Abstract
In our retrospective study, we aimed to investigate the relationship between urinary chloride (uCl−) and selected clinical and laboratory biomarkers, renal function, and patient outcomes in the acute heart failure (AHF) population. We divided 248 adult patients (≥ 18 years) with AHF into two groups: low uCl− (< 115 mmol/L) and high uCl−. The mean age of the patient group was 70.2 ± 12.6, and 182 patients were male (73.4%). Clinical endpoints included in-hospital mortality, one-year mortality, and a composite endpoint of one-year mortality and rehospitalization for heart failure. Patients were followed up for at least one year. Relevant clinical and baseline biomarker data were collected, including markers concerning inflammation, liver and kidney function, perfusion and congestion, iron status, cardiac remodeling, gasometry, renin and aldosterone. Low uCl− was associated with worse in-hospital outcomes, including higher in-hospital mortality (7.7% vs. 1.4%, p = 0.014), the need for inotropic support (20.19% vs. 2.08%, p ≤ 0.001), worsening of HF during therapy (17.31% vs. 4.86%, p ≤ 0.001), and the need for treatment in an intensive cardiac care unit (33.65% vs. 15.28%, p ≤ 0.001). Low uCl− was a significant predictor of one-year mortality (40.4% vs. 16.7%, p < 0.05) and the composite outcome (HR 2.42, 95% CI 1.43–4.08, p < 0.001). In the multivariable analysis, uCl− was independently associated with the risk of one-year mortality (HR 0.92, 95% CI 0.87–0.98, p < 0.05) and the composite outcome (HR 0.95, 95% CI 0.92–0.99, p < 0.05). Our findings suggest that low uCl− is a marker of more advanced heart failure, activation of the renin–angiotensin–aldosterone system and is related to worse one-year outcomes.
Similar content being viewed by others
Introduction
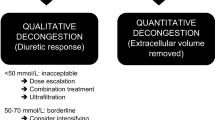
Acute heart failure (AHF) is a clinical condition in which the signs and symptoms of heart failure (HF) suddenly appear or worsen, requiring immediate evaluation and treatment1. Urine composition analysis has been shown to provide significant clinical information during the decongestion of AHF patients and is recommended as part of care in that population2. To date, chloride (Cl−) has often taken a back seat in terms of clinical studies concerning HF and fluid dynamics, with sodium (Na+) and potassium (K+) being the focus of those studies3,4,5. However, recently, this has changed, and increasing attention is being placed upon the role of Cl−, "chloride theory" and the potential roles of serum Cl− in kidney function and in fluid distribution within the body6,7,8,9,10. Urine Cl− (uCl−) is the main electrolyte involved in the regulation of renin secretion in the macula densa. Therefore, it is believed to be an instrument directly responsible for the adjustment of renal response and diuresis11,12,13,14. Thus, clinical data and studies analyzing the relationship between uCl− and worsening HF outcomes will be of evaluative, therapeutic, and prognostic value. Despite a strong pathophysiological background, data on Cl− (both urine or serum) in an AHF setting are scarce. Therefore, our study looks into the relationship between uCl− in terms of selected clinical and laboratory biomarkers, renal function, and patient outcomes in the AHF population. Finally, we suggest how these findings fit into our emerging understanding of the role of Cl− in HF pathophysiology.
Methods
Study population
This was a single-center, observational study that was undertaken in the Centre of Heart Diseases, 4th Military Hospital, Wroclaw, Poland between January 2016 and September 2017. All adult patients (≥ 18 years) hospitalized with AHF as the primary cause of hospitalization, treated with intravenous furosemide at admission, who expressed willingness to participate by signing an informed consent form, were enrolled. Exclusion criteria comprised the following: cardiogenic shock, diagnosis of acute coronary syndrome, severe liver disease, end-stage renal disease requiring renal replacement therapy, and evidence of infection. Patient treatment adhered to the ESC guidelines, as per the recommendations of attending physicians1,15. The research study was approved by the local ethics committee and was conducted in accordance with the Declaration of Helsinki.
For the purpose of this analysis, the patient population was divided into two groups based on urine Cl−: a low uCl− < 115 mmol/L and a high uCl− ≥ 115 mmol/L group, as determined from the first urine sample collected from the patients. Due to the lack of an available, clinically used cut-off point for uCl−, the cut-off was established arbitrarily as a value between the median and Youden index cut-point.
