Abstract
Moderately elevated albuminuria (30–300 mg/g) is a marker of renal dysfunction and a risk factor of cardiovascular disease. Additionally, several recent studies have reported a relationship between moderately elevated albuminuria and triglyceride (TG) levels. Therefore, we aimed to evaluate the relationship between the urine albumin-to-creatinine ratio (UACR) and total cholesterol (TC), TG, and high-density lipoprotein C (HDL-C) levels. We analyzed data from 19,340 patients from the 2011–2014 and 2019–2020 from the Korea National Health and Nutrition Examination Surveys. Multivariate linear regression analysis showed that the UACR was positively associated with TC and TG levels and negatively associated with HDL-C levels in both Korean women and men. These results were reanalyzed according to the degree of proteinuria (normal, moderately elevated albuminuria, and severely elevated albuminuria (≥ 300 mg/g)). We found a positive relationship between UACR and TC and TG levels, but a negative association with HDL-C levels, except for TC (moderately elevated albuminuria) and HDL-C (moderately elevated albuminuria) in Korean men and TC (severely elevated albuminuria), TG (severely elevated albuminuria), and HDL-C (normal range albuminuria) in Korean women. The correlation between albuminuria and lipid profiles became more evident as albuminuria shift from normal to the severely elevated albuminuria. Thus our multivariate linear regression analysis showed that lipid profiles (TG, TC, and HDL-C levels) were associated with the UACR.
Similar content being viewed by others
Introduction
An elevated urine albumin creatinine ratio (UACR) is a marker of renal dysfunction and an independent predictor of cardiovascular disease1,2. In addition, several studies have shown that moderately increased albuminuria (UACR < 30 mg/g) within the accepted normal range is associated with higher cardiovascular morbidity and mortality, even in the general population3,4. Cardiovascular disease (CVD) is a broad-spectrum disease associated with numerous pathophysiological mechanisms such as inflammation5, fatty acid metabolic processes, and cholesterol metabolic process6.
Several recent studies have also shown a correlation between triglycerides (TGs) and UACR7,8,9. A Japanese cohort study showed that increased TG levels were an independent risk factor for the development of proteinuria in both men (relative risk [RR], 1.032) and women (RR 1.007)7. The Korean National Health and Nutrition Examination Survey (KNHANES) study also showed that TG levels are correlated with the albumin-to-creatinine ratio in adults with hypertension9. The Risk Evaluation of cancers in Chinese diabetic Individuals: A longitudinal (REACTION) study also showed that higher TG levels (> 2.3 mmol/L) were associated with the UACR in both men and women8. Moreover, treatment of dyslipidemia may be beneficial in reducing albuminuria in patients with chronic kidney disease (CKD)10.
Cholesterol and TG levels are influenced by several factors, such as age, sex, diet style, and sleep; therefore, large datasets are required to identify reliable and generalized associations between UACR and lipid profiles. Consequently, we performed three assessments using a nationally representative Korean dataset. First, we conducted a univariate analysis, using Pearson’s and Spearman’s correlation analyses, Followed by multivariate linear regressions to evaluate the relationship between the UACR and total cholesterol (TC), TG, and high-density lipoprotein C (HDL-C). Second, stratification analysis was performed based on the UACR reference values recommended by the Kidney Disease Improving Global Outcome (KDIGO) to compare group-specific patterns of association between the UACR and lipid parameters. Third, we compared the categorized forms of the UACR (eight groups) with blood lipid levels. The UACR groups defined by the KDIGO guidelines were used to identify the associations between albuminuria and dyslipidemia in the multivariate analysis.
Results
All participants were divided into two based groups based on sex: men and women (Fig. S1), to assess sex-specific association pattern between UACR and lipid profiles groups. The UACR was sorted in ascending order and divided into five groups to check for a nonlinear relationship between the degree of albuminuria and lipid profile (Fig. 1 and Figs. S2, S3). Subsequently, as the UACR increased, a monotonically high prevalence of lipid-lowering medication use, and older age was observed in both men and women (Fig. 1). However, the lipid profiles did not exhibit straightforward increasing or decreasing patterns in relation to the UACR; instead, they showed ambiguous and nonlinear relationships that could not be easily explained (Tables S1, S2 and Fig. 1).
