Abstract
The thrust of the study was to determine the chemical composition of the essential oils extracted from Thymus pallescens de Noé and Cymbogon citratus Stapf. as well as to evaluate their efficacy in controlling Sitophilus zeamais Motschulsky and Tribolium castaneum (Herbst) in either single or combined populations. Carvacrol (56.04%) and geraniol (20.86%) were identified as the major constituents of T. pallescens and C. citratus respectively. The tested essential oils showed pronounced insecticidal activity against the pest species in relation with the applied doses. T. pallescens EO had the highest efficacy and S. zeamais was found to be more susceptible to both individual and combined treatments. With reference to the contact and fumigation assessments, T. pallescens EO effectuated corrected mortality rates ranging from 42.5–100% to 25–100% in S. zeamais with corresponding lethal concentration (LC50) values of 17.7 µl/ml and 15µL/L air respectively. Whereas, the T. pallescens EO exhibited corrected mortality rates of 42.5–100% and 20–100% with corresponding LC50 values of 18.1 µl/ml and 15.5 µL/L air against T. castaneum in contact and fumigation assessments, respectively. The corrected mortality rates increased for both insect species when using combination treatments, with significant increases in the LC50 values, ranging from 8.59 to 49.9% for both pest species. Analysis of energy biomarkers in the treated insects indicate significantly increased protein and carbohydrate contents and decreased lipids levels. The study therefore demonstrated the bio-insecticidal toxicity of the EOs from T. pallescens and C. citratus against two important maize post-harvest pests, concurrently revealing significant positive and negative insecticidal activity gradients in relation to single or combined populations.
Similar content being viewed by others
Introduction
Maize (Zea mays L.) is an important source of nutrients for both humans and livestock1. Maize production has increased significantly in recent years to keep up with the surging global demand2. However, several biotic and abiotic factors affect the productivity of maize, especially factors associated with post-production storage losses.
Pests are regarded as a principal cause of stored maize grain losses, estimated at 12–36% annually3,4. More than 37 species of insects have been reported to infest stored maize grains5. Notably, the major losses are mainly associated with Coleopteran species3,4,6.
The maize weevil Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) is considered as one of the most serious and damaging pests identified in stored corn worldwide. It affects the quantity and quality of the maize grains thus causing severe deterioration of the seed germination potential due to its ability to penetrate into the grain mass4,7. S. zeamais is a polyphagous pest species, which accounts for approximately 10–30% yield losses of the annual stored maize grains8.
Moreover, maize damaged by S. zeamais activity becomes highly susceptible to other saprophagous species especially Tribolium castaneum (Coleoptera: Tenebrionidae). In turn, infestation with T. castaneum is synonymous with increase in temperature and moisture of stored grains generating an environment that promotes fungal propagation leading to further grain degradation and deterioration9. Furthermore, co-existence of different species brings about interspecific competition which results in dramatic losses of the stored grain10,11.
To control insect pests during grain storage, chemical synthetic insecticides are frequently used since they can rapidly decimate dense insect populations. However, the indiscriminate use of these chemical compounds results in several adverse environmental impacts such as pest resistance, environmental pollution, disruption of ecological balance and destruction of non-target pollinator insects like bees12,13.
Indeed, resistance to several insecticides, such as malathion, pirimiphos-methyl, fenitrothion, and phosphine, has been reported in many storage insect-pests, including S. zeamais and T. castaneum14,15. Due to these adverse effects of synthetic chemical pesticides, biopesticides have been explored as a potential alternative16,17. In fact, natural insecticides have the advantage of low toxicity for humans and increased biodegradability18.
Several aromatic plants have been identified as potential organic pesticides that can control post-harvest insect pest species19,20,21,22,23. For example, Thymus pallescens essential oil finds extensive application in phytotherapy. Thyme is recognized for its antioxidative, antibacterial, insecticidal and anti-fungal properties24,25,26. More so, it has been documented that Cymbopogon citratus possesses various biological properties, encompassing insecticidal, antibacterial, antifungal and antioxidant activities26,27,28,29.
S. zeamais and T. castaneum have been found to be sensitive to various EOs and their active constituents30,31. Nonetheless, there is a scarcity of experimental work designed on the effects of EOs on simultaneous infestation of S. zeamais and T. castaneum. Therefore, the aim of this study was to determine the chemical compositions of T. pallescens and C. citratus EOs and to evaluate their insecticidal activity on 2 maize post-harvest pests, S. zeamais and T. castaneum, in single and combined populations. Consequently, we hypothesize that these EOs could negatively influence the physiological responses of the investigated pests.
Material and methods
Plant material collection and air curing
The aerial plant parts of T. pallescens and C. citratus were collected during the flowering season from various localities of Mascara and Algiers, North Algeria. All samples were partially dried for 15 days at room temperature (25 ± 3 °C).
Insect rearing
The study focused on two Coleopteran species: S. zeamais and T. castaneum, which were sampled from infested seed maize from farmers’ maize stocks. The insects were reared separately in plastic jars (60 cm × 40 cm × 12 cm) containing a mixture of 1 kg commercial maize and commercial flour according to the proportion of 9/1 (w/w), respectively. The trays were maintained at 26 ± 3 °C and, 70 ± 10% relative humidity (RH), under a 12:12 h (L;D) photoperiod. The adult insects used in all toxicity and biochemical studies were 2-week post-emergence insects of mixed sex13.
Essential oil extraction and GC–MS analysis
All EOs were obtained from the aerial parts of T. pallescens and C. citratus by hydrodistillation process for 3 h, using a Clevenger-type apparatus. Initially, a quantity of 100 g of plant material was added to 800 ml of distilled water in a 2-L flask. The set was placed in a balloon heater attached to a refrigerator to ensure condensation of EOs. The yields of EOs were expressed in g relative to 100 g of dry vegetable matter32. The T. pallescens leaves gave the highest yield of Eos, ranging from 1.8 to 2.1 g/100 g. The yield of C. citratus ranged from 0.8 to 1.1 g/100 g (mean value for four months). The extracted EOs were dried over anhydrous sodium sulfate and stored at 4°C until use.
Chemical analyses were performed using a system comprised of a 6890 gas chromatography (GC) apparatus using a VF WAX and HP-5MS capillary column (60 m × 0.25 mm × 0.5 µm film thickness) coupled with a Hewlett-Packard computerized 5973A mass spectrometry (MS) apparatus. All EOs were diluted 1/10 (v/v) in hexane, and 1 µL was injected by splitting at a ratio of 1:25. The GC column was a 30m (60m × 0.25mm × 0.5µm film thickness) using a VF WAX and HP-5 capillary column. The GC conditions were as follows: injector temperature, 250 °C; column temperature, isothermal at 60 °C and held for 6 min, then programmed to 250 °C at 6 °C/min and held at this temperature for 2 min; ion source temperature, 250 °C; and detector temperature, 320 °C. Helium was used as the carrier gas at a rate of 0.5 mL/min. The mass range varied from m/z 30 to 350 amu (atomic mass units). The EO components were identified by comparing their retention indices and mass spectra results against those for authentic samples and comparisons of the linear retention indices against a series of n-hydrocarbons. Computer matching was performed against both commercial (NIST 98 and ADAMS) and home-made library mass spectra, based on the analysis of pure substances and the components of known oils derived from data in the MS literature33.
