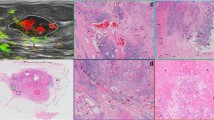
Figure 1 showcases the characteristics of breast tissue in Category A—almost entirely fatty as observed on different imaging modalities like in vivo mammogram (Fig. 1A), specimen ultrasound image (Fig. 1B), specimen histology (Fig. 1C), specimen PA imaging demonstrating the distribution of HbT (Fig. 1D) and overlaid lipids and collagen (Fig. 1E). Normal breast tissues of this category appeared to have continuous and intense lipid signals on PA-derived lipids distribution map (Fig. 1E). The occasional presence of traversing linear blood vessels was also noted on PA-derived HbT distribution map (Fig. 1D), but there was almost no collagen signal present, as verified with mammographic and histological findings. ROIs drawn for lipids and collagen gave an average signal intensity of 5276 a.u (SEM, 438.3; 95% CI [4392, 6159]) and 6.7 a.u (SEM, 1.2; 95% CI [4.2, 9.2]), respectively. As for distribution, lipids were noted to cover 95.66% (95% CI [91.8, 99.3]) of the area on average, while collagen covered approximately 24.55% (95% CI [13.84, 35.27]) of the area.
Multimodal imaging of a grade 2 invasive carcinoma of no special type (Category A) from a 61-year-old female. (A) Mammogram showing a spiculated mass (arrow) with involvement of the nipple areolar complex and the surrounding breast parenchyma being almost entirely fatty (*) falling into BIRADS Category A. Patient underwent neo-adjuvant chemotherapy which reduced the tumour size. (B) Post chemo, ex-vivo US (corregistered with PA images D-E) showing an irregular hypoechoic mass with spiculated margins (arrows). (C) Haematoxylin and eosin (H&E) stained microscopic image of tumour showing non-neoplastic breast tissue between the tumour (arrows) and the scanned margin, comprising mostly adipose tissue (green dotted circled area) admixed with a minor component of fibrous tissue likely secondary to post-chemotherapy changes (purple dotted circled areas). (D) PA image of the total hemoglobin (HbT) distribution map corresponding to (C) showing the presence of occasional traversing of linear blood vessels indicated by white arrow. (E) Composite PA image showing the distribution of lipid (green) and collagen (magenta). Collagen and lipid marked by purple and green dotted circle respectively correspond to the collagen and lipid marked by purple and green dotted circle on image (C). Scale bars C, 10 mm, scale bars (B, D-E), 5 mm.
Figure 2 showcases the characteristics of breast tissue in Category B — scattered fibroglandular tissue as observed on different imaging modalities like in vivo mammogram (Fig. 2A), specimen ultrasound image (Fig. 2B), specimen histology (Fig. 2C), specimen PA imaging demonstrating the distribution of HbT (Fig. 2D) and overlaid lipids and collagen (Fig. 2E). Normal breast tissue of this category appeared with abundant lipid signals, which were occasionally disrupted with linear or curvilinear strands of collagen (Fig. 2E). These strands correlated well with the normal fibrous stroma of the breast as noted on the histopathology (Fig. 2C). The appearance of collagen within the fibrous stroma has a similar appearance to that noted on the mammogram. ROIs drawn for lipids and collagen gave an average signal intensity of 3518 a.u (SEM, 202.8; 95% CI [3109, 3927]) and 9.3 a.u (SEM, 1.2, 95% CI [6.8, 11.8]), respectively. As for distribution, lipids were noted to cover 77.61% (95% CI [71.51, 83.71]) of the area, while collagen covers approximately 39.98% (95% CI [25.62, 54.33]) of the area on average.
Multimodal imaging of a grade 1 invasive carcinoma of no special type (Category B) from a 51-year-old female. (A) Mammogram showing the biopsy cavity with gas locules in the right outer breast from known underlying malignancy (arrow) and the surrounding breast parenchyma consisting of scattered fibroglandular tissue (*) falling in BIRADS Category B. Patient underwent hookwire guided wide local excision. (B), Ex-vivo US (coregistered with PA images D and E) confirmed excision of the index breast cancer as represented by a markedly hypoechoic mass with slight irregular margins (arrow). Central echogenic focus within the mass represents the hookwire. (C) H&E stained microscopic image of tumour showing the presence of non-neoplastic breast tissue between the tumour (black arrow) and the scanned margin, comprising mostly adipose tissue (green dotted circled area) admixed with a minor component of fibrous tissue containing breast lobules and ductules (purple dotted boxed area). (D) PA image of the total hemoglobin (HbT) distribution map corresponding to (C) shows expected thin layer of blood on specimen surface and few tiny traversing blood vessels. (E) Composite PA image showing the distribution of lipid (green) and collagen (magenta). Collagen and lipid marked by purple dotted box and green dotted circle respectively correspond to the collagen and lipid marked by purple dotted box and green dotted circle on image (C). Scale bars C, 10 mm, scale bars (B, D-E), 5 mm.