Study design
Following admission to hospital, detailed information concerning patient demographics (including history of HF), clinical history, comorbidities, previous therapies, and physical findings was collected. Dyspnea was measured using a self-reported 10-point Likert scale at the time of hospital admission, where a score of 0 means ‘absence of dyspnea’ and 10 points indicates ‘the most severe dyspnea/maximal dyspnea’. Clinical assessments, alongside venous blood and urine samples, were collected at baseline and the following subsequent timepoints: day 1, day 2, and discharge. The samples were also centrifuged and frozen at − 70 °C for additional prespecified analysis. A thorough literature review was undertaken, and biomarkers were selected on the basis of their relation to pertinent HF and hypochloremic pathophysiological pathways. The selected biomarkers are those involved in inflammation (CRP, IL-6, IL-22, WBC), liver and kidney function (AST, ALT, bilirubin, albumin, creatinine, blood urea), perfusion and congestion (NT-proBNP, lactate), iron status (Fe, total iron binding capacity, sTfR, Ferritin) and cardiac remodeling (follistatin, lipocalin, GDF-15, Galectin-3), gasometry (Na+, Osmolarity, Bicarbonate, Total Carbon Dioxide, pH), and RAAS markers (Renin, Aldosterone). Urine biomarkers of interest included sodium, potassium, chloride, urea, and creatinine.
In our study, baseline urine samples were obtained prior to the first in-hospital dose of intravenous furosemide. The only exception was when the patient was unable to provide a sample before furosemide administration, in which case the sample was collected at the first available moment. Samples were subsequently taken at day 1, day 2, and at discharge.
In accordance with the standard clinical practice at the hospital, all patients were instructed to limit their fluid intake to 1.5–2 L over a 24-h period alongside being advised to limit their daily sodium intake during their hospitalization.
The properties of each marker and evidence in HF and hypochloremia have been previously described elsewhere16,17,18,19,20,21,22. Information regarding the biomarkers of interest was not available to the treating physicians, and neither clinical nor therapeutic decisions were influenced by these results.
Laboratory measurements in peripheral blood and urine
Laboratory parameters were assessed using standard methods in our laboratory, including plasma NT-proBNP (N-Terminal Pro-B-Type Natriuretic Peptide) (method: immunoenzymatic, Siemens, Marburg, Germany) and troponin (TNI) (method: immunoenzymatic, single Dimension RxLMax, Siemens). Serum sTfR (mg/L) was measured from plasma samples frozen at − 70 °C using immunonephelometry (Siemens Healthcare Diagnostics, Inc., Deerfield, IL, USA). Both renin and aldosterone were assessed at day 1 and at discharge from frozen samples. The upper limit of normal (ULN) for renin was 46 (µIU/mL) (method: chemiluminescent immunoassay-CLIS, LIASON), and for aldosterone, it was 23.2 (ng/dL) (method: chemiluminescent immunoassay-CLIS, LIASON). The remaining biomarkers of interest were quantified using the Quantikine ELISA Immunoassay kit by R&D Systems. This assay employs the quantitative sandwich enzyme immunoassay technique to assess the following proteins: GDF-15 (also known as macrophage inhibitory cytokine-1 (MIC-1)) (n = 79), Follistatin (FS), Lipocalin-2 (NGAL) (n = 159), Il-6, and Il-22 (n = 159). The measurement of absorbance was performed using the Synergy/HTX multi-mode reader analyzer. The research was carried out in the laboratory of the Department of Clinical Pharmacology at Wroclaw Medical University.
Study outcomes
The clinical endpoints of the study were:
-
(i)
In-hospital mortality
-
(ii)
One-year mortality
-
(iii)
Composite endpoint of one-year mortality and rehospitalization for heart failure
Clinical follow-up
Discharged patients were followed-up at the HF clinic for at least one year. Information regarding rehospitalizations and survival status was obtained directly from patients or their relatives (telephone contact), from the HF clinic database, from the hospital system or from the national citizen registry by the investigators, who were blinded to the biomarker results. There were no instances of patient loss during the follow-up period.
Statistical analysis
Continuous variables with a normal distribution were presented using means and ± standard deviation, variables with skewed distribution were described by medians with [upper and lower quartiles], and categorized variables were given as numbers and percentages. The statistical significance of differences between groups was assessed using the t-test, Mann–Whitney U-test, or Kruskal–Wallis test. The normality of the distributions for these variables was evaluated by three different statistical tests: the Kolmogorov–Smirnov test, Lillefors test, and the Shapiro–Wilk test. To determine the suggested cutoff value of uCl− in our population, we performed an Area Under Curve (AUC) analysis and determined the Youden index cut-point. Variance Inflation Factor was calculated to establish the degree of collinearity between analyzed variables (uNa+ and uCl−). Cox proportional hazards models were used to calculate the hazard ratios (HR) with their corresponding 95% confidence intervals (95% CI) for all-cause mortality. Multivariable analyses were adjusted for age, ejection fraction, systolic blood pressure at admission, hemoglobin, NT-proBNP, and blood urea nitrogen. The impact of uCl− on the study outcomes (one-year mortality and composite outcome) was analyzed both as a continuous variable and as a categorical variable. Kaplan–Meier survival curves were constructed to demonstrate survival probability. The p < 0.05 level was considered statistically significant. Statistical analyses were performed using STATISTICA 12 (StatSoft Polska Sp. Z o.o., Krakow, Poland).