Insecticidal activity
The insecticidal activity of the EOs was evaluated against two pest species using two approaches: a separate treatment (each species was treated individually) and a combined treatment (the two species in coexistence). All bioassays were performed under controlled conditions (26 ± 1 °C, 65 ± 5% RH, and a 12:12 h L:D photoperiod).
Contact toxicity
Contact toxicity assays were conducted in accordance with a previously described protocol34. Each EO was dissolved in acetone to prepare a serial dilution. Preliminary tests were run to determine the appropriate concentrations that can cause mortality rates ranging from 20 to 80% mortality. For both species, concentrations of 1, 5, 10, 20, 30, 40, 50, 100, 200, 400 µL/mL of each EO were tested. Guided by this assessment the following concentrations were selected for the study: 10, 20, 30, 40, and 50µL/mL. The EOs were applied on the filter paper discs (9 cm diameter) using 2 mL for each trial. Acetone was allowed to evaporate for 10 min prior to the introduction of pest species. Twenty unsexed adults of S. zeamais and T. castaneum were introduced, either separately or together, into Petri dishes containing treated filter paper. The control was maintained under the same conditions without EOs. Four replicates were performed for each treatment. The mortality rates were recorded 72 h after treatment and corrected using Abbott’s formula (1925). The insects were considered dead when no movement was recorded. Half-maximal lethal concentration (LC50) values were assessed using probit analysis.
Fumigant toxicity
The fumigant toxicity of EOs was evaluated against S. zeamais and T. castaneum adults in accordance with a previously described technique13. Glass jars (1 L) were used as fumigation chambers. As for contact toxicity assay, preliminary tests were conducted in order to determine the concentrations that can effectuate 20 to 80% mortality rates in each of the insect populations. The tested concentrations were 1, 5, 10, 20, 30, 40, 50, 100, 200, 400 µL/L air. Subsequently the following concentrations of EOs were selected and used in the study: 10, 20, 30, 40, and 50 µL/L air. Filter papers (Whatman No. 1) were cut into 9 mm diameter pieces and sprayed with EOs then strongly affixed onto the undersides of the screw caps of the jars. The insides of the jars were brushed with Vaseline to prevent direct contact between the insects and the EO. Caps containing the treated filter paper were tightly screwed onto jars containing twenty adults of S. zeamais and T. castaneum either individually or together. The cover was well-sealed with parafilm. Control insects were maintained under the same conditions with affixed filter paper without EOs. The treatments were replicated four times. Mortality rates were recorded after 72 h of treatment and corrected using Abbott’s formula (1925). The LC50 values were assessed by probit analysis.
Effect of EOs on energy biomarkers, including proteins, carbohydrates, and lipids
To determine proteins, lipids, and total carbohydrate contents, adult insects were treated with three concentrations (30, 40, and 50 µL/mL) of T. pallescens and C. citratus EOs. The control insects used in these experiments were insects that suffered natural deaths. Four replicates were performed for each analysis.
Effect of EOs on proteins
A technique described by Bradford35 was used for the extraction and quantification of protein reserves. Coomassie Brilliant Blue G-250 (100 mg) was dissolved in 50 mL 95% ethanol (Prolabo; 96% Purity), and 100 mL 85% (w/v) phosphoric acid (Sigma) was added. The resulting solution was diluted to a final volume of 1 L. After crushing the individual insects in 400 µL of the Tris–HCl (20mM) (Sigma; Purity:99.5%) solution, the samples were incubated at 4 °C for 30 min to allow the proteins time to dissolve. An aliquot of 0.1 mL was transferred to a 12 × 100-mm test tube, 5 mL of Bradford reactive was added to the test tube and the contents were mixed by vortexing. The protein concentration was determined by spectrophotometry at 595 nm. The protein concentrations of each sample were determined against a standard curve constructed using 125, 250, 500, 1000 and 2000 µg bovine immunoglobulin G (IgG) dissolved in the same buffer as the samples. Before reading, the plates were gently stirred for 5 s to separate the protein aggregates.
Effect of EOs on carbohydrates
For the extraction and quantification of total carbohydrates, previously described methods were used36,37. Insects were ground in 400 µL of sodium sulfate (Prolabo) for 2 min, followed by the addition of 2 mL methanol (Prolabo; 99.8 purity). The tubes containing the homogenate were then centrifuged at 4 °C for 4 min at 2000 × g for 2 min. An aliquot of 100 µL was transferred to a new 12 × 75-mm tube, and 2 mL anthorone reactive was added followed by incubation in a 95 °C water bath for 17 min. The tubes were then placed in an ice bath for 10 min and the optical density at 625 nm was measured. For carbohydrates, a calibration standard curve was generated using a standard glucose solution (1 g/L). The blank was a 0.5 mg/mL glucose solution (5 mg of glucose in 10 mL of distilled water). A series of dilutions were performed to obtain the following glucose concentrations: 10, 20, 40, 60, 80, 100, and 200 µg/mL.
Effect of EOs on lipids
Lipid content was determined using methods described by Van Handel38 and Plaistow et al.39. The insects were crushed in 400 µL chloroform/methanol (1:1, v:v) solution. The supernatant was transferred to a clean tube (16 × 100 mm), which was retained in a water bath at 95 °C, placed inside a fume cupboard to allow the remaining solvent to evaporate. Then, 200 µL of concentrated (95%) sulfuric acid was added, and the solvent was allowed to evaporate at 90 °C for approximately 10 min. The sample was removed from the heating bath, allowed to cool, and 5 mL vanillin-phosphoric acid reagent (85%) was added. The samples were vortexed and then exposed to open air for 5 min to allow coloration to develop. The optical density of each sample was measured at 525 nm read after 25 min. The lipid concentration for each sample was determined against a standard curve constructed using 25, 50, 100, 200, 400, 800, and 1200 µg of commercial vegetable oil.
