Abstract
Gut microbiota can regulate the metabolic and immunological aspects of ischemic stroke and modulate the treatment effects. The present study aimed to identify specific changes in gut microbiota in patients with large vessel occlusion (LVO) ischemic stroke and assess the potential association between gut microbiota and clinical features of ischemic stroke. A total of 63 CSVD patients, 64 cerebral small vessel disease (CSVD) patients, and 36 matching normal controls (NCs) were included in this study. The fecal samples were collected for all participants and analyzed for gut microbiota using 16S rRNA gene sequencing technology. The abundances of five gut microbiota, including genera Bifidobacterium, Butyricimonas, Blautia, and Dorea and species Bifidobacterium_longum, showed significant changes with high specificity in the LVO patients as compared to the NCs and CSVD patients. In LVO patients, the genera Bifidobacterium and Blautia and species Bifidobacterium_longum were significantly correlated with the National Institutes of Health Stroke Scale (NIHSS) scores at the admission and discharge of the patients. Serum triglyceride levels could significantly affect the association of the abundance of genus Bifidobacterium and species Bifidobacterium_longum with the NIHSS scores at admission and modified Rankin Scale (mRS) at discharge in LVO patients. The identification of five gut microbiota with high specificity were identified in the early stage of LVO stroke, which contributed to performed an effective clinical management for LVO ischemic stroke.
Similar content being viewed by others
Introduction
Acute stroke is a life-threatening disease and a major cause of serious disability1. Large vessel occlusion (LVO) is typically caused by a thrombus or embolus that lodges into one of the large vessels of the intracranial circulation, diminishing cerebral blood flow to cause brain cell death and acute ischemic stroke2,3. Several scales [e.g., National Institutes of Health Stroke Scale (NIHSS)] have been considered to predict the occurrence of LVO stroke4; however, no proper means have proven sensitive and feasible enough yet. Meanwhile, although large artery atherosclerosis and cardioembolism are the two main reasons, the variable and complex pathogenesis can also cause LVO, such as cerebral vasculitis, dissecting aneurysm5,6. Furthermore, endovascular treatment and intravenous thrombolysis are the common treatments for acute LVO7,8; however, the subsequent drug therapy and clinical prognosis have not been sufficiently investigated. Therefore, it is essential to study the underlying pathological mechanisms and predictors of clinical outcomes in stroke patients with LVO.
The gut microbiota is the largest reservoir of microorganisms in the human body and plays an important role in regulating the metabolic and immunological aspects of ischemic stroke9,10. Previous studies have demonstrated an altered composition and abundance of the gut microbiota in various complications and poor outcomes post-stroke, including Streptococcus, Lactobacillus, and Oscillospira11,12. Additionally, the dysregulation of gut microbiota, by causing abnormal metabolism of short-chain fatty acids, can mediate immune and inflammatory reactions within the central nervous system and further affect stroke outcomes13,14. However, the investigation into the role of the gut-brain axis in a stroke is in its initial stage, and the association of the gut microbiota with the development and clinical outcome of an ischemic stroke needs to be clarified.
The present study aimed to identify specific gut microbiota in LVO stroke, which differed from the cerebral small vessel disease (CSVD) and normal state. Meanwhile, the association between gut microbiota and LVO stroke, including clinical symptoms, blood indices levels, and therapeutic effect, was also assessed.
Results
Characteristics of participants
Table 1 represents no difference in age, sex, body mass index (BMI), and complications (hypertension, diabetes, and coronary heart disease) among normal control (NC), CSVD and LVO groups.
Compositional analysis of gut microbiota in CSVD and HC groups
A total 8,439,651 sequences were obtained from 165 samples using QIIME2 software (version 2023.02; URL link: https://qiime2.org/), including 1,863,972 sequences in the NC group, 3,313,728 sequences in the CSVD group, and 3,261,951 sequences in the LVO group.
The operational taxonomic units (OTUs) were assigned with a 95% sequence similarity threshold. The LVO group exhibited a higher number of OTUs than the NC and CSVD groups (5028 vs. 2626 vs. 3455), including 956 similar OTUs (Supplementary Fig. S1). Samples’ curves in the rarefaction curves based on the amplicon sequence variant reached saturation plateau at the depth of 51,777 reads, signifying that the sequencing depths were sufficient for the majority of microbe species, and the sample size was reasonable (Supplementary Fig. S2).
Diversity analysis of gut microbiota among three groups
Alpha diversity analysis
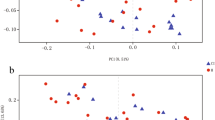
Six α-diversity indices, i.e., Observed species, Chao1, ACE, Shannon, Simpson and Coverage, were included in the present study (Fig. 1A). The scores of Observed species, Chao1, and ACE showed significant difference among NC, CSVD and LVO groups (FObserved species = 4.322; FChao1 = 4.553; FACE = 4.739; FCoverage = 29.301). Using the post-hoc analysis, LVO patients had significant higher Observed species, Chao1, and ACE scores than CSVD patients. Meanwhile, among NC, CSVD, and LVO groups, Coverage scores showed significantly difference (FCoverage = 29.301), however, there were no significant differences found in Shannon and Simpson scores.