Figure 3 showcases the characteristics of breast tissue in Category C — heterogeneously dense breast tissue as observed on different imaging modalities like in vivo mammogram (Fig. 3A), specimen ultrasound image (Fig. 3B), specimen histology (Fig. 3C), specimen PA imaging demonstrating the distribution of HbT (Fig. 3D) and overlaid lipids and collagen (Fig. 3E). Normal breast tissue of this category no longer shows the dominant lipid signals as compared to categories A and B. The collagen in Category C (Fig. 3E) is noted to be more clumped instead of the thin patterns in Category B. The ratio of lipids to collagen was also noted to decrease as compared to categories A and B. ROIs drawn for lipids and collagen gave an average signal intensity of 1625 a.u (SEM, 254, 95% CI [1112, 2137]) and 15.65 a.u (SEM, 1.6, 95% CI [12.4, 18.9]), respectively. As for distribution, lipids were noted to cover 52.23% (95% CI [40.51, 63.95]) of the area, while collagen covers approximately 54.77% (95% CI [44.63, 64.90]) of the area on average.
Multimodal imaging of a high grade ductal carcinoma in-situ (DCIS) (Category C) from a 64-year-old female. (A) Mammogram showing a dense mass occupying the retroareolar region of the right breast (dotted box) and the surrounding breast parenchyma being heterogeneously dense (*), falling into Category C. (B) Ex-vivo US showing a cluster of dilated ducts filled with solid content (dotted box). (C) H&E stained microscopic image corresponding to (B) showing extensive in-situ tumour (purple dotted boxed area), featuring multiple dilated ducts with minimal surrounding stromal reaction and some non-neoplastic breast tissue near the scanned margin (green dotted circled area), comprising a mixture of adipose tissue and fibrous tissue. The loose fibrous tissue in Category C is slightly denser and occupies a larger area as compared to Category B. (D) PA image of the total hemoglobin (HbT) distribution map corresponding to (C). The HbT intensity is higher and thicker than expected for normal blood/bleeding at surface of specimen and is seen to be around the known tumor (purple dotted boxed area). Overall findings are in keeping with increased peritumoral vascularity. Note the intensity of HbT is markedly lower in the area of non-neoplastic breast tissue (green dotted circle) as compared to its surroundings. (E) Composite PA image showing the distribution of lipid (green) and collagen (magenta). Collagen and lipid marked by purple dotted box and green dotted circle respectively correspond to the collagen and lipid marked by purple dotted box and green dotted circle on image (C). Scale bars C, 10 mm, scale bars (B, D-E), 5 mm.
Figure 4 showcases the characteristics of breast tissue in Category D —extremely dense breast tissue as observed on different imaging modalities like in vivo mammogram (Fig. 4A), specimen ultrasound image (Fig. 4B), specimen histology (Fig. 4C), specimen PA imaging demonstrating the distribution of HbT (Fig. 4D) and overlaid lipids and collagen (Fig. 4E). Normal breast tissue of this category showed abundant collagen signals as the dominant chromophore on PA image. Little fat was noticeable, and the areas of collagen signals are conglomerated with little interspersing fat (Fig. 4E). ROIs drawn for lipids and collagen gave an average signal intensity of 1017 a.u (SEM, 106, 95% CI [801, 1232]) and 20.11 a.u (SEM, 2.68, 95% CI [14.69, 25.54]), respectively. As for distribution, lipids were noted to cover 50.83% (95% CI [41.02, 60.64]) of the area, while collagen covers approximately 75.78% (95% CI [66.14, 85.42]) of the area on average.