Gender-specific linear trends in lipid profiles according to the UACR (mg/g) quintiles. ALM anti-dyslipidemia medication, HDL-C high density lipoprotein C (mg/dL), TC total cholesterol (mg/dL) TG triglyceride (mg/dL), UACR urine albumin-to-creatinine ratio (mg/g). *The age on the vertical axis is in log values of years.
Owing to the trend of urinary proteinuria following a long normal distribution (Fig. S1), we performed log transformation of UACR values. Subsequently, we conducted correlation analyses with the lipids (Figs. 2 and 3). The UACR in Korean men was positively correlated with TC based on both Pearson’s and Spearman’s correlation analyses (Fig. 2). Analysis of the association between UACR and TG and HDL-C levels in Korean men showed positive and negative correlations, respectively (Fig. 2A). The association patterns between UACR and lipid profiles in Korean women were consistent with those in Korean men (Fig. 3A).
Associational analyses of the UACR and lipid profiles in Korean men. (A) Upper scatter plots illustrating the distributions of UACR and lipid profiles, matched with the independent and dependent variables used in the linear regression. For the associations of UACR with three lipid profiles, Pearson’s and Spearman’s correlations (PCC and SCC) were used to calculate the degree of association. (B) Linear regression was implemented for association analysis between UACR and lipid profiles. Model 1 included age as the confounder. Model 2 included age, AHM, diabetes, and ALM as covariates. AHM antihypertensive medication, ALM anti-dyslipidemia medication, HDL-C high density lipoprotein C (mg/dL), PCC Pearson’s correlation coefficient, TC total cholesterol (mg/dL), TG triglyceride (mg/dL), SCC Spearman’s correlation coefficient, UACR urine albumin-to-creatinine ratio (mg/g).
Association between UACR and lipid profiles in Korean women. Two methods (PCC and SCC) were implemented to identify the relationships of UACR level with three lipid profiles. Multivariate linear models were utilized to identify the independent associations of UACR with three lipids. HDL-C high-density lipoprotein C (mg/dL), PCC Pearson’s correlation coefficient, TC total cholesterol (mg/dL), TG triglyceride (mg/dL), SCC Spearman’s correlation coefficient, UACR urine albumin-to-creatinine ratio (mg/g).
A multivariate linear regression analysis was conducted to investigate the robust associations between urinary proteinuria and blood lipid profiles (Figs. 2b and 3B). First, we set the log-transformed continuous value of UACR as an independent variable and examined its relationship with TC among Korean men. Proteinuria was found to be negatively correlated with TC, without including covariates, which is consistent with the results of correlation analyses using Pearson’s and Spearman’s correlation analyses. After adding age as a confounding variable, the beta coefficients became positive. In the final linear regression analysis, a significant positive correlation was observed after adjusting for age, antihypertensive medication (AHM), diabetes, and anti-dyslipidemia medication (ALM) (Fig. 2B). TG levels showed a clear positive correlation in the correlation analysis (Fig. 2A) and independent positive associations in both univariate and multivariate analyses (Fig. 2B). HDL-C exhibited a somewhat nonlinear relationship with UACR, but consistently showed a negative correlation in both univariate and multivariate analyses (Fig. 2B). The UACR exhibited an independent positive association with TC and TG and a negative relationship with HDL-C for Korean women in the multivariate analysis (Fig. 3B).
Stratified analysis based on the classification of proteinuria according to the KDIGO guidelines2 was conducted to determine whether there were significant and consistent patterns of correlation between the continuous variables proteinuria and lipid profiles Figs. S4 and S5). Specifically, we classified the patients into three groups based on thresholds of 30 and 300 mg/g UACR. The UACR showed an independent positive relationship with TC and TG and a negative association with HDL-C in most Korean men, except for TC (UACR: 30–300 mg/g) and HDL-C (UACR: 30–300 mg/g) (Fig. 4 and Fig. S4). These patterns observed in Korean men were mostly replicated in Korean women, except for TC (UACR > 300 mg/g), TG (UACR > 300 mg/g), and HDL-C levels (normal range) (Fig. 5 and Fig. S5).