Data analysis
Generalized linear models (GLMs) were used to analyze the corrected mortality (CM%) and energy biomarker values. The CM% was subjected to probit analysis to obtain LC50 values with their confidence limits. The insect corrected mortality and energy biomarkers data for contact and fumigant tests were separately subjected to Multivariate Analysis of Variance (MANOVA) with pest species, essential oil type, insect occurrence (single or combined) and concentrations of EOs as main effects. Mortality was the response variable. Mean comparison was performed using Tukey’s post hoc test at 5% probability level. All analyses were performed using the statistical software R Studio 1.2.5019- R version 3.6.1.
Results
Chemical composition of EOs
Chemical compositions of T. pallescens and C. citratus EOs are given in Table 1. The chemical analysis of T. pallescens EO revealed more than 18 compounds, representing 92.38% of the total EO, and the major constituents were carvacrol (56.64%), p-cymene (16.36%) and thymol (8.71%). The chemical profile of C. citratus EO revealed more than 30 compounds, comprising 82.75% of the total EO components, with geraniol (20.86%), limonene (10.5%), and camphene (7.8%) identified as the major constituents.
Insecticidal activity
The tested EOs exhibited strong insecticidal activity against S. zeamais and T. castaneum adults. A behavioral change was also noticed in both treated insect pests, as indicated by the observation of increased aggregation and couplings between males and females.
Contact toxicity
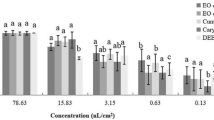
During contact toxicity bioassay, both T. pallescens and C. citratus EOs showed strong insecticidal activity towards S. zeamais and T. castaneum adults (Table 2). The intensity of CM% varied according to the target pest (F = 70.1; P = 0.000*), the type of EO (F = 31.6; P = 0.000*), the applied concentration (F = 138.2; P = 0.000*), and whether the species were alone or combined (F = 7; P = 0.09*). It was found that both insect pests were more sensitive to T. pallescens EO compared to C. citratus EO. The CM% gradually increased with increasing EO concentrations. Under separate population treatments, T. pallescens EO (10–50 µL/mL) resulted in CM% values of 35.0–100% and 42.5–90% for S. zeamais and T. castaneum, respectively, whereas CM% values for C. citratus EO (10–50 µL/mL) were 12.5–65% and 5–42.5%, respectively. When the contact tests were carried out on combined populations (S. zeamais and T. castaneum), the results revealed a significant adulticidal activity of EOs against these insect species. At the same application rates and the same exposure period, the CM% values for T. pallescens and C. citratus EOs (10–50 µL/mL) on S. zeamais adults were 10–100% and 5–77.5%, respectively, whereas the CM% values for T. castaneum adults were 15–80% and 5–35%, respectively.
The results of the contact toxicity trial expressed as LC50 of S. zeamais and T. castaneum adults, when tested separately or in combination are shown in Table 3. In the separate population treatments, T. pallescens EO was more active on S. zeamais (LC50 = 17.7 µL/mL) and T. castaneum adults (LC50 = 18.1 µL/mL). C. citratus EO was more effective against S. zeamais adults than against T. castaneum with LC50 values of 46.2 and 57.4 µL/mL, respectively. The LC50 values increased significantly under combined population treatment with levels of 8.59–49.9% for both insect pests. Similarly, T. pallescens EO demonstrated the highest insecticidal activity against S. zeamais and T. castaneum in the combined population with LC50 values of 20.8 and 36.1 µL/mL, respectively.
Fumigant toxicity
The CM% values determined from the fumigant toxicity test clearly indicated that both populations of insect pest species were greatly affected following their exposure to the tested EOs (Table 4). The intensity of CM% varied according to the target pest (F = 7.9; P = 0.006*), the type of EO (F = 110.7; P = 0.000*), the applied concentration (F = 204.9; P = 0.000*), and whether the species were alone or combined (F = 8.7; P = 0.04*).
Interestingly, the results of fumigant toxicity evaluation showed a pronounced increase in CM% compared with those of contact toxicity test. According to the GLM analysis, the population of S. zeamais appeared to have higher sensitivity to both EOs as compared to T. castaneum. The treatment of both separate and combined populations with T. pallescens EO showed higher CM% especially on S. zeamais which was more susceptible than T. castaneum.
Moreover, both pest species were more susceptible when treated alone than when treated together for both studied EOs. In the separate population, T. pallescens EO was found to be more active, with LC50 values of 15 and 15.5 µL/L air against S. zeamais and T. castaneum, respectively (Table 5). Under separate population treatments, T. pallescens EO (10–50 µL/mL) caused in CM% values of 25.5–100% and 20–95% for S. zeamais and T. castaneum, respectively, whereas CM% values for C. citratus EO (10–50 µL/mL) were 20–100% and 17.5–90%. A clear increase in the LC50 values (11.76 to 44.83%) was observed during the treatments of the combined population compared with the values measured in the separate population. Sitophilus zeamais was more sensitive than T. castaneum to the toxic effects of T. pallescens and C. citratus EOs, with LC50 values of 17 and 29.6µL/L air, respectively. When using contact test on combined populations (S. zeamais and T. castaneum), the CM% values for T. pallescens and C. citratus EOs (10–50 µL/mL) on S. zeamais adults were 17.5–100% and 15–85% for, whereas the CM% values for T. castaneum adults were 27.5–92.5% and 22.5–95%, respectively.
Effect of EOs on energy biomarkers
Protein content, lipid content, and carbohydrate levels were determined in S. zeamais and T. castaneum treated with T. pallescens as well as C. citratus EOs, and the results are shown in Tables 6 and 7.
Protein content values were significantly affected by the type (F = 98.54; P = 0.000**) and the concentration of the used EO (F = 20.09; P = 0.000*), the pest species (F = 7.08; P = 0.000*), and the toxicity test type (F = 22.28; P = 0.000*). In contact toxicity, S. zeamais and T. castaneum treated with both EOs showed protein levels of 12.57–15.81 µg/mg and 12.12–15.71 µg/mg, respectively. Whereas, in fumigant toxicity S. zeamais and T. castaneum treated with both EOs presented protein levels of 15.54–22.86 µg/mg and 11.55–18.42 µg/mg, respectively. Overall, all tested EO concentrations reduced the protein content values in both pest insect species when compared to the control. Significant decreases in protein contents (18.47 to 48.87%) were observed for S. zeamais treated with T. pallescens and C. citratus EOs during contact and fumigant tests. The protein levels in T. castaneum under the same conditions decreased by approximately 9.77 to 33.67%.
The carbohydrate contents in S. zeamais and T. castaneum were significantly affected by treatment with T. pallescens and C. citratus EOs (F = 137.8; P = 0.0000*) but were not affected by pest species (F = 0.31; P = 0.56), the type of toxicity test (F = 0.11; P = 0.1), or the EO concentrations (F = 0.3; P = 0.73). In contact toxicity assay, the carbohydrate levels of S. zeamais and T. castaneum treated with both EOs were 1.09–1.51 and 1.0–1.43 µg/mg, respectively. The values recorded during fumigation were 1.29–1.68 and 1.01–1.73 µg/mg, respectively. These results were significantly lower than control levels. Carbohydrates contents were significantly decreased in S. zeamais (12.78–43.32%) and T. castaneum (13.34–49.33%) following during fumigant and contact toxicity tests using both EO treatments.