Institutional review board statement
The research study was approved by the ethics committee of Wroclaw Medical University (387/2015) and was conducted in accordance with the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all subjects involved in the study.
Results
Baseline characteristics
The study population consisted of 248 patients, of which 182 (73.4%) were male, with a mean (± SD) age of 70.1 ± 12.6 years. The mean left ventricle ejection fraction (LVEF) was 37 ± 14%, and ischemic HF etiology was present in 124 (50%) patients. On admission, the mean systolic blood pressure, serum Na+, hemoglobin, and serum creatinine were 134 ± 31 mmHg, 139 ± 4 mmol/L, 13.3 ± 2.0 g/dL, and 1.36 ± 0.52 mg/dL, respectively. The median [upper and lower quartiles] plasma concentrations of NT-proBNP and troponin I were 5618 (3431–11,750) pg/mL and 0.06 (0.03–0.16) ng/mL, respectively. Baseline laboratory tests and concomitant medications are presented in Table 1. The mean uCl− level at admission was 130.7 ± 65.6 mmol/L, with low uCl− observed in 104 (41.9%) patients. The cutoff to define the low uCl− group was set at < 115 mmol/L. The suggested Youden cut-point was 113 mmol/L (refer to Supplementary Fig. 1).
Comparison of clinical and basic laboratory characteristics by uCl−
Lower levels of systolic and diastolic blood pressure on admission were observed in the low uCl− group: 128.6 ± 28.8 vs 138.1 ± 32.9 mmHg, p = 0.02 and 75.8 ± 15.8 vs. 81.4 ± 16 mmHg, p < 0.005, respectively. There were no statistically relevant differences in heart rate (HR) or reported dyspnea on admission between both groups. However, patients with low uCl− presented significantly lower serum Na+ and lower serum Cl− when compared with the high uCl− group with 138 ± 4.5 vs. 140 ± 4 mmol/L, p ≤ 0.001 and 110.2 ± 6.0 vs 112.9 ± 4.8 mmol/L, p = 0.003, respectively. The analysis of kidney function tests revealed statistically significant differences between the low uCl− and high uCl− groups, and so, in terms of creatinine at admission, creatinine at 24 h after admission and blood urea at admission, these values were significantly higher in the low uCl− group with 1.47 ± 0.6 vs. 1.28 ± 0.4 mg/dL, p = 0.005; 1.4 ± 0.6 vs. 1.2 ± 0.4 mg/dL, p = 0.006 and 68 ± 36 vs. 53 ± 25 mg/dL, p ≤ 0.001, respectively. No significant differences in liver function tests, blood count and troponin, all analyzed on admission, could be observed between both groups. However, albumin was lower in the low uCl− group, with values of 3.7 ± 0.4 vs. 3.8 ± 0.4 (g/dL), p = 0.031. There was a statistically significant difference in the length of hospitalization between both groups, with 10 ± 8 in the low uCl− group vs. 7 ± 4 days, p = 0.001 (Table 1).
Comparison of selected biomarkers between uCl− profiles
No significant difference was observed in selected biomarkers related to inflammation, including WBC, CRP, IL-6, and IL-12, between the low uCl− and high uCl−groups (Table 2). Of the several remodeling markers examined, lipocalin/NGAL and follistatin differed significantly between the low uCl− and high uCl− groups. Lipocalin/NGAL levels were higher in the low uCl− group (93.6 ± 54.8 vs. 76.3 ± 51.6 ng/mL, p = 0.04), while follistatin levels were lower in the high uCl− group (2102.7 ± 1391.8 vs. 2714.9 ± 1815.5 pg/mL, p = 0.005). NT-proBNP was significantly lower on admission in the low uCl− group, with values of 5258 (2958–9980) vs 6283 (4074–12,496) pg/mL, p = 0.033. However, the reverse was observable at discharge with higher NT-proBNP levels in the low uCl− group (3545 (2143–7750) vs 2903 (1694–5211) pg/mL, p = 0.024).