Subgroup association analysis between UACR and lipid profiles according to the KDIGO guidelines2 in Korean men. Linear regression was implemented for subgroup association analysis between UACR and lipid profiles. Model 1 included age as the confounder. Model 2 included age, AHM, diabetes, and ALM as covariates. Stratification analysis was conducted according to normal (A), moderately elevated albuminuria (B), and severely elevated albuminuria (C) status. AHM antihypertensive medication, ALM anti-dyslipidemia medication, HDL-C high-density lipoprotein C (mg/dL), UACR urine albumin-to-creatinine ratio (mg/g).
Subgroup association analysis between UACR and lipid profiles according to the KDIGO guidelines2 in Korean women. AHM antihypertensive medication, ALM anti-dyslipidemia medication, HDL-C high-density lipoprotein C (mg/dL), UACR urine albumin-to-creatinine ratio (mg/g).
The UACR were sorted in ascending order and divided into eight groups (Fig. S3). Furthermore, we designated the variables added to Model 3 as confounding variables and conducted a multivariate analysis of the association between the UACR in the eight groups and lipid concentrations. An S-shaped increasing trend was observed for TC in both men and women. A monotonically increasing pattern of TG levels was observed in Korean men. Finally, analysis of HDL-C levels in both men and women revealed, an inverse U-shaped decreasing trend (Figs. S6 and S7).
All UACR cases were categorized into the following three groups according to the KDIGO guidelines2: normal, moderately elevated albuminuria (30–300 mg/g), and severely elevated albuminuria (≥ 300 mg/g). Subsequently, multivariate linear regression was performed using the lipid profiles and UACR as the dependent and independent variables, respectively. Monotonic increases in TC and TG levels were observed in both Korean men and women (Fig. 6). In addition, a significant negative relationship was observed between HDL-C levels and UACR in both sexes (Fig. 6).
Discussion
Our study integrated and analyzed Korean-representative cohorts and demonstrated that the UACR correlated positively with TG and TC and negatively with HDL in both men and women. These results are consistent with stratified analysis. Additionally, we observed the association patterns of the UACR and lipid profiles according to sex and found nonlinear relationships between the UACR and lipid profiles in most lipid profiles; however, a monotonically positive correlation was observed between TG and UACR.
The correlation between TG levels and proteinuria was first reported by Tozawa et al.7. They showed that the TG level was a risk factor for the development of proteinuria among 4326 Japanese participants. However, there is limited understanding of the correlation between proteinuria and TG levels as proteinuria was measured using dipstick test. Tien et al. evaluated albuminuria as a categorical variable (normal, moderately elevated, or severe elevated) and TG levels in 2349 Chinese patients with diabetes from two medical centers11. Wang et al. evaluated the correlation between TG levels and UACR in 34,569 Chinese participants from eight centers8. Moreover, the TG-glucose index recently found to be positively associated with albuminuria in patients with hypertension12; however, the UACR measurement units were not unified; therefore, the UACR could not be evaluated as a continuous variable. Conversely, our study analyzed both parameters as continuous variables to provide a more precise analysis.
Protein loss from glomeruli activates several signals through the renal tubules to increase TG production13. TG-rich (Apoprotein B) ApoB-containing lipoproteins promote kidney disease progression14. Additionally, high TG levels are associated with factor VII and plasminogen activator inhibitor levels, which are in turn associated with intravascular fibrin deposits, thrombosis, and renal fibrosis, resulting in increased urinary albumin excretion15,16. Increased TG levels caused by insulin resistance impair glucose metabolism and, trigger oxidative stress and chronic inflammation, which can lead to microvascular permeability and increased glomerular urinary albumin excretion17,18. Impaired HDL-C mediated reverse cholesterol transport promotes glomerulosclerosis and tubulointerstitial damage19. Moreover, higher HDL-C levels were associated with a reduced rate of microalbuminuria in patients with diabetes20. Although the exact mechanism has not yet been identified, we believe that these changes in TG and HDL-C levels affect TC levels, resulting in a positive correlation between UACR and TC levels. Consequently, we observed a strong positive correlation between TG levels and UACR among the lipid profiles.