Alpha-diversity and Beta-diversity indices for the species in the gut microbiota among NC, CSVD and LVO groups. (A) α-diversity indices. Same letter (a or b) on the box indicates no significant difference in the index between two groups. (B) β-diversity indices. Principal coordinates (PCoA) analysis (Left). Axis. 1 and 2 are two main components with the most interpretation of differences between samples. PLS-DA analysis (Right). The scale stands for the relative distance without practical significance. The NC, CSVD and LVO subjects are colored in blue, orange and green, respectively. NC, normal control; CSVD, cerebral small vessel disease; LVO, large vessel occlusion.
Beta diversity analysis
The β-diversity was calculated by Principal Coordinate Analysis (PCoA) analysis based on the weighted UniFrac distances at OTU level (Fig. 1B). Meanwhile, the Partial Least Squares-Discriminant Analysis (PLS-DA) analysis was also conducted for the reduction of the impact of intergroup differences (Fig. 1B).
Compositional analysis of gut microbiota at genus and species level levels among three groups
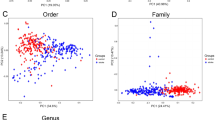
The top one most dominant bacterial genera with the highest relative abundances in these three groups were consistent, i.e., Bacteroidetes (Supplementary Fig. S3). Prevotella, as the second highest abundant bacterium, only appeared in the LVO group, however, the second highest abundance bacterium of the NC and CSVD groups was Faecalibacterium (Supplementary Fig. S3). Meanwhile, in species level, Bacteroides_vulgatus as the recognizable bacteria showed the consistently highest relative abundances in three groups (Supplementary Fig. S4). Bacteroides_uniformis was the second highest abundance bacterium in both the NC and CSVD groups, but Bacteroides_coprocola was the second highest abundance bacterium in the LVO group (Supplementary Fig. S4).
There were 20 genera and 17 species, having significant differences in their relative abundances, among the NC, CSVD and LVO groups (Supplementary Figs. S5 and S6). Using the MetaStats analysis, four genera and one species with specifically changed relative abundances in the LVO group (Fig. 2). In genus level, relative abundances of Bifidobacterium and Butyricimonas significantly increased and relative abundances of Blautia and Dorea significantly reduced in the LVO group when compared to HC and CSVD groups (Fig. 2). Moreover, LVO patients also exhibited significantly higher relative abundances in Bifidobacterium_longum than HC and CSVD participants in species level (Fig. 2). Therefore, these five gut microbes showed significant changes with high specificity in LVO patients. Moreover, LVO patients were further divided into two subgroups, i.e., cardioembolism and large-artery atherosclerosis groups (Supplementary Table 1). The relative abundances of these five gut microbes showed no significant difference between cardioembolism and large-artery atherosclerosis subgroups (Supplementary Fig. S7). Furthermore, gender as an important factor was also considered for the expression of gut microbes. There was no significant difference in the relative abundances of these five gut microbes between male and female subgroups in LVO group (Supplementary Fig. S8).
Taxa with significant differences at phylum and species level among NC, CSVD and LVO groups. “*” and “#” indicate significant changes in the LVO group as compared to the NC or CSVD group, respectively. ***/###p-value < 0.001; **/##p-value < 0.01; */#p-value < 0.05. NC, normal control; CSVD, cerebral small vessel disease; LVO, large vessel occlusion.
Results of linear discriminant analysis effect size (LEfSe) analysis
Using the LEfSe analysis, there were 91 taxa been identified with Linear Discriminant Analysis (LDA) scores of > 2 and p-value of < 0.05. Supplementary Fig. S9A showed a cladogram for all the taxonomic levels abundance, and the top 10 taxa with the highest LDA scores in each group are shown in Supplementary Fig. S9B. As shown in Supplementary Fig. S9, at genus level, the LVO group had significantly changed relative abundance of Actinomyces, Bifidobacterium, Scardovia, Atopobium, Cryptobacterium, Bacteroides, Parabacteroides, Alloprevotella, Prevotella, Enterococcus, Lactobacillus, Ezakiella, Hungatella, Anaerotruncus, Megasphaera, Fusobacterium, Campylobacter, and Succinivibrio as compared to the HC and CSVD groups. Meanwhile, compared with the NC and CSVD groups, species Clostridium_symbiosum and Sutterella_wadsworthensis also showed significant difference in relative abundance in the LVO group (Supplementary Fig. S9).