Regarding iron status, only sTfR at admission displayed a statistically relevant difference between the two groups, with higher levels recorded in the low uCl− group (2.2 ± 0.9 vs. 1.8 ± 0.7 (mg/L), p ≤ 0.001).
The renin–angiotensin–aldosterone system in different uCl− profiles
The renin–angiotensin–aldosterone system (RAAS) demonstrated significant differences in the majority of variables analyzed. Renin levels at day 1, but not at discharge, were higher in the low uCl− group (70.0 (9.1–300.2) vs 17.6 (4.4–61.9) µIU/mL, p = 0.001 and 47.6 (13.0–343.6) vs 44.7 (10.9–152.2) µIU/mL, p = 0.159, respectively). Aldosterone levels at day 1 and discharge were significantly higher in the low uCl− group (13.1 (7.9–27.4) vs 9.4 (6.2–13.5) and 11.3 (6.6–18.4) vs 8.8 (5.8–13.8) ng/dL), respectively, both p < 0.001 (Table 2).
In the multivariable analysis (corrected for systolic and diastolic blood pressure at admission, renin, serum Na+, serum osmolarity, serum creatinine, and NT-proBNP), only aldosterone assessed at admission to the hospital was found to be significantly associated with urine chloride (beta- 0.22), p = 0.018.
Comparison of urine biomarkers between the low uCl− and high uCl− groups
We observed in the low uCl− group, the following lower biomarker levels: uNa+; 67.3 ± 28.1 vs. 100.6 ± 33.6 mmol/L, p ≤ 0.001, uK+; 26.9 ± 13.4 vs. 33.1 ± 16.2 mmol/L, p = 0.002, urine cations (Na+ + K+); 88.9 ± 21 vs 161 ± 70 mmol/L, p < 0.001 and uCl−/urine cations index; 0.96 ± 0.19 vs 1.33 ± 0.99, p < 0.001 (Table 3). The correlation between uCl− and uNa+ in our population is high r ~ 0.7, p < 0.001, however the variance inflation factor in the model was low, ranging between 1.02 and 1.03.
Differences in in-hospital outcomes between the low uCl− and high uCl− groups
All in-hospital outcomes were significantly worse in the low uCl− group. In terms of in-hospital mortality, eight (7.7%) patients experienced the event in the low uCl− group vs. two (1.4%) in the high uCl− group, p = 0.014. Other clinical outcomes were also worse in the low uCl− group, such as the need for inotropic support (20.19% low uCl− vs. 2.08% high uCl−, p ≤ 0.001), worsening of HF during therapy (17.31% low uCl− vs. 4.86% high uCl−, p ≤ 0.001), and the need for treatment in an intensive cardiac care unit (33.65% low uCl− vs. 15.28% high uCl− group, p ≤ 0.001) (Fig. 1).
The prognostic significance of low uCl− in AHF
One-year mortality in the low uCl− group compared to the high uCl− group was 40.4% (42 events) vs. 16.7% (24 events), p < 0.05. The probability of survival in both groups is presented in Fig. 2, and the probability of survival without any event in both groups is presented in Fig. 3. The low uCl− group was at significantly higher risk of one-year mortality and the composite outcome, with a hazard ratio (HR) of 95% confidence interval (95% CI) 2.42 (1.43–4.08) and 2.20 (1.44–3.36), respectively, both p < 0.001 (Table 4). In the multivariable model (after adjustments for systolic blood pressure, serum creatinine, ejection fraction, age, bilirubin, and NT-proBNP at admission) uCl− , evaluated as a continuous variable (per 10 mmol/L change) was related to the risk of both one-year mortality and the composite outcome, with HRs of (95% CI) 0.92 (0.87–0.98) and 0.95 (0.92–0.99), respectively, both p < 0.05 and when evaluated per standard deviation, comparable, although weaker, results were observed with HRs of (95%CI) 0.56 (0.38–0.84) and 0.74 (0.57–0.98), respectively, both p < 0.05 (Table 4). Comparable results were observed when the multivariable model adjusted for gender, estimated glomerular filtration rate (eGFR) at admission, atrial fibrillation at admission, NT-proBNP at admission, chronic obstructive pulmonary disease (COPD) and troponin I (TNI) at admission (Supplementary Table A).
In a subgroup of patients (n = 172) that underwent simultaneous assessment of Cl− in urine and serum we examined the impact of these markers on prognosis. In a multivariable model that included uCl−, serum Cl− as well as other variables (SBP, serum creatinine, ejection fraction, age and NT-proBNP at admission), only serum creatinine and uCl− were independent prognostic markers, the HR (95% CI) for uCl− in the model was 0.99 (0.98–0.99), p = 0.008 (see Supplementary Table B).