Therefore, we can consider proteinuria not only as an indicator of kidney dysfunction but also as a marker for dyslipidemia1. Additionally, the moderately elevated albuminuria measurement using urine analysis has the advantage of being less invasive and simpler than dyslipidemia measurements using fasting blood tests. The Honolulu Heart Program, which was followed for 27 years, showed that proteinuria, detected by urine dipstick, could independently predict an increased risk of incident CVD21. Excessive protein reabsorption in the proximal tubules of the kidney promotes an inflammatory response22 and reflects the increased transvascular leakage of various proteins, including lipoproteins23. Inflammation elicits local and systemic endothelial dysfunction and CVD24.
Nonetheless, this study had some limitations. First, since this was a cross-sectional study, we were unable to establish a causal relationship; only associations between variables could be identified. Second, several parameters (such as past history and prescribed medications) were obtained from the questionnaire that may have led to biased results. Third, we analyzed the KNHANES, which is a previously archived public dataset and does not include information on the detailed type of medication for chronic disease. Therefore, we could not consider the use of medications, such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or sodium glucose transport protein 2 inhibitors, which may reduce albuminuria. Moreover, the KNHANES we used does not include information on the type of lipid-lowering medication but does include a categorical variable for whether a subject is taking medication. Fourth, we used a single urine spot sample to assess UACR rather than a 24-h urine collection. Fifth, because the KNHANES only evaluated low density lipoprotein cholesterol (LDL-C) in hypertriglyceridemia group (TG > 200 mg/dL), it is challenging to identify a generalized relationship between UACR and LDL-C. Moreover, we were unable to obtain data on the factors influencing blood lipid levels, such as thyroid function, body shape, and digestive tract diseases. Nevertheless, we investigated the correlation between albuminuria and lipid profiles in a single representative group using nationally normalized data.
In conclusion, we showed that lipid profiles, especially TG levels, were associated with UACR. This correlation between albuminuria and dyslipidemia indicates that simple spot urine measurement is a highly useful predictor of dyslipidemia and renal function.
Methods
Study population
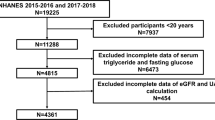
We used data from the KNHANES, a nationwide dataset from Korea. The Division of Chronic Disease Surveillance of the Korea Centers for Disease Control and Prevention in the Ministry of Health and Welfare assesses and monitors nutritional and medical health status through the KNHANES25,26. Thus the KNHANES dataset includes nutritional surveys, health examinations, and health interviews that implement a complex multistage probability sample design to obtain nationally representative data. Because the 2015–2018 KNHANES were designed not to evaluate information about UACR, among the approximately 20 datasets containing the clinical information of different participants each year, the KNHANES (2011–2014 and 2019–2020 datasets), including the UACR levels, were used. Participants aged < 40 years and those with missing data were excluded, resulting in 19,340 participants. All KNHANES participants agreed to participate in our study and provided informed consent. The analysis of KNHANES data were performed in compliance with the Declaration of Helsinki. This study was approved by the Institutional Review Board of Wonju Severance Christian Hospital (IRB No. CR321375).
Covariates
The association between the UACR and CVD or individual predictors in the CVD model has been demonstrated previously4,27. Leading groups worldwide have constructed cardiovascular disease prediction models using readily available demographic, physiological, and blood test indicators obtained from clinical settings28. Furthermore, advanced models that can better represent complex clinical data have recently been developed through dimensionality transformations such as kernel methods29,30. We referenced important studies such as the discovery of the New World by Columbus to select seven variables as covariates.
Six demographic, anthropometric, and laboratory predictors were selected as covariates: age, systolic blood pressure, AHM, current smoking status, and diabetes. Moreover, lipid profiles were considered as dependent variables in linear or logistic regression; therefore, ALMs, such as statins, were included as confounders.