Functional predictions for gut microbiota
Using the PICRUSt2 analysis tool, the underlying functions in the gut microbiota of the three groups were predicted and annotated based on the Kyoto Encyclopedia of Genes and Genomes database (Fig. 3A). The differences in the functional prediction among the three groups were further analyzed using Kruskal–Wallis test. The pathway of “Xylene degradation”, “Proteasome”, “Aminobenzoate degradation”, “Lipoic acid metabolism”, “Flavonoid biosynthesis”, “Staphylococcus aureus infection”, “Geraniol degradation”, “African trypanosomiasis”, “Ubiquinone and other terpenoid-quinone biosynthesis”, “Naphthalene degradation”, “Valine, leucine and isoleucine degradation”, “Citrate cycle (TCA cycle)”, “Lipopolysaccharide biosynthesis”, “Biosynthesis of siderophore group nonribosomal peptides” and “Linoleic acid metabolism” showed significant difference among three groups (Fig. 3B). Additionally, using Wilcoxon test, the relative abundances of “Xylene degradation” were significantly reduced and the relative abundances of “Proteasome”, “Lipoic acid metabolism”, “Aminobenzoate degradation”, “African trypanosomiasis”, and “Citrate cycle (TCA cycle)” were significantly increased in the LVO group as compared to the NC or CSVD group (Fig. 3B).
Functional predictions of the gut microbiota. (A) Heat-map of functional genes in gut microbiota of all participants, showing top 30 genes with maximum relative abundances. The Abscissa stands for the samples, and the ordinate is functional genes. The colors represent the abundance of function, and the gradual change in color from light to deep indicates the relative abundance of function from low to high. (B) Significant difference in Kyoto Encyclopedia of Genes and Genomes pathways for gut microbiota in three groups using Kruskal–Wallis test. ***p-value < 0.001; **p-value < 0.01; *p-value < 0.05. The red boxes indicate significant changes in the LVO group as compared to the NC and CSVD group. NC, normal control; CSVD, cerebral small vessel disease; LVO, large vessel occlusion.
Association analysis of environmental factors in LVO patients
Correlation analysis
In order to determine the potential correlations between the composition of gut microbiota and environmental factors in LVO patients, one correlation matrixes were generated using partial correlation analysis with adjusting age, sex, BMI, and complications (Fig. 4A). Five gut microbes that showed specific changes in patients with LVO were included in the present analysis. In LVO patients, the relative abundance of genera Bifidobacterium and species Bifidobacterium_longum showed significant positive correlations with brain infarction volume (BIV) and triglyceride (TG), and homocysteine (HCY) levels. Meanwhile, LVO patients had significant negative correlations between the TG, fasting blood-glucose (FBG), and hemoglobin A1c (HbAlc) levels.
Associations of clinical features with gut microbiota at genus and species level in LVO patients. (A) Heatmap shows the correlation coefficients between clinical features at admission and gut microbiota. Covariates were age, sex, BMI, and complications. (B) Correlation analyses of NIHSS scores at admission and discharge with gut microbiota. Original NIHSS score was normalized to [0, 1] using Min–Max Normalization. Changed value = admission score − discharge score. Covariates were age, sex, BMI, and complications. (C) Difference in gut microbiota at genus and species level between good functional outcome and poor functional outcome groups. Good functional outcome was defined as a mRS score of 0–2, and poor functional outcome was defined as a mRS score of 3–6. LVO, large vessel occlusion, NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale; BIV, brain infarct volume; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; FBG, fasting blood-glucose; HbAlc, hemoglobin A1c; HCY, homocysteine; UA, Uric Acid; BMI, body mass index.
To better analyze the data, the original NIHSS score was normalized to [0,1] using Min–Max Normalization. The relative abundance of genera Bifidobacterium and species Bifidobacterium_longum showed significant positive correlations with admission and discharge NIHSS scores and negatively correlated with changed value of NIHSS (Fig. 4B). Meanwhile, there were significant negative correlation between the relative abundance of genera Blautia and admission and discharge NIHSS scores in the LVO group (Fig. 4B).
Furthermore, LVO patients were further divided into the good functional outcome group (score of 0–2) and the poor functional outcome group (score of 3–6) according to the discharge modified Rankin Scale (mRS) scores15,16. No significant difference in age, sex, BMI, and complications (hypertension, diabetes and coronary heart disease) between these two groups (data not shown). The relative abundance of genera Bifidobacterium and species Bifidobacterium_longum were significantly increased and the relative abundance of genera Blautia were significantly reduced in the poor functional outcome group as compared to the good functional outcome group in LVO patients (Fig. 4C).
Mediation analysis
Further mediation analyses in LVO patients depicted that environmental factor significantly regulated the relative abundance of gut microbiota on the severity and outcome of LVO stroke. Those indices that had statistically significance in previous correlation analyses were included in the present mediation analysis. Age, sex, BMI, and complications were used as covariants. The serum TG levels could affect the relative abundance of genera Bifidobacterium and species Bifidobacterium_longum on the normalized admission NIHSS scores (Fig. 5A).
Mediation and interactive effect analyses of gut microbiota in LVO patients. (A) The mediation effects of serum TG levels on the relative abundance of genera Bifidobacterium and species Bifidobacterium_longum and admission NIHSS scores. (B) Assessment of interactive effect between serum TG levels and relative abundance of species Bifidobacterium_longum on discharge mRS score in LVO patients using the line regression analysis. Covariates were age, sex, BMI, and complications. LVO, large vessel occlusion, NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale; TG, triglyceride; BMI, body mass index.