Lipid content values were significantly affected by the type (F = 102.1; P = 0.0000*) and concentration of EO (F = 36.1; P = 0.000*), the pest species (F = 27.5; P = 0.009*), and the type of toxicity test (F = 23; P = 0.000*). In contact toxicity, the highest levels of lipids were recorded in S. zeamais treated with T. pallescens (1.60–2.59 µg/mg) and C. citratus (1.52–2.02 µg/mg) EOs. While, in fumigant toxicity the highest levels of lipids were recorded in S. zeamais treated with T. pallescens (1.44–2.95 µg/mg) and C. citratus (1.20–2.39 µg/mg) EOs. This response was more pronounced with T. pallescens EO than C. citratus EO. The obtained results indicated a significant increase in the lipids contents of S. zeamais and T. castaneum treated with both EOs, with values ranging from 3.56 to 37.68% and from 2.8 to 52.81%, respectively.
Discussion
In the conducted investigations, the chemical composition as well as insecticidal activity of T. pallescens and C. citratus EOs against S. zeamais and T. castaneum adults were assessed in separate and combined populations. According to the results, the major components of T. pallescens were identified as carvacrol (56.64%), p-cymene (16.36%), and thymol (8.71%). Our previous study revealed that carvacrol, thymol, γ-terpinene, and p-cymene were the major components of T. pallescens EO with percentage yields of 54.09, 16.24, and 8.47%, respectively, which is in quite agreement to the obtained results in this study40. The EO of the same plant (T. pallescens) has been reported to have similar composition41. Regarding C. citratus EO, geraniol (20.86%), limonene (10.50%), and camphene (7.80%) were found to be the major compounds, which is in accordance with previous findings40,42. However, the chemical composition of the C. citratus EO was different to that reported by Kumar et al.43 and Brügger et al.44 where neral, citral, 1,8-cineole, and geranyl acetate was detected at higher concentrations. These differences are probably due to the crop season and country origin45.
The studied EOs demonstrated significant insecticidal activity. The observed insecticidal activity and the differences observed in the efficiencies of the tested EOs might be attributable to the different bioactive compounds present in the different plant species. Chemical compounds with a wide spectrum of insecticidal effects include phenols (1,8 cineole and carvacrol), alcohols (α-terpineol, terpinen-4-ol and linalool), aldehydes and ketones (camphor and citronellal), and monoterpene hydrocarbons (Camphene, α-pinene and p-cimene)43,46,47,48,49. In a related study, α-pinene and β-pinene presented high insecticidal activity against T. castaneum50,51, whereas carvacrol, thymol, and p-cymene were more active against T. castaneum46. In a study conducted by Yildirim et al.47, twenty-eight monoterpenes, including monoterpene hydrocarbons and oxygenated monoterpenes, were found to display significant insecticidal effects against S. zeamais.
The tested EOs in our study are likely rich sources of these components. Benchabane et al.41 have studied the insecticidal activity of T. pallescens EO and reported that thymol and carvacrol were the components most responsible for this bioactivity. In this study, geraniol (20.86%), limonene (10.50%), and camphene (7.80%) were found to be the most abundant components in C. citratus EO. These compounds have also been reported to have insecticidal activities against a variety of pest insect species50,52,53.
Oyedejiet al.54 reported fumigant toxicity and contact activity of Citrus sinensis essential oil, and their constituents (including terpineol and linalool) against S. zeamais, with LC50 of 80.01 μL/L air and 95.63 μg/, respectively. Aryal et al.55 showed the stronger contact toxicity of Acorus calamus with LD50 value of 2.29 and 0.16% of EO concentration in scintillating vial bioassay to Maize weevil (S. zeamais). In the same technique, at 10% concentration S. zeamais adult showed highest repellent activity (98.75%) as compared to 5, 2.5 and 1.25% concentrations after 24 h of treatment. EO from inflorescences of Etlingera elatior had fumigant activity (LC50 = 0.83 μL/L of air) and contact activity (LD50 = 1.42 μg/adult) against adult forms of S. zeamais56. The EO from Lippia javanica (Burm. f.) showed fumigant toxicity to S. zeamais adult, with LD50 = 216 μg/cm3 air. This same EO also exhibited contact toxicity (LC50 = 6.22 mg/mL) and its major constituents (perillaldehyde (LC50 = 1.07 mg/mL) and linalool (LC50 = 1.82 mg/mL)) as well as a 1:1 mixture of these compounds (LC50 = 0.85 mg/mL), the mixture of the major compounds proved the most efficient57. The EO from Croton rudolphianus leaves exhibited toxicity against S. Zeamais by contact (LC50=70.64 μL/mL) and fumigation (64 μL/L in the air) caused the highest mortality (43.75%)58. Fouad et al.59 reported insecticidal activity of five EO (Citrus aurantifolia and Citrus reticulate, Eucalyptus citriodora, Melaleuca alternifolia and Mentha piperita), and their constituents (including Limonene, α-pinene and -α-pinene) against S. zeamais. The mean lethal concentrations (LC50) estimated for the EOs is variable between 1.04 and 2.56 μL g–1. Compared with 0.046 μL g–1 prorogued with deltamethrin-piperonyl butoxide insecticide. The contact and fumigant toxicity of EO against C. maculatus was comparable to Rosmarinus officinalis EO60, greater than Petroselinum sativum EO61 and less than Thymus persicus EO62.
Zhang et al.63 found that EO extracted from the leaves of Chamaecyparis obtusa demonstrated contact and Fumigant toxicity against T.castaneum adults, with respective LC50 values was 52.54 μg/adult of 7.09 μg/L air. In previous studies, it had been reported that the EO of Artemisia herba alba Asso, Juniperus phoenicea L and Rosmarinus officinalis exerted significant fumigant toxicity against T. castanum (LD50 = 12.4 μg/adult)64. LC50 values of EOs isolated from Curcuma aromatica salisb showed more contact (at 24 h, LC50 = 10.40 mg/cm2) and fumigant (at 24 h, LC50 = 18.18 mg/L air) toxicity against T. castaneum65. The contact toxicity studied with Cinnamomum glanduliferum (Wall.) leaf EO showed contact effect against T. castaneum, indicating a low LD50/LC50 value (12.13 μg/adult and 104.67 μg/cm2, respectively)66. The fumigant studies with C. austroindica and C. aromatica EOs showed higher toxicity against T. castaneum at the exposure time of 24 h (LC50= 35.65 & 29.10 μL/L), respectively67. Pervious study reported better contact activity for Thymus capitatus and Origanum compactum against T. castaneum. In the contact toxicity, LC50 values were 0.58 and 0.35 μL/cm2 after 24 h of exposure, respectively68.