Statistics
Continuous variables are expressed as means and standard deviations and were analyzed using ANOVAR. The average values of the variables according to the UACR quartiles were used as representative values for each group to test for linear trends in continuous features. Categorical variables are expressed as frequencies and percentages were analyzed using the Chi-squared test. The sampling weights determined by the data constructors were used to estimate the total population represented by the data. After employing the weight values, univariate and multivariate linear regression analyses were used to identify the association between UACR and lipid profiles, such as TG, HDL-C, and TC.
Data availability
This study analyzed the data obtained from the KNHANES. The KNHANES database contains publicly available data (https://knhanes.kdca.go.kr/knhanes/sub03/sub03_02_05.do).
References
Gerstein, H. C. et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286, 421–426. https://doi.org/10.1001/jama.286.4.421 (2001).
Stevens, P. E. & Levin, A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 158, 825–830. https://doi.org/10.7326/0003-4819-158-11-201306040-00007 (2013).
Mogensen, C. E. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N. Engl. J. Med. 310, 356–360. https://doi.org/10.1056/nejm198402093100605 (1984).
Kim, Y. J., Hwang, S. W., Lee, T., Lee, J. Y. & Uh, Y. Association between urinary albumin creatinine ratio and cardiovascular disease. PLoS One 18, e0283083. https://doi.org/10.1371/journal.pone.0283083 (2023).
Lee, T. et al. Identification of cardiovascular disease-related genes based on the co-expression network analysis of genome-wide blood transcriptome. Cells 11, 2867. https://doi.org/10.3390/cells11182867 (2022).
Koplev, S. et al. A mechanistic framework for cardiometabolic and coronary artery diseases. Nat. Cardiovasc. Res. 1, 85–100. https://doi.org/10.1038/s44161-021-00009-1 (2022).
Tozawa, M. et al. Triglyceride, but not total cholesterol or low-density lipoprotein cholesterol levels, predict development of proteinuria. Kidney Int. 62, 1743–1749. https://doi.org/10.1046/j.1523-1755.2002.00626.x (2002).
Wang, Y. X. et al. Elevated triglycerides rather than other lipid parameters are associated with increased urinary albumin to creatinine ratio in the general population of China: a report from the REACTION study. Cardiovasc. Diabetol. 18, 57. https://doi.org/10.1186/s12933-019-0863-8 (2019).
Lee, S. H. et al. Relationship between dyslipidemia and albuminuria in hypertensive adults: A nationwide population-based study. Medicine (Baltimore) 95, e3224. https://doi.org/10.1097/md.0000000000003224 (2016).
Zhang, Z., Wu, P., Zhang, J., Wang, S. & Zhang, G. The effect of statins on microalbuminuria, proteinuria, progression of kidney function, and all-cause mortality in patients with non-end stage chronic kidney disease: A meta-analysis. Pharmacol. Res. 105, 74–83. https://doi.org/10.1016/j.phrs.2016.01.005 (2016).
Tien, K. J., Tu, S. T., Chen, H. C., Hsiao, J. Y. & Hsieh, M. C. Triglycerides are independently associated with albuminuria in Taiwanese Type 2 diabetic patients. J. Endocrinol. Investig. 35, 800–803. https://doi.org/10.3275/8060 (2012).
Tian, Y. et al. Association between the triglyceride-glucose index and albuminuria in hypertensive individuals. Clin. Exp. Hypertens. 45, 2150204. https://doi.org/10.1080/10641963.2022.2150204 (2023).
Moorhead, J. F., Chan, M. K., El-Nahas, M. & Varghese, Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet 2, 1309–1311. https://doi.org/10.1016/s0140-6736(82)91513-6 (1982).
Samuelsson, O. et al. Complex apolipoprotein B-containing lipoprotein particles are associated with a higher rate of progression of human chronic renal insufficiency. J. Am. Soc. Nephrol. 9, 1482–1488. https://doi.org/10.1681/asn.V981482 (1998).
Tan, K. et al. Hypertriglyceridaemia and vascular risk. Report of a meeting of physicians and scientists, University College London Medical School. Lancet 342, 781–787. https://doi.org/10.1016/0140-6736(93)91544-V (1993).
Kamgar, M. et al. Impaired fibrinolytic activity in type II diabetes: correlation with urinary albumin excretion and progression of renal disease. Kidney Int. 69, 1899–1903. https://doi.org/10.1038/sj.ki.5000355 (2006).