Interaction effect analysis
Additionally, line regression analyses further indicated that higher serum TG levels and higher relative abundance of species Bifidobacterium_longum were significantly associated with the worse discharge mRS scores, which suggested that the interactive effects of serum and relative abundance of species Bifidobacterium_longum on the discharge mRS scores may occur in LVO patients. Age, sex, BMI, and complications were used as covariants. In order to depict the specific interactive pattern, we divided subjects into a “mRS 0–2” group (good functional outcome group) and a “mRS 3–6” group (poor functional outcome group). LVO patients with higher discharge mRS score showed higher serum TG levels and relative abundance of species Bifidobacterium_longum (Fig. 5B).
Discussion
The main findings of the present study included: (1) the gut microbiota showed altered diversities among these three groups, indicating a higher richness of gut microbiota in the LVO group; (2) the genera Bifidobacterium, Butyricimonas, Blautia, and Dorea and the species Bifidobacterium_longum showed significantly higher specificity in LVO patients as compared to the NCs and CSVD patients; (3) in LVO patients, the genera Bifidobacterium and Blautia and species Bifidobacterium_longum were significantly associated with the severity of LVO stroke as well as lipid and glucose metabolism; (4) serum TG levels, as an important mediator, could affect the association of the relative abundance of genus Bifidobacterium and species Bifidobacterium_longum with the NIHSS scores at admission in LVO patients; (5) The relative abundance of genera Bifidobacterium and Blautia and species Bifidobacterium_longum were associated with the functional outcome at discharge in LVO patients. Therefore, the five intestinal microbes were identified in LVO stroke with high specificity, which could reflect the LVO stroke symptoms and function outcome.
To the best of our knowledge, this is the first study, investigating the differences in gut microbiota among NC, CSVD, and LVO stroke. The present study found that the relative abundances of genera Bifidobacterium and Butyricimonas and species Bifidobacterium_longum were significantly higher, while those of genera Blautia and Dorea were significantly lower in LVO patients than the NC and CSVD groups, suggesting specific changes in the composition and expression of the gut microbiota in LVO stroke. Compared to the single comparison group of NC, a CSVD group was first added to analyze the difference in gut microbiota between the LVO and CSVD groups. CSVD represents the vasculopathy of the small arteries, arterioles, venules, and capillaries of the brain17. CSVD is closely correlated with an increased risk of stroke in patients with intracranial arterial stenosis18, and high CSVD burden is related to the fast infarct growth rate and poor cerebral collateral circulation in LVO patients19,20. This suggests that CSVD is strongly associated with the clinical outcome of LVO stroke. Furthermore, large artery atherosclerosis stroke can affect downstream arterioles and brain tissue hypoperfusion, causing the occurrence of CSVD21. Ischemia hypoxia in LVO may induce cellular energy imbalance, oxidative stress, inflammatory response, and other mechanisms to lead to endothelial dysfunction, blood-cerebrospinal fluid barrier injury, and cell apoptosis, which are the primary pathogeny of CSVD22. Previous study has also found that the CSVD is more prevalent in the large artery atherosclerosis stroke subtype of LVO stroke23. Based on the comparison of three groups, the current study showed that these gut microbiotas had the specific expression in LVO stroke patients, which suggested that the influence of LVO rather than CSVD leaded to these changes in gut microbiotas.
A previous study indicated that the patients with cerebral infarction exhibited a higher abundance of Bifidobacterium than the healthy volunteers24, which was similar to our findings. However, the findings in the present study were inconsistent with another previous study25, which reported a reduced relative abundance of genus Bifidobacterium in stroke patients as compared to normal subjects. Due to the modulating effects of oral aspirin and atorvastatin on the human gut microbiota, especially genus Bifidobacterium and species Bifidobacterium_longum26, the collections of fecal samples in the current study were scheduled into 24 h after a thrombolytic therapy with alteplase to reduce the effects of following medication, which represented the gut microbial status closest to the stroke onset. The results showed that the increased relative abundance of genera Bifidobacterium and species Bifidobacterium_longum were positively correlated with the severity of LVO stroke and negatively correlated with the therapeutic effect of stroke in the LVO group. LVO patients with poor functional outcome exhibited an increased expression of genera Bifidobacterium and species Bifidobacterium_longum at admission. These indicated that LVO patients with higher relative abundances of genus Bifidobacterium and species Bifidobacterium_longum at admission might exhibit more severe stroke symptoms and imply a worse efficacy of drug treatment. Furthermore, the present study found significant correlations between the relative abundances of genus Bifidobacterium and species Bifidobacterium_longum and the serum TG and HCY levels. This suggested that these microbes might be associated with lipid and homocysteine metabolisms in the early stage of LVO stroke24,27,28,29. Meanwhile, in our analyses, the serum TG levels of LVO patients could mediate the effects of species Bifidobacterium_longum on the ischemic stroke severity and functional outcome at discharge, which also provided a new insight on high TG as the risk factor of stroke30,31. Additionally, ingestion of Bifidobacterium was thought to be beneficial for the middle cerebral artery occlusion-induced neurological dysfunctions32. The current study showed that the early abundance of Bifidobacterium in LVO patients might play a protective role to compete stroke risk factors (hyperlipidemia and hyperhomocysteinemia) and ischemic brain. This protective benefit from Bifidobacterium might be exhausted prematurely during treatment if probiotics/prebiotics are not given as dietary supplements, leading to a poor functional outcome.