The toxicity of T. pallescens and C. citratus EOs against two stored-product pests, S. zeamais and T. castaneum, were determined. The CM% results were found to vary with the type and concentration of the tested EOs and the insect species. Thymus pallescens EO demonstrated high toxicity compared to C. citratus EO. Adults of S. zeamais were more susceptible to both plant EOs than T. castaneum. The different responses observed between the two insect species could be attributed to morphological and behavioral differences. It was found that the insecticidal activity of both EOs was highly influenced by the tested toxicity method. Our results are in accordance with previous findings regarding insecticidal activities of various EOs and their major components against S. zeamais and T. castaneum34,46,58,69,70.
Furthermore, the obtained results indicated that LC50 values increased in the combined populations compared with the separate populations. The observed increase in LC50 values for both pest insects could be due to the increase in the total number of individuals. In addition, the synchronous presence of multiple primary pest species in a mixed-species population can affect control measures, particularly for targeted species11.
In fact, EO toxicity can be attributed to different mechanisms of action against the physiological processes in insect pests. Previous studies have shown that natural compounds can cause symptoms associated with neurotoxic activity, such as hyperactivity, seizures, and tremors, often followed by paralysis and death71. EOs and their major components also reported to result in the strong inhibition of AChE in congeneric insect pest species, such as S. oryzae13. This could explain the toxicity effects of T. pallescens and C. citratus EOs against S. zeamais and T. castaneum. The bioactive properties of EOs can vary depending on the molecules contained in the EOs, the insect species, the toxicity test used, and the dose used72.
Plant-derived EOs affect insect metabolism and development through various biochemical and physiological processes26,73. In this study, significant decreases in the levels of proteins and carbohydrates, and a significant increase in lipids have been recorded. The decreased protein contents observed for both pest insects may be associated with several factors, including a decrease in protein synthesis or an increase in protein breakdown to detoxify the bioactive molecules of EOs74,75. Yazdani et al.75 obtained similar results using EOs from Thymus vulgaris L. and Origanum vulgare L. against the hemolymph protein of lesser mulberry pyralid Glyphodes pyloalis Walker.
The carbohydrate contents significantly decreased following EO treatments in the tested pest insects. Similar results were obtained by Yazdani et al.76 in G. pyloalis following treatment with T. vulgaris and O. vulgare EOs and by Tarigan et al.77 in T. castaneum and Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae) treated with cardamom, cinnamon, and nutmeg EOs.
Insects typically convert carbohydrates into lipids and glycogens37, which could explain the decrease in carbohydrate levels and the increased lipid levels observed in the treated insects. The increase in lipid levels can also be explained by the establishment of insect teguments to prevent the harmful effects of EOs. According to Morgan78, the external covers of insects consist of an impermeable lipid layer, which typically contains alkanes, methyl branched alkanes, and alkenes. The observed increase in the lipid levels of the insect body may represent the activation of resistance mechanisms to counteract the stress of EOs. The obtained results agree also with the observations of Bouzar Essaïdi et al.79, who found that treatments with extracts from Lantana camara L. significantly enhanced lipid contents of the pine processionary moth Thaumetopea pytiocampa.
Conclusion
EOs provide an effective and ecologically sustainable solution for the control of insect pests. Our study demonstrated that T. pallescens and C. citratus EOs were effective against S. zeamais and T. castaneum and could be used as an alternative and “safe” control method for reducing the negative economic impacts associated with these insect pests, as part of an integrated pest management strategy for stored products. Our results also revealed that the synchronized occurrence of S. zeamais and T. castaneum reduced EO efficiencies and, consequently, increased the LC50 values.
Data availability
The data for this study is available from the second corresponding author upon reasonable request.
References
Klopfenstein, J., Ericksona, G. E. & Bergerb, L. L. Maize is a critically important source of food, feed, energy and forage in the USAT. Field Crops Res. 153(2013), 5–11. https://doi.org/10.1016/j.fcr.2012.11.006 (2012).
Ranum, P., Peña-Rosas, J. P. & Garcia-Casal, M. N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 1312(1), 105–112. https://doi.org/10.1111/nyas.12396 (2014).
Sallam, M.N. Insect damage. Damage on post-harvest. In Mejia, D., Lewis, B. (Eds) In Pho - Post-harvest Compendium. Food and Agriculture Organization of the United Nations, p. 37 (1999).
López-Castillo, L. M. et al. Postharvest insect resistance in maize. J. Stored Prod. Res. 77, 66–76. https://doi.org/10.1016/j.jspr.2018.03.004 (2018).
Napoleao, T. H., Agra-Neto, A. C., Pontual, E. V., Belmonte, B. R. & Paiva, P. M. G. Biology, ecology and strategies for control of stored-grain beetles: A review. In Beetles: Biodiversity, Ecology and Role in the Environment (ed. Stack, C.) 105–122 (Nova Science Publishers Inc, 2015).
De Oliveira, A. P. S. et al. Evaluation of the insecticidal activity of Moringa oleifera seed extract and lectin (WSMoL) against Sitophilus zeamais. J. Stored Prod. Res. 87, 1–8. https://doi.org/10.1016/j.jspr.2020.101615 (2020).
de Araújo, A. M. N. et al. Lethal and sublethal responses of Sitophilus zeamais populations to essential oils. J. Pest Sci. 90(2), 589–600. https://doi.org/10.1007/s10340-016-0822-z (2017).
Mikami, A. Y., Carpentieri-Pípolo, V. & Ventura, M. U. Resistance of maize landraces to the maize weevil Sitophilus zeamais Motsch. (Coleoptera: Curculionidae). Neotrop. Entomol. 41(5), 404–408. https://doi.org/10.1007/s13744-012-0054-8 (2012).
Yun, T. S., Park, S. Y., Yu, J., Hwang, Y. & Hong, K. J. Isolation and identification of fungal species from the insect pest Tribolium castaneum in rice processing complexes in Korea. Plant Pathol. J. 34(5), 356–366 (2018).
Trematerra, P., Ianiro, R., Athanassiou, C. G. & Kavallieratos, N. G. Behavioral interactions between Sitophilus zeamais and Tribolium castaneum: The first colonizer matters. J. Pest Sci. 88(3), 573–581. https://doi.org/10.1007/s10340-014-0633-z (2014).