Multimodal imaging of a grade 3 invasive carcinoma of no special type (Category D) from a 50-year-old female. (A) Mammogram showing a lobulated dense breast mass (arrows) in the central breast and the surrounding breast parenchyma is extremely dense (*) falling into Category D. (B) Ex-vivo US confirming the excision of the index breast cancer as represented by a lobulated hypoechoic mass (arrows). (C) H&E stained microscopic image of tumour corresponding to B revealing densely arranged non-neoplastic breast tissue between the tumour and the scanned margin, comprising relatively more fibrous tissue (purple dotted boxed areas) as compared to adipose tissue. (D) PA image of the total hemoglobin (HbT) distribution map corresponding to (C) shows thin layer of expected blood at surface of excised specimen. (E) Composite PA image showing the distribution of lipid (green) and collagen (magenta). Collagen and lipid marked by purple dotted boxes corresponding to the collagen and lipid marked by purple dotted boxes on image (C). Scale bars C, 10 mm, scale bars (B, D-E), 5 mm.
US-PA image interpretation for abnormal breast tissues (i.e., breast cancers or regions of fibrosis)
For specimens with breast cancers, ROIs were also drawn within the cancers. Of the 12 lumpectomy specimens obtained for breast cancers, there was no signal obtained within 3 of the cancers. One of the specimens had a complete pathologic response to neo-adjuvant chemotherapy, another specimen had a deep-seated tumor that was out of the achievable depth of PA imaging, and the last specimen had imaging artifacts from a metal wire (i.e., hookwire), which was used for guided localization prior to lumpectomy. These characteristics of the tumor are summarized in Table S1.
For the other 9 cancers, the ROIs drawn for collagen and hemoglobin gave an average signal intensity of 21.03 a.u (SEM, 2.6 a.u, 95% CL [15.82,26.23]) and 0.15 a.u (SEM, 0.03 a.u, 95% CI [0.09,0.22]), respectively. A representative specimen is presented in Fig. S5. For areas of scarring/fibrosis, ROIs were drawn within the areas of fibrosis for collagen and hemoglobin. These gave an average signal intensity of 16.86 a.u (SEM, 3.012, 95% CI [10.64, 23.07]) and 0.18 a.u (SEM, 0.02 a.u, 95% CI [0.13, 0.22]) for collagen and hemoglobin, respectively, in comparison with the average collagen signal intensity of 12.67 a.u (SEM, 0.95 a.u, 95% CI [10.79,14.55]) and HbT signal intensity of 0.11 a.u (SEM, 0 a.u., 95% CI [0.09,0.12]) for normal tissue. A representative specimen is presented in Fig. S6.
Semiquantitative analysis of PA-derived chromophores across the specimens
To better understand the relevance of PA features for clinical translation, we did a semiquantitative analysis of PA-derived lipids, collagen and HbT across different breast densities in the specimen's normal, fibrotic and cancerous regions. A one-way ANOVA analysis showed the intensities of PA-derived lipids decreased with increasing breast densities, with a significant difference (P < 0.001) in their means (Fig. 5A). Tukey’s multiple comparison tests showed a significant difference between the individual categories (P < 0.001), except for categories C and D. The intensities of PA-derived collagen increased with increasing breast densities, with a significant difference (P < 0.001) in their means upon analysis by one-way ANOVA test (Fig. 5B). However, Tukey’s multiple comparison tests showed significant differences between categories A and C (P < 0.01), categories A and D (P < 0.001), and categories B and D (P < 0.001), but not for the rest of the pairs.
Quantification of tissue/tumor parameters compared across various categories of breast densities (Cat A-D). (A) Mean intensities of lipid distribution on PA images showing significant decreasing trend across breast tissues of increasing density (categories A-D). (B) Mean intensities of collagen distribution on PA images showing significant increasing trend across breast tissues of increasing density (cat A-D). Mean area occupied by lipids (C) and collagen (D) showed similar trends to that of mean lipids and collagen intensities but with varying significance. (E) Mean collagen intensities plotted across normal, scarring and cancer tissues and their corresponding HBT (F) values. ns—P > 0.05, *—P < 0.05, **—P < 0.01, ***—P < 0.001.
We also looked at the area occupied by PA-derived lipids and collagen across various categories of breast densities. The area occupied by lipids decreasing with increasing breast densities, with a significant difference (P < 0.001) in their means (Fig. 5C). Upon Tukey’s multiple comparison tests, we noted that there were significant differences between each pair of breast density categories (Category A vs. B, P < 0.01, rest of the pairs, P < 0.001) but not between categories C and D. Likewise, by investigating the area occupied by PA-derived collagen, we noted that the collagen area increased as the breast density increased with a significant difference (P < 0.001) across all 4 categories (Fig. 5D). Post-hoc analysis using Tukey’s multiple comparisons revealed a significant difference (P < 0.001) between categories A and C, categories A and D, and categories B and D. The rest of the pairs showed no significant difference in their means.