Guerrero-Romero, F. et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 95, 3347–3351. https://doi.org/10.1210/jc.2010-0288 (2010).
Bolton, C. H. et al. Endothelial dysfunction in chronic renal failure: Roles of lipoprotein oxidation and pro-inflammatory cytokines. Nephrol. Dial. Transplant. 16, 1189–1197. https://doi.org/10.1093/ndt/16.6.1189 (2001).
Vaziri, N. D. Lipotoxicity and impaired high density lipoprotein-mediated reverse cholesterol transport in chronic kidney disease. J. Ren. Nutr. 20, S35-43. https://doi.org/10.1053/j.jrn.2010.05.010 (2010).
Sun, X. et al. Association of serum high-density lipoprotein cholesterol with microalbuminuria in type 2 diabetes patients. Lipids Health Dis. 17, 229. https://doi.org/10.1186/s12944-018-0878-2 (2018).
Madison, J. R. et al. Proteinuria and risk for stroke and coronary heart disease during 27 years of follow-up: The Honolulu heart program. Arch. Intern. Med. 166, 884–889. https://doi.org/10.1001/archinte.166.8.884 (2006).
Remuzzi, G., Ruggenenti, P. & Perico, N. Chronic renal diseases: Renoprotective benefits of renin-angiotensin system inhibition. Ann. Intern. Med. 136, 604–615. https://doi.org/10.7326/0003-4819-136-8-200204160-00010 (2002).
Jensen, J. S. Renal and systemic transvascular albumin leakage in severe atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 15, 1324–1329. https://doi.org/10.1161/01.atv.15.9.1324 (1995).
Paisley, K. E. et al. Endothelial dysfunction and inflammation in asymptomatic proteinuria. Kidney Int. 63, 624–633. https://doi.org/10.1046/j.1523-1755.2003.00768.x (2003).
Kweon, S. et al. Data resource profile: The Korea national health and nutrition examination survey (KNHANES). Int. J. Epidemiol. 43, 69–77. https://doi.org/10.1093/ije/dyt228 (2014).
Kim, Y. The Korea National Health and nutrition examination survey (KNHANES): Current status and challenges. Epidemiol. Health 36, e2014002. https://doi.org/10.4178/epih/e2014002 (2014).
Arnlöv, J. et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: The Framingham Heart Study. Circulation 112, 969–975. https://doi.org/10.1161/circulationaha.105.538132 (2005).
D’Agostino, R. B. Sr. et al. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 117, 743–753. https://doi.org/10.1161/circulationaha.107.699579 (2008).
Goff, D. C. Jr. et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129, S49-73. https://doi.org/10.1161/01.cir.0000437741.48606.98 (2014).
Yadlowsky, S. et al. Clinical implications of revised pooled cohort equations for estimating atherosclerotic cardiovascular disease risk. Ann. Intern. Med. 169, 20–29. https://doi.org/10.7326/m17-3011 (2018).
Author information
Authors and Affiliations
Contributions
Conceptualization, S.W.H. and J.Y.L.; methodology, S.W.H., T.L., Y.U. and J.Y.L.; software, S.W.H., T.L., Y.U. and J.Y.L.; validation, S.W.H., T.L., Y.U. and J.Y.L.; formal analysis, S.W.H., T.L., Y.U. and J.Y.L.; investigation, Y.U. and J.Y.L.; resources, Y.U. and J.Y.L.; data curation, S.W.H., T.L., Y.U. and J.Y.L.; writing—original draft preparation, S.W.H., T.L., Y.U. and J.Y.L.; writing—review and editing, S.W.H., T.L., Y.U. and J.Y.L.; visualization, S.W.H., T.L., Y.U. and J.Y.L.; supervision, Y.U. and J.Y.L.; project administration, J.Y.L.; funding acquisition, J.Y.L. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hwang, S.W., Lee, T., Uh, Y. et al. Urinary albumin creatinine ratio is associated with lipid profile. Sci Rep 14, 14870 (2024). https://doi.org/10.1038/s41598-024-65037-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-65037-w
- Springer Nature Limited