In a bivariable model that included uNa+ and uCl−, both variables were independent prognostic markers in our population (see Supplementary Table C). Moreover, both markers were independent prognosticators of one-year mortality, but uNa+ outperformed uCl− in terms of identifying patients reaching the composite endpoint (see Supplementary Tables D−G).
Importantly, uCl− was significantly related to outcomes even when analyzed as per 10 mmol/L change, per standard deviation change and as a continuous variable (see Supplementary Material).
Discussion
The primary outcome of our study is the adverse association of low uCl− levels with various clinical outcomes when compared to the high uCl− group. In patients with AHF, the current standard for renal function monitoring includes serum creatinine analysis, glomerular filtration rate (eGFR) estimation, and urine excretion volumes23,24. Recently, there has been a growing interest in incorporating urine composition, particularly spot uNa+, into the early assessment algorithm for AHF patients1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25. Our study aligns with previous reports that highlight the prognostic potential of uNa+ in predicting response to diuretics, one-year mortality, and rehospitalizations, thereby establishing the significant diagnostic value of uCl−. This diagnostic value is further supported by our observation that uNa+ and uCl− both function as independent prognostic markers.
While uNa+ has received substantial attention as an indicator of resistance to diuretic treatment and worsening HF symptoms leading to increased mortality28,29,30, uCl− has been overlooked as a valuable biomarker, despite its significant role in HF pathophysiology31. Most previous studies regarding Cl− have primarily focused on serum concentration7, and to the best of our knowledge, our study is among the few to explore the relationship between uCl− and patient outcomes32,33. Our analysis of urine biomarkers reveals a positive correlation between all analyzed electrolytes, consistent with trends observed in serum levels. Significantly, after multivariable analysis of uCl− and serum Cl− only uCl− remained an indicator of prognostic value.
Recent emerging studies6,7,34 have introduced the “chloride theory,” which posits that changes in serum chloride concentration are the primary determinant of alterations in plasma volume and the RAAS in worsening HF and its therapeutic resolution. Our observations regarding uCl− correspond with this emerging theory, particularly through our identification of low uCl− being associated with increased RAAS activation and fluid balance dysfunction, as evidenced by statistically significant decreases in serum Na+ and osmolarity in the low uCl− group. Notably, the most prominent differentiation between the low uCl− and high uCl− groups pertains to the RAAS, showing marked differences in relevant parameters. Cl− plays a pivotal role in the RAAS within the kidney and is known to exhibit an inverse relationship with renin35,36,37. The persistent activation of the RAAS in HF is linked to adverse long-term effects, such as ventricular remodeling, water and salt retention, and higher mortality38,39,40,41,42,43. Since the drafting of this manuscript, a further study regarding ‘chloride theory’ has been published. In the study, which comprised a cohort of 29 patients with acute HF, uCl− concentration demonstrated an inverse correlation with RAAS activity44. Additionally, the patients involved in the study were then divided into two groups based on the difference between serum and uCl− concentrations, namely an excretion group (low renal Cl− avidity, higher uCl−) and an absorption group (high renal Cl− avidity, lower uCl−). The absorption group was associated with impaired renal function, increased cardiac burden, and neurohormonal activity, thereby further substantiating the importance of Cl− in HF pathophysiology.
Similarly, in our study, patients with low uCl− concentration exhibited several characteristics indicative of a higher HF burden or more advanced disease stage, including RAAS activation, lower blood pressure, hyponatremia, and elevated NT-proBNP levels at discharge. Additionally, the low uCl− group demonstrated worse renal function, as evidenced by higher creatinine, blood urea, and lipocalin/NGAL values. These findings are consistent with the well-established relationship between kidney function and heart failure, although kidney health during the HF treatment phase remains somewhat ambiguous45.
HF triggers a detrimental cycle of cardiac burden, neurohormonal and inflammatory responses, and progressive multiorgan dysfunction leading to mortality38,46,47,48,49,50,51. Low uCl− levels appear to be indicative of an organism under distress and, within the context of HF, may serve as a prognostic indicator for unfavorable outcomes. In fact, patients with a low uCl− concentration exhibited several characteristics indicative of a higher HF burden or more advanced disease stage and in-hospital indices of poor prognosis. This underscores the importance of monitoring uCl− levels in HF patients and suggests its potential utility as a valuable biomarker for predicting outcomes in this patient population.