In the present study, the decrease in the relative abundance of genus Blautia was negatively correlated with NIHSS scores at admission and discharge of LVO patients, and LVO patients with good functional outcome exhibited an increased expression of Blautia at admission, suggesting that genus Blautia abundance was associated with short-term neurological outcomes in stroke. Likewise, Chang et al. indicated that acute ischemic stroke patients with good outcomes exhibited an increased abundance of Blautia33. Furthermore, the current study observed negative correlations between the relative abundance of genera Blautia and TG, FBG, and HbAlc levels in the LVO patients, which suggested that Blautia might be involved in the glucose and lipid homeostasis in stroke. Previous studies also indicated that beneficial Blautia could alleviate hyperglycemia and hyperlipidemia in type 2 diabetes34,35. Hence, a supplement of Blautia might be beneficial for the treatment of stroke and the improvement of glucose and lipid metabolisms.
The current study showed an increase in the abundance of genus Butyricimonas and a decrease in that of genus Dorea in LVO patients, which exhibited high specificity for LVO stroke. The patients with middle cerebral artery occlusion-induced ischemic stroke showed an increased abundance of Butyricimonas, a pathogenic bacterium, which was significantly correlated with the infarct size and plasma levels of inflammatory markers36. Moreover, the abundance of Butyricimonas was associated with early neurological deficits and could predict recanalization therapy outcomes in ischemic stroke37. In addition, a recent study indicated that Dorea could mediate anti-inflammatory metabolites to affect the functional outcomes in ischemic stroke and may be used as a potential target in the clinical therapy of stroke38. However, more details on the association between Butyricimonas/Dorea and stroke are lacking.
There were certain limitations to the current study. (1) The present findings just supported these identified intestinal microbes as a consequence of LVO stroke, and it was unclear whether their changes were the key cause of inducing LVO stroke. (2) The fecal samples were not collected at the end of treatment from the LVO patients. (3) Independent verifications are necessary to determine the specificity of these five gut microbiota in LVO stroke. In a subsequent study, we will further expand the sample size and perform multi-center verifications to identify the characteristics of gut microbiota for large artery atherosclerosis and cardio-embolism stroke subtypes. Moreover, multiple fecal samples should be collected during treatment to explore the dynamic changes in the abundance of gut microbiota, which might contribute to assessing the potential of gut microbiota as a therapeutic target for ischemic stroke. Furthermore, animal experiments are essential to determine whether these changes in gut microbiota can induce the occurrence of LVO stroke.
In conclusion, the present study demonstrated five gut microbes with high specificity in patients with LVO and found several microbes associated with the severity and functional outcome of LVO stroke as well as lipid, homocysteine, and glucose metabolisms. These findings revealed the characteristics of gut microbes in the early stage of LVO stroke and provided a new insight on the stroke clinical management.
Materials and methods
Participants
All the participants or their legal guardians provided written informed consent. The Ethics Committee of the Affiliated Wuxi People’s Hospital of Nanjing Medical University approved the current study (approval number: KY21088). All clinical investigations were conducted in strict adherence to the principles outlined in the Declaration of Helsinki, and all experiments were performed in accordance with relevant guidelines and regulations.
A total of 63 LVO patients were recruited from the stroke center of the Affiliated Wuxi People’s Hospital of Nanjing Medical University, and 36 matching NCs were recruited through community health screening in Wuxi City. Moreover, 64 CSVD patients from the Affiliated Wuxi People’s Hospital of Nanjing Medical University, who had been reported in our previous studies, also were included in the present study. The standardized clinical interview was performed for all the participants, involving demographic inventory as well as physical and mental health examination. The brain magnetic resonance imaging (MRI) along with three-dimensional T1-weighted, T2-weighted, fluid-attenuated inversion recovery, susceptibility-weighted images, diffusion-weighted imaging, and magnetic resonance angiography (MRA), were also conducted for each participant. The dietary structure of each participant is similar.
The inclusion criteria for all the participants were as follows: (1) > 18 years old and (2) no contraindication in MRI scan. The LVO patients showed first-ever stroke symptoms, and the time of stroke was less than 4.5 h. All LVO patients were anterior circulation single LVO on computed tomography angiography or MRA. The Pullicino formula was used to calculate the BIV of LVO patients based on the cranial MRI scan. Furthermore, the CSVD patients had periventricular and deep white matter hyperintensity, lacunar infarcts, and cerebral microbleeds but not any new brain infarcts. All the NCs did not have any stroke, which was reflected by consistent imaging results. Participants in the NC group were randomly recruited during the same period. The NC group matched with the LVO and CSVD groups for age, sex, BMI, and complications. Meanwhile, all the participants had no habit of taking antiplatelet drugs or statins prior to enrollment.