On comparing collagen (Fig. 5E) and HbT (Fig. 5F) intensities across the normal, fibrotic and tumorous regions in the different categories, we noted significant differences in their means upon one-way ANOVA analysis. Post-hoc comparison using Tukey’s multiple comparison tests showed that there was a significant difference in collagen values between normal and tumorous tissue (P < 0.001) and HbT values between normal and fibrotic tissues (P < 0.05). In contrast, no significant differences were found between the remaining pairs.
Discussion
Herein, we demonstrated that lipids and collagen distribution seen on PA images concur with what routine imaging modalities (i.e., mammography) and histopathologic examination show. Lipids' intensity and area/distribution decrease as breast density increases from category A to D. Conversely, collagen's intensity and area/distribution increase as breast density increases from category A to D, aligning well with mammographic breast density categories. While one-way ANOVA statistical analysis picked up significant differences in the mean intensity and area/distribution of lipids and collagen distribution in all breast density categories, post-hoc Tukey’s multiple comparison tests showed significant differences between most pairs (Fig. 5). The likely reason for this is due to the ex vivo nature of this study. In a breast lumpectomy, only the breast tumor and a small area of surrounding normal tissue is surgically removed. In a normal breast, the intensity and distribution of chromophores are likely to be non-uniform. This is well demonstrated by studies showing that breast tissue is typically most concentrated in the upper outer quadrants compared to the rest of the breast18. Hence, lumpectomy specimens obtained in different quadrants of the breast would give different results for both intensity and area/distribution of chromophores. Nonetheless, our study results demonstrate a visible trend for both intensities and distribution of lipids and collagen chromophores progressing from categories A to D. These findings are more pronounced when comparing slightly distant categories (e.g., Category A vs. C and B vs. D) due to the reduction in the overlap between closely opposed categories and concur with mammographic and histopathologic findings.
The verification of these PA findings could serve as a milestone for future PA breast studies. In our opinion, the results from this study demonstrate that PA signal intensities of individual chromophores (i.e., collagen and lipids) in breast tissue should not be read or interpreted alone without the knowledge of the background parenchymal density category obtained from routine imaging modalities. For example, the signal intensity of collagen in normal breast tissue can vary from 0 to 75.54 a.u progressing from categories A to D. Similarly, the lipids to collagen ratio/distribution should not be interpreted alone as there is also a vast difference across different breast densities.
With the understanding of these heterogeneous values and patterns across different breast densities, we proceeded to use this as a benchmark and measured and compared these values with those of other breast conditions (i.e., benign (normal + fibrosis) vs. malignant breast lesions). We found that collagen signal intensities in cancerous breast tissue (21.03 a.u) were ~ 66% higher than in normal breast tissue (12.67 a.u) with statistical significance (p < 0.001). This finding is interesting as collagen could be used as an additional biomarker to diagnose breast cancers accurately. Herein, we are not able to suggest whether breast density is related to malignancy as this is a complex and contentious issue within the field of breast imaging. While some studies have suggested a potential correlation between higher breast density and an increased risk of malignancy9, others have found that the association may not be as marked as it was thought to be10,11. Addressing the potential causal link between breast density and malignancy would necessitate a different study design, likely involving a larger cohort and longitudinal data to assess the progression from dense breast tissue to malignant transformation.
As breast cancers are known to demonstrate increased angiogenesis, known biomarkers such as blood/Hb or oxygen saturation (sO2) have been well studied in vivo7. However, the pitfall of using blood biomarkers is the potential to misdiagnose slow-growing breast cancers such as Ductal Carcinoma in-situ (DCIS). The combination of collagen with blood biomarkers could theoretically overcome this pitfall and reduce the number of false negative results using Hb or sO2 as the only biomarker. However, not to forget the challenges in translating collagen imaging in in vivo settings. Firstly, melanin on the skin will limit the light absorbed by other chromophores below the skin. Secondly, while the use of D2O as a couplant in the handheld probe for in vivo can mitigate the masking of signals from water, there are a few things to be tested to extract collagen signals efficiently – 1) use many wavelengths across 700 nm to 1100 nm to unmix for collagen, 2) focus on/include the longer wavelengths like 1200 nm where the collagen absorption is at its peak compared to water or heavy water.
