Abstract
Binge drinking (BD) contributes strongly to the harms of alcohol use disorder. Most rodent models do not result in binge-level blood alcohol concentrations (BACs), and to better understand individual and sex differences in neurobiological mechanisms related to BD, the use of outbred rat strains would be valuable. Here, we developed a novel BD model where after 3+ months of intermittent access to 20% alcohol Wistar rats drank, twice a week, with two 5-min intake (what we called Two-shot) separated by a 10-min break. Our findings showed during Two-Shot that most animals reached ≥ 80 mg% BAC levels (when briefly food-restricted). However, when increasing alcohol concentrations from 20 to 30%, 40%, or 50%, rats titrated to similar intake levels, suggesting rapid sensing of alcohol effects even when front-loading. Two-Shot drinking was reduced in both sexes by naltrexone (1 mg/kg), validating intake suppression by a clinical therapeutic agent for human problem drinking. Further, both propranolol (β-adrenergic receptor antagonist) and prazosin (α1-adrenergic receptor antagonist) reduced female but not male BD at the lower dose. Thus, our results provide a novel model for BD in outbred rats and suggest that female binging is more sensitive to adrenergic modulation than males, perhaps providing a novel sex-related therapy.
Similar content being viewed by others
Introduction
Alcohol use disorder (AUD) is among the most prevalent mental diseases globally, and excessive alcohol drinking is now one of the leading causes of death, accounting for 1 in 5 deaths among 20–49 year old in the United States1. Binge drinking (BD), an episodic heavy use of alcohol, is a major obstacle to treating AUD2,3,4,5, and 3/4th of costs of binge intake are due to individuals who binge2. Reducing levels of BD can reduce health risks3,6 and incidence of relapse4, while BD can increase risk of developing more serious alcohol problems in non-dependent subjects7,8,9. In addition, rates of excessive alcohol drinking in women have risen dramatically in recent years10,11,12,13, and women have greater risk for alcohol problems including comorbidities14,15,16,17, and identification of sex-specific drivers would help personalize therapies to reduce drinking harms.
Thus, there is a considerable urgency to discover brain mechanisms that underlie BD, and this would be aided by rodent models with reliable high drinking levels. Several mouse lines show higher drinking and have provided many valuable insights5, while outbred rats would also have considerable utility when seeking to understand individual differences in alcohol drives. However, rats generally do not readily consume enough alcohol to reach high intoxication and blood alcohol concentrations (BAC) ≥ 80 mg%, which meets the criteria of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) for BD in humans3,5,18. During the past years, considerable advances were made using forced administration of alcohol through alcohol vapor, gavage, or liquid diet methods, and by studying strains with a genetic predisposition to alcohol (reviewed by Jeanblanc and colleagues5). In addition, hybrid operant models have provided useful BD paradigms in rats5 including sex differences19,20. Thus, we sought to develop a technically simple BD model using alcohol consumed from the bottle21, with the goal of setting a foundation to better understand individual and sex differences in underlying mechanisms, including where rats generally allow broader behavioral investigations.
Another useful feature of a BD model would be to detect changes after a long-term drinking history, as often occurs in humans. We and others have used an intermittent access two-bottle choice paradigm (IA2BC), where, after 3+ months of alcohol intake, animals exhibit several features related to human AUD including escalation22,23, sensitivity to drugs that reduce human drinking22,24,25, withdrawal signs (although moderate)26,27, front-loading5,21,28,29,30, and compulsive intake (consumption despite negative consequences31,32). However, in this model, BAC levels range around 40–60 mg% in 20–30 min22,33, suggesting need for a paradigm where binge-level intake is achieved.