The exclusion criteria for each participant were as follows: (1) hemorrhagic disorder or bleeding tendency (e.g., cerebral hemorrhage and gastrointestinal hemorrhage); (2) any severe psychiatric disorders (e.g., schizophrenia and major depressive disorder); (3) any infectious disease (e.g., pulmonary infection); (4) brain trauma or other neurologic diseases (e.g., Parkinson’s disease and Alzheimer’s disease); (5) any significant medical problems (e.g., tumor, significantly impaired liver or kidney functions, digestive system disease, and metabolic disease); (6) record of antibiotic use in the last six months or during treatment; and (7) usage of prebiotics or probiotics within one month before admission or during treatment.
At 24 h after a thrombolytic therapy with alteplase, the LVO patients were arranged for further drug therapies. According to “Chinese Stroke Association Guidelines for Clinical Management of Cerebrovascular Disorders (2019)”39, all the ischemic stroke patients with LVO received dual antiplatelet aggregation therapy, lipid-lowering treatment, and improving cerebral microcirculation. The duration of treatment was 8 ± 1 days for each LVO patient. Only those LVO patients, who met the current treatment protocol, were included in this study. Meanwhile, each LVO patient obtained a standard Mediterranean diet during their duration of hospital stay without the use of a nasogastric tube. Notably, the present study was not a clinical trial but an observational study.
Furthermore, NIHSS40 scale was used to evaluate the symptoms of stroke, and the mRS41 scale was used as a measure of post-stroke physical disability. These two assessments were conducted at the time of admission and discharge of the patients.
Collection of fecal samples and 16S rRNA gene sequencing
After thrombolytic therapy (within 24 h), the fecal sample was collected after LVO participant’s defecation using stool collection tubes with stool DNA stabilizer (Genstone Biotech, Beijing, China), and then stored at − 80 °C. Furthermore, fecal samples were collected at 7:00–8:00 a.m. for other participants.
FastDNA Spin Kit For Soil (MP Biomedicals, Santa Ana, CA) was used for the DNA extractions of fecal samples. Subsequently, the compositional analysis of gut microbiota were conducted by Genesky Biotechnologies Inc. (Shanghai, China). Details of sequencing and data analysis could be found in Supplementary Materials and previous studies42,43.
Collection of serum samples and detection of blood indexes
For LVO patients, peripheral venous blood was collected using a vacutainer tube (without anticoagulant) and an EDTA-coated tube at 6:00–7:00 a.m. on the first day after admission. The serum samples were further obtained by centrifugation at 3500 rpm at 4 °C for 10 min. All blood samples were measured immediately after collection. Due to the main goal of the present study is to investigate specific gut microbiota of LVO and potential association of gut microbiota with the pathologic change in LVO stroke, blood samples were collected only in the LVO group.
The total cholesterol, TG, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, FBG and uric acid were detected in serum using a fully automatic biochemical analyzer (UniCel DxC 600 Synchron, Beckman Coulter, California, United States). Hemoglobin A1c (HbA1c) level was assayed in whole-blood samples and HCY level was assayed in serum samples using the high-performance liquid chromatography (Agilent 1200 device, Agilent Technologies, Waldbronn, Germany).
Statistical analyses
The data analyses were conducted using SPSS version 23.0 (SPSS Inc. Chicago, IL, USA) and R software package (version 4.2.1).
Alpha and beta diversity analyses were performed for the diversity analysis of gut microbiota. In alpha-diversity analysis, Observed species, Chao1, and ACE indices were used for assessing the community richness, and Shannon, Simpson, and Coverage indices were used for assessing the community diversity. Beta diversity was used to analyze differences in the composition of gut microbiota using PCoA and PLS-DA. LEfSe was performed to identify the markers to interpret the difference among groups where the threshold score of LDA was 2. The functions of species in the gut microbiota were predicted using PICRUSt2 analysis tool and Kyoto Encyclopedia of Genes and Genomes database (https://www.genome.jp/kegg/pathway.html).
The continuous variables were shown as mean ± standard deviation and were analyzed using the Kruskal–Wallis H test for non-normal distribution, or the One-way ANOVA test for normal distribution. The categorical variables were analyzed using the Chi-square test. Partial correlation analysis was used to determine the correlation between the taxonomies of gut microbiota and clinical data in LVO patients, with adjusting age, sex, BMI, and complications. Furthermore, the mediation analysis in LVO patients could determine whether environmental factors mediated the association between the relative abundance of gut microbiota on the severity of stroke based on a standard three-variable mediation model44,45. Line regression was used to evaluate the interaction between two variables on the functional outcome46,47. Changed value = admission score − discharge score. The statistically significant differences were considered as p-value < 0.05.
Institutional review board statement
The Ethics Committee of the Affiliated Wuxi People’s Hospital of Nanjing Medical University approved the current study (approval number: KY21088).
Informed consent
A signed written informed consent form and acceptance to participate in the study were received from all participants in the study.
Data availability
The data supporting this study’s findings are available on request from the corresponding author.
References
Morotti, A., Poli, L. & Costa, P. Acute stroke. Semin. Neurol. 39(1), 61–72 (2019).