In addition, we also demonstrated that benign conditions such as stromal fibrosis show significantly (P < 0.05) higher HbT signal intensity (mean, 0.18 a.u; SEM, 0.02 a.u) as compared to normal breast tissue (mean, 0.11 a.u; SEM, 0.01 a.u). This is unsurprising as these benign fibrotic conditions are proliferative with increased microvascular supply of tiny capillaries19. However, the HbT signal intensity of breast cancers (mean, 0.15 a.u; SEM, 0.03 a.u) was higher than in normal breast tissue but lower than in benign fibrotic conditions. This finding is atypical as breast malignancies are known to have increased angiogenesis, and the authors hypothesize that the ex vivo nature of this study likely explains the results. As breast cancers are typically supplied by larger feeding vessels with fast flow, the blood signal would be expected to drop significantly upon excision due to the lack of active blood flow. However, blood signals may be retained within benign fibrotic/scar tissue due to the smaller size of blood vessels (i.e., tiny capillaries) and slower flow, respectively. These findings could be further investigated and validated in future in vivo studies.
In previous in-vivo studies1,7, predictions on whether a histology is benign or malignant have been based on photoacoustic patterns of blood. These predictions are typically correlated with final histopathology results obtained through small needle biopsies. In contrast, this ex-vivo study evaluates the entire excised breast tissue, thus differing from the limited tissue samples obtained via needle biopsies. Additionally, the PA images we generated were nearly identical to pathology images, allowing us to evaluate not only the breast lesion of concern but also the surrounding tissues. Furthermore, our study explores new chromophores such as lipid and collagen, in addition to hemoglobin. This would also be particularly useful in the intra-operative setting for assessing breast margins during lumpectomy. In the ex vivo setting, there is the presence of bleeding due to surgery, and active metabolization starts to cease upon excision of breast cancer. Hb as a biomarker hence becomes less reliable in this setting, and the importance of other chromophores (i.e., lipids and collagen) begin to show in these situations. In the clinical setting, the addition of these biomarkers on top of gray-scale US may provide additional confidence in the intra-operative assessment of breast cancers and potentially reduce the rates of positive margin after BCS and future re-operation for patients20.
This study is not without its limitations. Firstly, the sample is small, with only 12 patients. As a pilot study, the study was performed primarily as a proof-of-concept. Future studies with larger sample sizes will have to be performed to validate these findings. Next, the imaging depth obtained from this study has a maximum of 5 mm. Hence, the validation of PA findings with histopathology remains limited to the depths/range of PA imaging. As this is a proof-of-concept study, an off-the-shelf imaging probe was used, and this may not be the optimal probe for breast cancer imaging application. While the arc-shaped transducer offers superior photoacoustic image quality, we acknowledge that ultrasound image quality may be compromised to some extent compared to a linear array. In our ongoing efforts to optimize imaging probes, we plan to develop a hybrid-shape transducer that capitalizes on the strengths of both linear arrays and arc arrays21.
The new hybrid-shape transducer aims to address this compromise by leveraging lower centre frequency transducers for high-quality photoacoustic imaging and higher centre frequency transducers for high-quality ultrasound imaging. By combining the advantages of both types of transducers, we anticipate achieving improved overall imaging performance and versatility for a range of clinical applications.
Conclusion
In conclusion, this study provides strong evidence that PA imaging effectively captures the distribution of lipids and collagen in breast tissue, yielding results consistent with mammograms and histopathologic findings. These findings provide valuable insights into the diverse composition of breast tissue across different density categories. While individual analysis of lipids, collagen, and blood distribution may not be sufficient for accurate differentiation, their combined assessment may show potential in distinguishing benign breast conditions from malignant ones and in evaluating positive and negative margins in BCS. By harnessing the capabilities of PA imaging, personalized and precision medicine approaches can be enhanced, leading to more targeted and effective interventions. Continued exploration of PA imaging techniques and their integration with other diagnostic modalities hold great promise for advancing the field of breast cancer management.
Data availability
The data generated in this study are available upon request from the corresponding author.
References
Diot, G. et al. Multispectral optoacoustic tomography (MSOT) of Human breast cancer. Clin. Cancer Res. 23(22), 6912–6922 (2017).
Kim, J. et al. Multiparametric photoacoustic analysis of human thyroid cancers in vivo. Cancer Res. 81(18), 4849–4860 (2021).
Kothapalli, S.-R. et al. Simultaneous transrectal ultrasound and photoacoustic human prostate imaging. Sci. Transl. Med. 11(507), eaav2169 (2019).
Roll, W. et al. Multispectral optoacoustic tomography of benign and malignant thyroid disorders: a pilot study. J. Nucl. Med. 60(10), 1461–1466 (2019).