Athanassiou, C. G., Kavallieratos, N. G. & Campbell, J. F. Competition of three species of Sitophilus on rice and maize. PLoS One 12(3), 1–12. https://doi.org/10.1371/journal.pone.0173377 (2017).
Corrêa, A. S., Pereira, E. J. G., Cordeiro, E. M. G., Braga, L. S. & Guedes, R. N. C. Insecticide resistance, mixture potentiation and fitness in populations of the maize weevil (Sitophilus zeamais). Crop Prot. 30(12), 1655–1666. https://doi.org/10.1016/j.cropro.2011.08.022 (2011).
Abdelgaleil, S. A. M., Mohamed, M. I. E., Shawir, M. S. & Abou-Taleb, H. K. Chemical composition, insecticidal and biochemical effects of essential oils of different plant species from Northern Egypt on the rice weevil, Sitophilus oryzae L. J. Pest Sci. 89(1), 219–229. https://doi.org/10.1007/s10340-015-0665-z (2016).
Ribeiro, B. M., Guedes, R. N. C., Oliveira, E. E. & Santos, J. P. Insecticide resistance and synergism in Brazilian populations of Sitophilus zeamais (Coleoptera: Curculionidae). J. Stored Prod. Res. 39(1), 21–31. https://doi.org/10.1016/S0022-474X(02)00014-0 (2002).
Sousa, A. H., Faroni, L. R. D. A., Guedes, R. N. C., Tótola, M. R. & Urruchi, W. I. Ozone as a management alternative against phosphine-resistant insect pests of stored products. J. Stored Prod. Res. 44(4), 379–385. https://doi.org/10.1016/j.jspr.2008.06.003 (2008).
Isman, M. B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 51, 45–66. https://doi.org/10.1146/annurev.ento.51.110104.151146 (2006).
Rebouh, N. Y. et al. Environmentally friendly wheat farming: biological and economic efficiency of three treatments to control fungal diseases in winter wheat (Triticum aestivum L.) under field conditions. Plants 11, 1566. https://doi.org/10.3390/plants11121566 (2022).
Lengai, G. M. W., Muthomi, J. W. & Mbega, E. R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 7, 1–3. https://doi.org/10.1016/j.sciaf.2019.e00239 (2020).
Ziaee, M. & Moharramipour, S. Effectiveness of medicinal plant powders on Sitophilus granarius and Tribolium confusum. J. Crop Prot. 2, 43–50 (2013).
Ziaee, M. The effects of topical application of two essential oils against Sitophilus granarius (Coleoptera: Curculionidae) and Tribolium confusum (Coleoptera: Tenebrionidae). J. Crop Prot. 3, 589–595 (2014).
Campolo, O., Giunti, G., Russo, A., Palmeri, V. & Zappalà, L. Essential oils in stored product insect pest control. J. Food Qual. 2018, 1–18. https://doi.org/10.1155/2018/6906105 (2018).
Trivedi, A., Nayak, N. & Kumar, J. Recent advances and review on use of botanicals from medicinal and aromatic plants in stored grain pest management. J. Entomol. Zool. Stud. 6(3), 295–300 (2018).
Yang, Y., Isman, M. B. & Tak, J. H. Insecticidal activity of 28 essential oils and a commercial product containing Cinnamomum cassia bark essential oil against Sitophilus zeamais Motschulsky. Insects 11(8), 1–15 (2020).
Rota, M. C., Herrera, A., Martinez, R. M., Sotomayor, J. A. & Jordán, M. J. Anti-microbial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control. 19(7), 681–687 (2008).
Giordani, R., Hadef, Y. & Kaloustian, J. Compositions and antifungal activities ofessential oils of some Algerian aromatic plants. Fitoterapia 79, 199–220 (2008).
Moutassem, D., Bellik, Y. & Sannef, M. E. H. Toxicity and repellent activities of Thymus pallescens and Cymbopogon citratus essential oils against Sitophylus granarius. Plant Prot. Sci. 57(4), 297–309. https://doi.org/10.17221/185/2020-PPS (2021).
Widelska, G., Stelmasiewicz, M., Skalicka-Woźniak, K., Oniszczuk, A. & Ludwiczuk, A. Antioxidant activity of lemongrass essential oil and its constituents. Facta Univ. FU Phys. Chem. Technol 16, 132 (2018).
Martinazzo, A. P., de Oliveira, F. D. S. & de Souza Teodoro, C. E. Antifungal activity of Cymbopogon citratusessential oil against Aspergillus flavus. Ciênc. Nat. 2019(41), 20 (2019).
Shendurse, A. M. et al. Phytochemical screening and antibacterial activity of lemongrass (Cymbopogon citratus) leaves essential oil. J. Pharmacogn. Phytochem. RJPP 10, 445–449 (2021).
Regnault-Roger, C. The potential of botanical essential oils for insect pest control. Integr. Pest Manag. Rev. 2(1), 25–34. https://doi.org/10.1023/A:1018472227889 (1997).
Rajendran, S. & Sriranjini, V. Plant products as fumigants for stored-product insect control. J. Stored Prod. Res. 44(2), 126–135. https://doi.org/10.1016/j.jspr.2007.08.003 (2008).
Elyemni, M. et al. Extraction of essential oils of Rosmarinus officinalis L. by two different methods: Hydrodistillation and microwave assisted hydrodistillation. Sci. World J. 2019, 1–6. https://doi.org/10.1155/2019/3659432 (2019).
Tuberoso, C. I. G., Kowalczyk, A., Sarritzu, E. & Cabras, P. Determination of antioxidant compounds and antioxidant activity in commercial oil seeds for foods use. Food Chem. 103, 1494–1501 (2007).
Tapondjou, A. L., Adler, C., Fontem, D. A., Bouda, H. & Reichmuth, C. Bioactivities of cymol and essential oils of Cupressus sempervirens and Eucalyptus saligna against Sitophilus zeamais Motschulsky and Tribolium confusum du Val. J. Stored Prod. Res. 41(1), 91–102. https://doi.org/10.1016/j.jspr.2004.01.004 (2005).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72(1–2), 248–254. https://doi.org/10.1016/0003-2697(76)90527-3 (1976).
Van Handel, E. Rapid determination of glycogen and sugars in mosquitoes. J. Am. Mosq. Control Assoc. 1(3), 299–301 (1985).
Sönmez, E. & Gülel, A. Effects of different temperatures on the total carbohydrate, lipid and protein amounts of the bean beetle, Acanthoscelides obtectus Say (Coleoptera: Bruchidae). Pak. J. Biol. Sci. 11(14), 1803–1808 (2008).
Van Handel, E. Rapid determination of total lipids in mosquitoes. J. Am. Mosq. Control Assoc. 1(3), 302–304 (1985).