Nevertheless, it is essential to acknowledge the limitations of our study. It was a single-center post hoc analysis constrained by a limited sample size, drawn solely from the Polish resident population. The study was designed to optimally assess the first in-hospital urine sample before any initial intravenous dose of furosemide, which occurred in the majority of cases. However, there were instances where certain patients received diuretics while in transit in the ambulance (n = 12, 5%), or the first available urine sample was obtained upon admission to the ward (after receiving furosemide). Since the initial urine samples exhibited relatively high levels of uNa+ and uCl−, it is important to consider that various confounding factors may have already affected chloride excretion during the pre-hospital phase. This could possibly be attributed to an increased dosage of oral loop diuretics routinely taken by many patients once their congestion status started to deteriorate. Furthermore, the analyzed biomarkers may introduce selection bias, and as is common in comparable studies, male patients were overrepresented. In our paper the cut-off value to distinguish between high and low uCl− was chosen arbitrarily, but this cut-off has almost the same specificity and sensitivity to the cut-off of 113 mmol/L calculated from the ROC analysis. A longitudinal assessment (trajectory) of urine chloride during hospitalization could provide further insights into its role in AHF pathophysiology. Additionally, confirmation of the differentiation between the low uCl− and high uCl− groups necessitates multicenter studies and a more extensive patient cohort.
Conclusion
Chloride has transitioned from being an overlooked “cousin” of sodium (Na+) to becoming one of the more intriguing markers for assessing acute heart failure (AHF) and predicting prognosis. Our study has shown that low urine Cl− excretion during an AHF episode serves as an indicator of more advanced HF and activation of the RAAS and is related to worse one-year outcomes. A simple urine Cl− test, therefore, could be used to identify those patients who are in need of more intensive and specific treatment.
Data availability
All relevant data are contained within the manuscript.
References
McDonagh, T. A. et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 42(36), 3599–3726. https://doi.org/10.1093/eurheartj/ehab368 (2021).
Mullens, W. et al. The use of diuretics in heart failure with congestion: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 21, 137–155. https://doi.org/10.1002/ejhf.1369 (2019).
Warren, J. V. & Stead, E. A. Fluid dynamics in chronic congestive heart failure: An interpretation of the mechanisms producing the edema, increased plasma volume and elevated venous pressure in certain patients with prolonged congestive failure. Arch. Intern. Med. 73(2), 138–147. https://doi.org/10.1001/archinte.1944.00210140028004 (1944).
Mullens, W., Verbrugge, F. H., Nijst, P. & Tang, W. H. W. Renal sodium avidity in heart failure: From pathophysiology to treatment strategies. Eur. Heart J. 38(24), 1872–1882. https://doi.org/10.1093/eurheartj/ehx035 (2017).
Núñez, J. et al. Long-term potassium monitoring and dynamics in heart failure and risk of mortality. Circulation 137(13), 1320–1330. https://doi.org/10.1161/CIRCULATIONAHA.117.030576 (2018).
Kataoka, H. Chloride in heart failure syndrome: Its pathophysiologic role and therapeutic implication. Cardiol. Ther. 10, 407–428. https://doi.org/10.1007/s40119-021-00238-2 (2021).
Zandijk, A. J. L. et al. Chloride in heart failure: The neglected electrolyte. JACC 9(12), 904–915. https://doi.org/10.1016/j.jchf.2021.07.006 (2021).
Kataoka, H. Mechanistic insights into chloride-related heart failure progression according to the plasma volume status. ESC Heart Fail. 9, 2044–2048. https://doi.org/10.1002/ehf2.13927 (2022).
Seo, M. et al. Prognostic significance of serum chloride level in heart failure patients with preserved ejection fraction. ESC Heart Fail. 9, 1444–1453. https://doi.org/10.1002/ehf2.13840 (2022).
Llàcer, P. et al. Prognostic significance of plasma chloride in elderly patients hospitalized for acute heart failure. ESC Heart Fail. 10, 2637–2647. https://doi.org/10.1002/ehf2.14434 (2023).
Wesson, D. E. Glomerular filtration effects of acute volume expansion: Importance of chloride. Kidney Int. 32, 238–245. https://doi.org/10.1038/ki.1987.216 (1987).
Hanberg, J. S. et al. Hypochloremia and diuretic resistance in heart failure: Mechanistic insights. Circulation 9(8), e003180. https://doi.org/10.1161/CIRCHEARTFAILURE.116.003180 (2016).
Briggs, J. The macula densa sensing mechanism for tubuloglomerular feedback. Fed. Proc. 40, 99–103 (1981).
Briggs, J. P. & Schnermann, J. B. Whys and wherefores of juxtaglomerular apparatus function. Kidney Int. 49, 1724–1726. https://doi.org/10.1038/ki.1996.253 (1996).
Ponikowski, P. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 37, 2129–2200 (2016).