Beume, L. A. et al. Large vessel occlusion in acute stroke. Stroke 49(10), 2323–2329 (2018).
Keigher, K. M. Large vessel occlusion in the acute stroke patient: Identification, treatment, and management. Crit. Care Nurs. Clin. N. Am. 32(1), 21–36 (2020).
Duvekot, M. H. C. et al. Comparison of eight prehospital stroke scales to detect intracranial large-vessel occlusion in suspected stroke (PRESTO): A prospective observational study. Lancet Neurol. 20(3), 213–221 (2021).
Bossone, E. et al. Stroke and outcomes in patients with acute type A aortic dissection. Circulation 128(11 Suppl 1), S175–S179 (2013).
Topakian, R., Stieglbauer, K., Nussbaumer, K. & Aichner, F. T. Cerebral vasculitis and stroke in Lyme neuroborreliosis. Two case reports and review of current knowledge. Cerebrovasc. Diseases (Basel, Switzerland). 26(5), 455–461 (2008).
Campbell, B. C. V. & Khatri, P. Stroke. Lancet (London, England) 396(10244), 129–142 (2020).
Zhu, L. et al. Fluid-attenuated inversion recovery vascular hyperintensity as a potential predictor for the prognosis of acute stroke patients after intravenous thrombolysis. Front. Neurosci. 15, 808436 (2021).
Li, N. et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 19(1), 191 (2019).
Tan, B. Y. Q., Paliwal, P. R. & Sharma, V. K. Gut microbiota and stroke. Ann. Indian Acad. Neurol. 23(2), 155–158 (2020).
Pluta, R., Januszewski, S. & Czuczwar, S. J. The role of gut microbiota in an ischemic stroke. Int. J. Mol. Sci. 22(2), 915 (2021).
Wang, J., Zhang, H., He, J. & Xiong, X. The role of the gut microbiota in the development of ischemic stroke. Front. Immunol. 13, 845243 (2022).
Ratajczak, W. et al. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim. Pol. 66(1), 1–12 (2019).
Lee, J. et al. Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circ. Res. 127(4), 453–465 (2020).
Nam, H. S. et al. Intensive vs conventional blood pressure lowering after endovascular thrombectomy in acute ischemic stroke: The OPTIMAL-BP randomized clinical trial. Jama 330(9), 832–842 (2023).
Zi, W. et al. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: The DEVT randomized clinical trial. Jama 325(3), 234–243 (2021).
Li, Q. et al. Cerebral small vessel disease. Cell Transplant. 27(12), 1711–1722 (2018).
Kwon, H. M. et al. Frequency, risk factors, and outcome of coexistent small vessel disease and intracranial arterial stenosis: Results from the stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis (SAMMPRIS) trial. JAMA Neurol. 73(1), 36–42 (2016).
Sohn, J. H. et al. Effect of cerebral small vessel disease burden on infarct growth rate and stroke outcomes in large vessel occlusion stroke receiving endovascular treatment. Biomedicines 11(11), 3102 (2023).
Huo, L. et al. Impact of leukoaraiosis severity on the association of outcomes of mechanical thrombectomy for acute ischemic stroke: A systematic review and a meta-analysis. J. Neurol. 268(11), 4108–4116 (2021).
Moroni, F. et al. Carotid atherosclerosis, silent ischemic brain damage and brain atrophy: A systematic review and meta-analysis. Int. J. Cardiol. 223, 681–687 (2016).
Li, S. et al. Endothelial dysfunction and hyperhomocysteinemia-linked cerebral small vessel disease: Underlying mechanisms and treatment timing. Front. Neurol. 12, 736309 (2021).
Lee, S. J. et al. The leukoaraiosis is more prevalent in the large artery atherosclerosis stroke subtype among Korean patients with ischemic stroke. BMC Neurol. 8, 31 (2008).
Zhang, W. T., Niu, J. Y. & He, C. Associations of OSAHS complicated by cerebral infarction with intestinal flora, inflammatory factors, homocysteine and adiponectin expression. Eur. Rev. Med. Pharmacol. Sci. 24(24), 12993–12999 (2020).
Wang, Z., Xu, K. & Zhou, H. Characteristics of gut virome and microbiome in patients with stroke. Nan Fang Yi Ke Da Xue Xue Bao 41(6), 862–869 (2021).
Chen, G. et al. Effects of long-term regular oral aspirin combined with atorvastatin to prevent ischemic stroke on human gut microbiota. Eur. J. Pharmacol. 951, 175800 (2023).
Chaiyasut, C. et al. Influence of bifidobacterium breve on the glycaemic control, lipid profile and microbiome of type 2 diabetic subjects: A preliminary randomized clinical trial. Pharmaceuticals (Basel) 16(5), 695 (2023).
Wang, Q. et al. Bifidobacterium breve and Bifidobacterium longum attenuate choline-induced plasma trimethylamine N-oxide production by modulating gut microbiota in mice. Nutrients 14(6), 1222 (2022).