Male and female Wistar rats have a rapid initial intake after IA2BC (front loading) with around 80% of alcohol consumption occurring during the first 5 min of exposure28,29,30, and others have noted the importance of rate of intake for rodent BD models5. Based on this evidence, we sought to develop a novel BD animal model where we restricted alcohol access and determined whether giving rats two 5-min alcohol access periods, separated by 10 min (which we call the Two-Shot model). Importantly, most animals reached ≥ 80 mg% BAC levels during Two-Shot. We also examined whether increasing the standard 20% alcohol to 30%, 40%, or 50%, would lead to higher BACs and thus binge intake levels. Further, to pharmacologically validate that the Two-Shot model was impacted by a compound that reduces human drinking, we tested naltrexone, an FDA-approved drug to treat AUD22,34. Finally, due to the urgent need to develop novel pharmacotherapies for problem drinking, we investigated the impact of α1 adrenergic receptor (AdrR) antagonists and β AdrR antagonists through injections of prazosin and propranolol, respectively. Inhibiting α1 AdrRs reduces several aspects of drive for alcohol in humans, and represent a critical new therapeutic against excessive consumption25,35, while β AdrR inhibitors have also shown efficacy against aspects of alcohol behavior in humans but have been understudied (see “Discussion”). Thus, we tested whether propranolol or prazosin decreased BD in females and males. Together, our results showed that giving rats 3+ months of intermittent intake, then restricting access to two brief exposures of alcohol each drinking day, can be used as a new model of rat binge drinking.
Results
The Two-Shot drinking model
We previously found both sexes of Wistar rats have strong initial intake in a drinking session, also called front-loading28,29,30. To increase intake in outbred rats to BD levels, we developed a new restricted-access paradigm. Male and female Wistar rats first drank for ~ 3 months under IA2BC, and then switched to drinking twice a week in a Two-Shot model. In each Two-Shot drinking session (Fig. 1), rats had 5 min of access to alcohol (called “Shot-1”), followed by a 10-min break, and then had an additional 5 min access to alcohol (called “Shot-2”). Thus, each Two-Shot session was a total of 20 min.
Two-Shot alcohol led to binge-level BACs
We first evaluated whether consumption of 20% alcohol during the Two-Shot session would lead to binge-level BACs (≥ 80 mg%). Thus, male (n = 14) and female (n = 18) Wistar rats drank 20% alcohol in a Two-Shot session, and blood was collected through a saphenous vein 20 min after ending the Shot-2. For these studies, rats were food restricted for 3 h before the Two-Shot session, to remove variability associated with different amounts of food in the gut. While BAC without food restriction would be more variable, it is likely that rats would experience these higher level BACs on some intake days, especially since rats with ad-libitum access to food showed strong titration of intake levels when alcohol concentration was altered (following section).
Thus, these studies give an estimate of the maximum possible BAC that could be reached. Our findings showed that BAC was greater than 80 mg% for both sexes (Fig. 2A, female: 112.9 ± 9.6 mg%, male: 89.8 ± 9.2 mg%), with intake > 1 g/kg in 20 min (Fig. 2B). In addition, alcohol intake level (g/kg) and BACs were significantly correlated in both females (Fig. 2C; F(1,17) = 16.16, p = 0.0009, R2 = 0.4874) and males (Fig. 2D; F(1,13) = 13.41, p = 0.0029, R2 = 0.5078), with no sex difference in slopes (F(1,30) = 0.114, p = 0.7381). Male intake-BAC relations remained significant without the largest outlier value (n = 13, F(1,12) = 5.687, p = 0.0345, R2 = 0.3215). Thus, during Two-Shot model of 20% ethanol, both sexes exhibited high drinking levels and reached BACs greater than 80 mg%.
Two-Shot model with 20% alcohol leads to binge-level BACs. (A) BAC was greater than 80 mg% with 20% alcohol drinking in females (purple) and males (green) and (B) alcohol intake > 1 g/kg. Also, alcohol intake level (g/kg) and BACs were significantly correlated in females (C) and males (D). *p < 0.05; **p < 0.01; ***p < 0.001.