Demissei, B. G. et al. Optimizing clinical use of biomarkers in high-risk acute heart failure patients. Eur. J. Heart Fail. 18, 269–280 (2015).
Tromp, J. et al. Biomarker Profiles of Acute Heart Failure Patients With a Mid-Range Ejection Fraction. JACC Heart Fail 5, 507–517 (2017).
Zymliński, R. et al. Increased blood lactate is prevalent and identifies poor prognosis in patients with acute heart failure without overt peripheral hypoperfusion. Eur. J. Heart Fail. 20, 1011–1018. https://doi.org/10.1002/ejhf.1156 (2018).
Tanaka, K. et al. Follistatin Like 1 Regulates Hypertrophy in Heart Failure with Preserved Ejection Fraction. JACC Basic Transl Sci 1, 207–221 (2016).
Gruson, D., Ahn, S. A. & Rousseau, M. F. Biomarkers of inflammation and cardiac remodeling: The quest of relevant companions for the risk stratification of heart failure patients is still ongoing. Biochem. Med. 21, 254–263 (2011).
Verbrugge, F. H. Utility of urine biomarkers and electrolytes for the management of heart failure. Curr. Heart Fail. Rep. 16(6), 240–249. https://doi.org/10.1007/s11897-019-00444-z (2019).
Collins, S. P. et al. Early urine electrolyte patterns in patients with acute heart failure. ESC Heart Fail. 6(1), 80–88. https://doi.org/10.1002/ehf2.12368 (2019).
Rossignol, P., Coats, A. J., Chioncel, O., Spoletini, I. & Rosano, G. Renal function, electrolytes, and congestion monitoring in heart failure. Eur. Heart J. Suppl. 21, M25–M31. https://doi.org/10.1093/eurheartj/suz220 (2019).
Martens, P. & Mullens, W. Spot urinary sodium in decompensated heart failure as a prognostic metric for successful ambulatory decongestion. J. Card. Fail. 24(6), 355–356 (2018).
Zymliński, R. et al. Novel IVC doraya catheter provides congestion relief in patients with acute heart failure. J. Am. Coll. Cardiol. Basic Transl. Sci. 7, 326–327. https://doi.org/10.1016/j.jacbts.2022.02.013 (2022).
Biegus, J. et al. Renal profiling based on estimated glomerular filtration rate and spot urine sodium identifies high-risk acute heart failure patients. Eur. J. Heart Fail. 23(5), 729–739. https://doi.org/10.1002/ejhf.2053 (2021).
Biegus, J. et al. Spot urine sodium in acute heart failure: Differences in prognostic value on admission and discharge. ESC Heart Fail. 8(4), 2597–2602. https://doi.org/10.1002/ehf2.13372 (2021).
Biegus, J. et al. Serial assessment of spot urine sodium predicts effectiveness of decongestion and outcome in patients with acute heart failure. Eur. J. Heart Fail. 21(5), 624–633. https://doi.org/10.1002/ejhf.1428 (2019).
Cobo-Marcos, M. et al. Usefulness of natriuresis to predict in-hospital diuretic resistance. Am. J. Cardiovasc. Dis. 10(4), 350–355 (2020).
Ferreira, J. P. et al. Spot urine sodium excretion as prognostic marker in acutely decompensated heart failure: The spironolactone effect. Clin. Res. Cardiol. 105, 489–507 (2016).
De Bacquer, D., De Backer, G., De Buyzere, M. & Kornitzer, M. Is low serum chloride level a risk factor for cardiovascular mortality?. J. Cardiovasc. Risk 5(3), 177–184 (1998).
Xanthopoulos, A. et al. The prognostic role of spot urinary sodium and chloride in a cohort of hospitalized advanced heart failure patients: A pilot study. Life 13(3), 698. https://doi.org/10.3390/life13030698 (2023).
Kataoka, H. Clinical significance of spot urinary chloride concentration measurements in patients with acute heart failure: Investigation on the basis of the “Tubulo-glomerular feedback” mechanism. Cardiology https://doi.org/10.33140/COA.06.01.04 (2021).
Kataoka, H. The, “chloride theory,” a unifying hypothesis for renal handling and body fluid distribution in heart failure pathophysiology. Med. Hypotheses 104, 170–173. https://doi.org/10.1016/j.mehy.2017.06.005 (2017).
Kotchen, T. A., Welch, W. J., Lorenz, J. N. & Ott, C. E. Renal tubular chloride and renin release. J. Lab Clin. Med. 110(5), 533–540 (1987).
Salomonsson, M., Gonzalez, E., Kornfeld, M. & Persson, A. E. The cytosolic chloride concentration in macula densa and cortical thick ascending limb cells. Acta Physiol. Scand. 147, 305–313. https://doi.org/10.1111/j.1748-1716.1993.tb09503.x (1993).