Bell, A. G. The production of sound by radiant energy. Science 2(49), 242–253 (1881).
van Huizen, L. M. G. et al. Second and third harmonic generation microscopy visualizes key structural components in fresh unprocessed healthy human breast tissue. J. Biophoton. 12(6), e201800297 (2019).
Neuschler, E. I. et al. A pivotal study of optoacoustic imaging to diagnose benign and malignant breast masses: a new evaluation tool for radiologists. Radiology 287(2), 398–412 (2018).
Kruger, R. A. et al. Dedicated 3D photoacoustic breast imaging. Med. Phys. 40(11), 113301 (2013).
Lin, L. et al. Single-breath-hold photoacoustic computed tomography of the breast. Nat. Commun. 9(1), 2352 (2018).
Schoustra, S. et al. Twente Photoacoustic Mammoscope 2: system overview and three-dimensional vascular network images in healthy breasts. J. Biomed. Opt. 24(12), 121909 (2019).
Toi, M. et al. Visualization of tumor-related blood vessels in human breast by photoacoustic imaging system with a hemispherical detector array. Sci. Rep. 7(1), 41970 (2017).
Martinez-Hernandez, A., Francis, D. J. & Silverberg, S. G. Elastosis and other stromal reactions in benign and malignant breast tissue: an ultrastructural study. Cancer 40(2), 600–606 (1977).
Xu, S. et al. The role of collagen in cancer: from bench to bedside. J. Transl. Med. 17(1), 309 (2019).
Goh, Y. et al. Multispectral optoacoustic tomography in assessment of breast tumor margins during breast-conserving surgery: a first-in-human case study. Clin. Breast Cancer 18(6), e1247–e1250 (2018).
Goh, Y. et al. Biochemical “decoding” of breast ultrasound images with optoacoustic tomography fusion: first-in-human display of lipid and collagen signals on breast ultrasound. Photoacoustics 27, 100377 (2022).
Goh, Y. et al. Photoacoustic tomography appearance of fat necrosis: a first-in-human demonstration of biochemical signatures along with histological correlation. Diagnostics 12(10), 2456 (2022).
Yang, J., Choi, S. & Kim, C. Practical review on photoacoustic computed tomography using curved ultrasound array transducer. Biomed. Eng. Lett. 12(1), 19–35 (2022).
Chan, S. et al. Evaluation of the association between quantitative mammographic density and breast cancer occurred in different quadrants. BMC Cancer 17(1), 274 (2017).
Nassar, L. et al. Stromal fibrosis of the breast: a spectrum of benign to malignant imaging appearances. Radiol. Res. Pract. 2019, 5045908 (2019).
Goh, Y. et al. Ultrasound guided optoacoustic tomography in assessment of tumor margins for lumpectomies. Transl. Oncol. 13(2), 254–261 (2020).
Merčep, E., Deán-Ben, X. L. & Razansky, D. Combined pulse-echo ultrasound and multispectral optoacoustic tomography with a multi-segment detector array. IEEE Transact. Med. Imag. 36(10), 2129–2137 (2017).
Acknowledgements
The authors acknowldge the following funding agencies for their support to this research: Agency for Science, Technology and Research (A*STAR), for the intramural funding support, Central Research Fund (UIBR) 2021 and National Research Foundation, Singapore for awarding Translational Biophotonics Innovation Platform IAFPP (H19H6a0025) and National Medical Research Council for ClinicianScientist individual research grant- new investigator grant (CS-IRG-NIG).
Author information
Authors and Affiliations
Contributions
Y.G.G, G. B and H.M,T conceptualized the application of intrinsic biomarkers for breast imaging. Y.G.G performed clinical ultrasound imaging, coordinated imaging across various modalities, wrote the manuscript taking inputs from others. G.B. executed the PA imaging. RZ designed the imaging setup. G.B. analysed the data and correlated with other imaging modalities with Y.G.G. GB wrote the PA portions of the manuscript in discussion with RZ. H.M.T performed the pathology analysis and together with T.C wrote the pathology aspect of the manuscript. M.H, S.A.B, C.W.Q.N, S.A.L, E. F provided specimens for the imaging. Q. S. T and M.O supervised the work. All authors proof read the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Goh, Y., Balasundaram, G., Tan, H.M. et al. Ultrasound-guided photoacoustic (US-PA) tomography of the breast: Biochemical differentiation using intrinsic tissue markers—lipids, collagen and hemoglobin with histopathologic correlation. Sci Rep 14, 18054 (2024). https://doi.org/10.1038/s41598-024-65114-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-65114-0
- Springer Nature Limited