Plaistow, S. J., Bollache, L. & Cézilly, F. Energetically costly precopulatory mate guarding in the amphipod Gammarus pulex: Causes and consequences. Anim. Behav. 65(4), 683–691. https://doi.org/10.1006/anbe.2003.2116 (2003).
Moutassem, D., Belabid, L., Bellik, Y., Ziouche, S. & Baali, F. Efficacy of essential oils of various aromatic plants in the biocontrol of Fusarium wilt and inducing systemic resistance in chickpea seedlings. Plant Prot. Sci. 55(3), 202–217. https://doi.org/10.17221/134/2018-PPS (2019).
Benchabane, O., Hazzit, M., Mouhouche, F. & Baaliouamer, A. Influence of extraction duration on the chemical composition and biological activities of essential oil of Thymus pallescens de Noé. Arab. J. Sci. Eng. 40(7), 1855–1865. https://doi.org/10.1007/s13369-015-1694-x (2015).
Bassolé, I. H. N. et al. Chemical composition and antimicrobial activity of Cymbopogon citratus and Cymbopogon giganteus essential oils alone and in combination. Phytomedicine 18(12), 1070–1074. https://doi.org/10.1016/j.phymed.2011.05.009 (2011).
Kumar, P., Mishra, S., Malik, A. & Satya, S. Housefly (Musca domestica L) control potential of Cymbopogon citratus Stapf (Poales: Poaceae) essential oil and monoterpenes (citral and 1,8-cineole). Parasitol. Res. 112(1), 69–76. https://doi.org/10.1007/s00436-012-3105-5 (2013).
Brügger, B. P. et al. Bioactivity of the Cymbopogon citratus (Poaceae) essential oil and its terpenoid constituents on the predatory bug, Podisus nigrispinus (Heteroptera: Pentatomidae). Sci. Rep. 9(1), 1–8. https://doi.org/10.1038/s41598-019-44709-y (2019).
Bellik, Y. et al. Antioxidant activity of the essential oil and oleoresin of Zingiber Officinale Roscoe as affected by chemical environment. Int. J. Food Prop. 16, 1304–1313. https://doi.org/10.1080/10942912.2011.584257 (2013).
Kim, S. I. et al. Toxicity and repellency of Origanum essential oil and its components against Tribolium castaneum (Coleoptera: Tenebrionidae) adults. J. Asia-Pac. Entomol. 13(4), 369–373 (2010).
Yildirim, E., Emsen, B. & Kordali, S. Insecticidal effects of monoterpenes on Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). J. Appl. Bot. Food Qual. 86(1), 198–204. https://doi.org/10.5073/JABFQ.2013.086.027 (2013).
Pavela, R., Žabka, M., Bednář, J., Tříska, J. & Vrchotová, N. New knowledge for yield, composition and insecticidal activity of essential oils obtained from the aerial parts or seeds of fennel (Foeniculum vulgare Mill.). Ind. Crops Prod. 83, 275–282. https://doi.org/10.1016/j.indcrop.2018.01.021 (2016).
Park, J. H., Jeon, Y. J., Lee, C. H., Chung, N. & Lee, H. S. Insecticidal toxicities of carvacrol and thymol derived from Thymus vulgaris Lin. against Pochazia shantungensis Chou & lu., newly recorded pest. Sci. Rep. 7, 1–7. https://doi.org/10.1038/srep40902 (2017).
García, M., Donadel, O. J., Ardanaz, C. E., Tonn, C. E. & Sosa, M. E. Toxic and repellent effects of Baccharis salicifolia essential oil on Tribolium castaneum. Pest Manag. Sci. 61(6), 612–618. https://doi.org/10.1002/ps.1028 (2005).
Olivero-Verbel, J., Nerio, L. S. & Stashenko, E. E. Bioactivity against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) of Cymbopogon citratus and Eucalyptus citriodora essential oils grown in Colombia. Pest Manag. Sci. 66(6), 664–668. https://doi.org/10.1002/ps.1927 (2010).
Park, I. K., Lee, S. G., Choi, D. H., Park, J. D. & Ahn, Y. J. Insecticidal activities of constituents identified in the essential oil from leaves of Chamaecyparis obtusa against Callosobruchus chinensis (L.) and Sitophilus oryzae (L.). J. Stored Prod. Res. 39, 375–384 (2003).
Owolabi Moses, S., Ogundajo, A. L., Alafia, A. O., Ajelara, K. O. & Setzer, W. N. Composition of the essential oil and insecticidal activity of Launaea taraxacifolia (Willd.) Amin ex C. Jeffrey growing in Nigeria. Foods 9, 914. https://doi.org/10.3390/foods9070914 (2020).
Oyedeji, A. O., Okunowo, W. O., Osuntoki, A. A., Olabode, T. B. & Ayo-folorunso, F. Insecticidal and biochemical activity of essential oil from Citrus sinensis peel and constituents on Callosobrunchus maculatus and Sitophilus zeamais. Pestic. Biochem. Physiol. 168, 104643. https://doi.org/10.1016/j.pestbp.2020.104643 (2022).
Aryal, S., Poudel, A., Kafle, K. & Aryal, L. N. Insecticidal toxicity of essential oil of Nepalese Acorus calamus (Acorales:Acoraceae) against Sitophilus zeamais (Coleoptera: Curculionidae). Heliyon 9, e22130. https://doi.org/10.1016/j.heliyon.2023.e22130 (2023).
de Lira Pimentel, C. S. et al. Insecticidal potential of essential oil from inflorescences of Etlingera elatior and its major constituents against Sitophilus zeamais. Ind. Crops Prod. 203, 117154 (2023).
Kamanula, J. F. et al. Chemical variation and insecticidal activity of Lippia javanica (Burm. f.) Spreng essential oil against Sitophilus zeamais Motschulsky. Ind. Crops Prod. 110, 75–82. https://doi.org/10.1016/j.indcrop.2017.06.036 (2017).
de Araújo Ribeiro, I. A. T. et al. Chemical characterization and insecticidal effect against Sitophilus zeamais (maize weevil) of essential oil from Croton rudolphianus leaves. Crop Prot. 129, 105043 (2020).
Fouad, H. A., da Camara, C. A. G., de Moraes, M. M. & de Melo, J. P. R. The synergistic effects of five essential oils and eight chiral compounds on deltamethrin-piperonyl butoxide insecticide against Sitophilus zeamais (Coleoptera: Curculionidae). J. Asia-Pac. Entomol. 26, 102072. https://doi.org/10.1016/j.aspen.2023.102072 (2023).
Güdek, M. & Çetin, H. Fumigant toxicity on adults of Callosobruchus maculatus (F.)(Coleoptera: Chrysomelidae) of essential oil from Rosmarinus officinalis L. and its side effects on chickpea grains. J. Essent. Oil Bear. Plants 20(1), 272–281 (2017).