Thomson, S. C. & Blantz, R. C. Ions and signal transduction in the macula densa. J. Clin. Invest. 106(5), 633–635. https://doi.org/10.1172/JCI10930 (2000).
Biegus, J. et al. Distinct renin/aldosterone activity profiles correlate with renal function, natriuretic response, decongestive ability and prognosis in acute heart failure. Int. J. Cardiol. 345, 54–60. https://doi.org/10.1016/j.ijcard.2021.10.149 (2021).
Schrier, R. W. & Abraham, W. T. Hormones and hemodynamics in heart failure. N. Engl. J. Med. 341, 577–585. https://doi.org/10.1056/NEJM199908193410806 (1999).
Dzau, V. J., Colucci, W. S., Hollenberg, N. K. & Williams, G. H. Relation of the renin-angiotensin-aldosterone system to clinical state in congestive heart failure. Circulation 63, 645–651. https://doi.org/10.1161/01.CIR.63.3.645 (1981).
Hartupee, J. & Mann, D. L. Neurohormonal activation in heart failure with reduced ejection fraction. Nat. Rev. Cardiol. 14, 30–38. https://doi.org/10.1038/nrcardio.2016.163 (2016).
Levine, T. B., Francis, G. S., Goldsmith, S. R., Simon, A. B. & Cohn, J. N. Activity of the sympathetic nervous system and renin-angiotensin system assessed by plasma hormone levels and their relation to hemodynamic abnormalities in congestive heart failure. Am. J. Cardiol. 49, 1659–1666. https://doi.org/10.1016/0002-9149(82)90243-0 (1982).
Chonko, A. M., Bay, W. H., Stein, J. H. & Ferris, T. F. The role of renin and aldosterone in the salt retention of edema. Am. J. Med. 63, 881–889. https://doi.org/10.1016/0002-9343(77)90541-1 (1977).
Kataoka, H. Neurohormonal activation and renal chloride avidity in acute heart failure: Clinical evidence supporting the “chloride theory”. Cardiorenal. Med. https://doi.org/10.1159/000536293 (2024).
Mullens, W. et al. Evaluation of kidney function throughout the heart failure trajectory: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 22, 584–603. https://doi.org/10.1002/ejhf.1697 (2020).
Zymliński, R. et al. Multi-organ dysfunction/injury on admission identifies acute heart failure patients at high risk of poor outcome. Eur. J. Heart Fail. 21(6), 744–750. https://doi.org/10.1002/ejhf.1378.PMID:30561066 (2019).
Zymliński, R. et al. Elevated troponin I level assessed by a new high-sensitive assay and the risk of poor outcomes in patients with acute heart failure. Int. J. Cardiol. 230, 646–652. https://doi.org/10.1016/j.ijcard.2017.01.012 (2017).
Sokolski, M. et al. Urinary levels of novel kidney biomarkers and risk of true worsening renal function and mortality in patients with acute heart failure. Eur. J. Heart Fail. 19(6), 760–767. https://doi.org/10.1002/ejhf.746 (2017).
Biegus, J. et al. Impaired hepato-renal function defined by the MELD XI score as prognosticator in acute heart failure. Eur. J. Heart Fail. 18(12), 1518–1521. https://doi.org/10.1002/ejhf.644 (2016).
Biegus, J. et al. Liver function tests in patients with acute heart failure. Pol. Arch. Med. Wewn. 122(10), 471–479. https://doi.org/10.20452/pamw.1413 (2012).
Maeda, D. et al. Prognostic value of the liver fibrosis marker fibrosis-5 index in patients with acute heart failure. ESC Heart Fail. 9, 1380–1387. https://doi.org/10.1002/ehf2.13829 (2022).
Funding
This research was financially supported by Wroclaw Medical University, Wroclaw, Poland [SUBZ.A460.23.005].
Author information
Authors and Affiliations
Contributions
SNM, JB, MF, RZ, PP and JB conceived and designed the research and experiments. SNM performed the experiments. SNM and JB analyzed the data. JB, GM and GI performed the statistical analysis and prepared visual data. SNM, JB, MF, and RZ prepared the original draft. SNM, JB, MF, GM, GI, PP, and RZ completed the review and editing; PP and RZ were responsible for project supervision. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nawrocka-Millward, S., Biegus, J., Fudim, M. et al. The role of urine chloride in acute heart failure. Sci Rep 14, 14100 (2024). https://doi.org/10.1038/s41598-024-64747-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64747-5
- Springer Nature Limited