Brasili, E. et al. Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 induce different age-related metabolic profiles revealed by 1H-NMR spectroscopy in urine and feces of mice. J. Nutr. 143(10), 1549–1557 (2013).
Huang, Y. Q. et al. Relationship between triglyceride levels and ischaemic stroke in elderly hypertensive patients. Postgrad. Med. J. 96(1133), 128–133 (2020).
Antonios, N., Angiolillo, D. J. & Silliman, S. Hypertriglyceridemia and ischemic stroke. Eur. Neurol. 60(6), 269–278 (2008).
Rahman, Z., Bhale, N.A., Dikundwar, A.G., Dandekar, M.P. Multistrain probiotics with fructooligosaccharides improve middle cerebral artery occlusion-driven neurological deficits by revamping microbiota-gut-brain axis. Probiotics Antimicrob. Proteins. (2023).
Chang, Y. et al. Microbiota dysbiosis and functional outcome in acute ischemic stroke patients. Sci. Rep. 11(1), 10977 (2021).
Tong, X. et al. Structural alteration of gut microbiota during the amelioration of human type 2 diabetes with hyperlipidemia by metformin and a traditional Chinese herbal formula: A multicenter, randomized open label clinical trial. mBio 9(3), e02392-e2417 (2018).
Yan, Y., Li, Q., Shen, L., Guo, K. & Zhou, X. Chlorogenic acid improves glucose tolerance, lipid metabolism, inflammation and microbiota composition in diabetic db/db mice. Front. Endocrinol. 13, 1042044 (2022).
Hammond, T. C. et al. Gut microbial dysbiosis correlates with stroke severity markers in aged rats. Front. Stroke 1, 1026066 (2022).
Chou, P. S. et al. Predicting adverse recanalization therapy outcomes in acute ischemic stroke patients using characteristic gut microbiota. Microorganisms 11(8), 2016 (2023).
Guo, Q. et al. Integrated traditional Chinese medicine improves functional outcome in acute ischemic stroke: From clinic to mechanism exploration with gut microbiota. Front. Cell. Infect. Microbiol. 12, 827129 (2022).
Liu, L. et al. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: Executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc. Neurol. 5(2), 159–176 (2020).
Kwah, L. K. & Diong, J. National Institutes of Health Stroke Scale (NIHSS). J. Physiother. 60(1), 61 (2014).
Quinn, T. J., Dawson, J., Walters, M. R. & Lees, K. R. Reliability of the modified Rankin Scale: A systematic review. Stroke 40(10), 3393–3395 (2009).
Shi, Y. et al. Alteration and clinical potential in gut microbiota in patients with cerebral small vessel disease. Front. Cell. Infect. Microbiol. 13, 1231541 (2023).
Xu, Y. et al. Antipsychotic-induced gastrointestinal hypomotility and the alteration in gut microbiota in patients with schizophrenia. Brain Behav. immunity 99, 119–129 (2022).
Shi, Y. et al. Potential association of neutrophil extracellular traps with cognitive impairment in cerebral small vessel disease. J. Gerontol. A Biol. Sci. Med. Sci. 78(11), 1999–2006 (2023).
Shi, Y. et al. Potential clinical value of circular RNAs as peripheral biomarkers for the diagnosis and treatment of major depressive disorder. EBioMedicine 66, 103337 (2021).
Shi, Y. et al. Potential association of neutrophil extracellular traps with cognitive impairment in cerebral small vessel disease. J. Gerontol. Series A Biol. Sci. Med. Sci. 78(11), 1999–2006 (2023).
Zhao, E. et al. Value of serum brain-derived neurotrophic factor and glial fibrillary acidic protein for detecting depression in patients with Helicobacter pylori infection. Neurosci. Lett. 825, 137687 (2024).
Acknowledgements
The authors would like to thank all participants in the present study for their cooperation and relevant medical staff (e.g., Mr. Feng Wang, Mr. Xiangming Fang, Mr. Guangjun Xi, Miss. Jingyu Deng, Mrs. Yiping You, and Mrs. Qianqian Gao) from the Neurology and Imaging Department of the Affiliated Wuxi People’s Hospital of Nanjing Medical University for their assistance in this work. The authors would like to thank Biomaster Biotechnologies Inc. (Nanjing, China) for their technical assistance.
Funding
This study was funded by the WuXi Municipal Health Commission (No. Q202222), National Natural Science Foundation of China (No. 82301715), and Wuxi Taihu Lake Talent Plan, Supports for Leading Talents in Medical and Health Profession (2020THRC-DJ-SNW).
Author information
Authors and Affiliations
Contributions
YCS designed this study. PH and CJ draft the manuscript. ZFW, GJC, JH and ZD recruited the participants and collected the samples. JQN and LL analyzed the data. WJ performed the measurements of blood indicators. KFC contributed to the discussion and revised the manuscript. XXZ revised the manuscript and language. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, P., Jiang, C., Ni, J. et al. Identifying gut microbiota with high specificity for ischemic stroke with large vessel occlusion. Sci Rep 14, 14086 (2024). https://doi.org/10.1038/s41598-024-64819-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64819-6
- Springer Nature Limited