Massango, H. G. L. L. et al. Toxicity and metabolic mechanisms underlying the insecticidal activity of parsley essential oil on bean weevil, Callosobruchus maculatus. J. Pest Sci. 90, 723–733 (2017).
Moharramipour, S., Taghizadeh, A., Meshkatalsadat, M. H., Talebi, A. A. & Fathipour, Y. Repellent and fumigant toxicity of essential oil from Thymus persicus against Tribolium castaneum and Callosobruchus maculatus. Commun. Agric. Appl. Biol. Sci. 73(3), 639–642 (2008).
Zhang, L. et al. The component of the Chamaecyparis obtusa essential oil and insecticidal activity against Tribolium castaneum (Herbst). Pestic. Biochem. Physiol. 195, 105546. https://doi.org/10.1016/j.pestbp.2023.105546 (2023).
Boukraa, N. et al. Insecticidal and repellent activities of Artemisia herba alba Asso, Juniperus phoenicea L and Rosmarinus officinalis L essential oils in synergized combinations against adults of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Biocatal. Agric. Biotechnol. 45, 102513. https://doi.org/10.1016/j.bcab.2022.102513 (2022).
SulhathAminu, S. T. A., Visakh, N. U., Pathrose, B. & George, S. B. Investigating the insecticidal properties of essential oils extracted from wild turmeric (Curcuma aromatica salisb) leaves waste against three key stored product pests. Sustain. Chem. Pharm. 38, 101482. https://doi.org/10.1016/j.scp.2024.101482 (2024).
Qi, Y.-T. et al. Temporal variation of chemical profiles and insecticidal properties of Cinnamomum glanduliferum (Wall.) Nees leaf essential oil. Ind. Crops Prod. 211, 118166. https://doi.org/10.1016/j.indcrop.2024.118166 (2024).
Johnson, A. J. et al. Insecticidal properties of Clausena austroindica leaf essential oil and its major constituent, trans-anethole, against Sitophilus oryzae and Tribolium castaneum. Ind. Crops Prod. 182, 114854. https://doi.org/10.1016/j.indcrop.2022.114854 (2022).
Annaz, H. et al. Chemical profiling and bioactivities of essential oils from Thymus capitatus and Origanum compactum against Tribolium castaneum. Heliyon 10, e26102. https://doi.org/10.1016/j.heliyon.2024.e26102 (2024).
Li, W. Q., Jiang, C. H., Chu, S. S., Zuo, M. X. & Liu, Z. L. Chemical composition and toxicity against Sitophilus zeamais and Tribolium castaneum of the essential oil of Murraya exotica aerial parts. Molecules 15(8), 5831–5839. https://doi.org/10.3390/molecules15085831 (2010).
Chu, S. S., Feng Hu, J. & Liu, Z. L. Composition of essential oil of Chinese Chenopodium ambrosioides and insecticidal activity against maize weevil, Sitophilus zeamais. Pest Manag. Sci. 67(6), 714–718. https://doi.org/10.1002/ps.2112 (2011).
Kostyukovsky, M., Rafaeli, A., Gileadi, C., Demchenko, N. & Shaaya, E. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: Possible mode of action against insect pests. Pest Manag. Sci. 58(11), 1101–1106. https://doi.org/10.1002/ps.548 (2002).
Devi, M. A., Sahoo, D., Singh, T. B. & Rajashekar, Y. Toxicity, repellency and chemical composition of essential oils from Cymbopogon species against red flour beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). J. Consum. Prot. Food Saf. https://doi.org/10.1007/s00003-019-01264-y (2019).
Senthil-Nathan, S. Physiological and biochemical effect of neem and other Meliaceae plants secondary metabolites against Lepidopteran insects. Front. Physiol. 4(359), 1–17. https://doi.org/10.3389/fphys.2013.00359 (2013).
Vijayaraghavan, C., Sivakumar, C., Kavitha, Z. & Sivasubramanian, P. Effect of plant extracts on biochemical components of cabbage leaf webber Crocidolomia binotalis, Zeller. J. Biopestic. 3, 275–277 (2010).
Ranjini, K. D., Ranjini, K. R., Beegum, T. P. N. & Nambiar, J. G. Botanical extracts influence the protein and free amino acid concentration of fat body in the mango leaf webber Orthaga exvinacea Hampson (Lepidoptera: Pyralidae). J. Entomol. Zool. Stud. 4(1), 464–468 (2016).
Yazdani, E., Sendi, J. J. & Hajizadeh, J. Effect of Thymus vulgaris L. and Origanum vulgare L. essential oils on toxicity, food consumption, and biochemical properties of lesser mulberry pyralid glyphodes pyloalis walker (Lepidoptera: Pyralidae). J. Plant Prot. Res. 54(1), 53–61. https://doi.org/10.2478/jppr-2014-0008 (2014).
Tarigan, S. I., Dadang, S. & Harahap, I. Toxicological and physiological effects of essential oils against Tribolium castaneum (Coleoptera: Tenebrionidae) and Callosobruchus maculatus (Coleoptera: Bruchidae). J. Biopestic. 9(2), 135–147 (2016).
Morgan, E. D. Biosynthesis in Insects 226 (Royal Society of Chemistry, 2004).
Bouzar Essaïdi, K., Allal-Benfekih, L. & Djazouli, Z. E. Comparative effects of biological treatments alone or in combination on the energy reserve contents of caterpillars of the pine processionary Thaumetopea pytiocampa schiff (Lepidoptera, Notodontidae). Revue Agrobiol. 6, 47–52 (2014).
Acknowledgements
This work was supported by the Laboratory of Characterization and Valorization of Natural Resources, Faculty of Nature and Life Sciences, Mohamed El Bachir El Ibrahimi University.
Funding
This paper has been supported by the RUDN University Strategic Academic Leadership Program.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.D., T.B.; methodology, Y.B., M.D.; software, A.O.U.; validation, Y.B. and N.Y.R.; formal analysis, Z.R., O.D.K.; investigation, Z.R., A.O.U., O.D.K.; resources, M.D., T.B.; data curation, Y.B.; writing—original draft preparation, M.D., T.B.; writing—review and editing, Y.B., N.Y.R., N.J.K.; visualization, M.D., N.Y.R.; supervision, T.B., M.D., N.J.K. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moutassem, D., Boubellouta, T., Bellik, Y. et al. Insecticidal activity of Thymus pallescens de Noë and Cymbogon citratus essential oils against Sitophilus zeamais and Tribolium castaneum. Sci Rep 14, 13951 (2024). https://doi.org/10.1038/s41598-024-64757-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64757-3
- Springer Nature Limited