Rats maintained similar intake levels as alcohol concentration increased
Since rats show rapid initial intake (front-loading) during our typical 20 min/day, 5d/week intake paradigm (after the 3+ months of IA2BC)28,29,30, we considered the possibility that increasing the alcohol concentration from standard 20% to 30%, 40%, or 50% alcohol would lead to even higher BAC levels (assuming the same volume of intake), increasing the chance of exceeding binge-level BACs. Indeed, front-loading rats seem to consume alcohol faster than significant levels of alcohol can enter the bloodstream36,37. Thus, we examined n = 20 animals per sex, with each rat being exposed to each alcohol concentration at least twice, in randomized order across drinking days. Surprisingly, we found that drinking levels were similar regardless of alcohol concentration, although female consumption was overall higher than males. This perhaps suggests that rats have early interoceptive changes which they use to titrate to similar intoxication levels, regardless of the concentration of alcohol consumed (and see “Discussion”38).
Thus, a two-way repeated-measures ANOVA for Shot-1 (Fig. 3A,B) showed a main effect of sex (F(1,38) = 9.868, p = 0.0033), but no effect for alcohol concentration (F(2.224,84.50) = 0.467, p = 0.6489) or interaction (F(3,114) = 0.632, p = 0.5958). Thus, within the first 5-min intake period, rats had similar intake levels (g/kg) even when consuming different alcohol concentrations. However, we note that Shot-2 consumption (Fig. 3C,D) showed no effect of sex (F(1,38) = 0.000, p = 0.9894), alcohol concentration (F(2.909,110.5) = 0.594, p = 0.6153), or interaction (F(3,114) = 0.373, p = 0.7724). Also, Shot-1 intake was 2–3 × greater than Shot-2 drinking, consistent with front-loading. These suggest that titration of intake volume across different alcohol concentrations occurred within Shot-1, the first 5-min access period. Further, the analysis for Two-Shot session intake (Shot-1 plus Shot-2) (Fig. 3E,F) showed a main effect of sex (F(1,38) = 7.013, p = 0.0117) but not alcohol concentration (F(2.509,95.35) = 0.253, p = 0.8247) or interaction (F(3,114) = 0.077, p = 0.9723). Thus, rats overall maintained a similar average drinking level across different alcohol concentrations during the Shot-1 period. While these results suggested that increasing alcohol concentrations was not effective in further increasing drinking levels, they also indicated that rats may have some type of rapid interoception, which they utilize to titrate their drinking levels to approximately some preferred level in each session.
Female and male rats maintained similar intake levels as alcohol concentration increased. (A,B) During Shot-1, females consumed more alcohol than males and, for both sexes, there was no difference in intake levels between 20%, 30%, 40%, or 50% alcohol. (C,D). For Shot-2, there were no differences by sex or alcohol concentrations. (E,F) During the full Two-Shots intake period, females consumed more alcohol, and there was no difference between the alcohol concentrations. n = 20 per sex *p < 0.05.
To further evaluate if rats were titrating intake to reach a particular level, we examined whether intake level with 20% alcohol would correlate with consumption with higher alcohol concentrations. In females, 20% consumption levels correlated with 40% and 50% but not 30%, during the full Two-Shot period (Shot-1 plus Shot-2) (Fig. 4A; 30%: F(1,18) = 2.912, p = 0.1051, R2 = 0.1393; 40%: F(1,18) = 21.94, p = 0.0002, R2 = 0.5493; 50%: F(1,18) = 6.039, p = 0.0244, R2 = 0.2512). Also, females Shot-1 20% intake correlated with Shot-1 drinking for all other alcohol concentrations (Fig. 4B; 30%: F(1,18) = 9.937, p = 0.0055, R2 = 0.3557; 40%: F(1,18) = 39.79, p < 0.0001, R2 = 0.6885; 50%: F(1,18) = 8.003, p = 0.0111, R2 = 0.3078). In males, 20% alcohol intake correlated with drinking levels with 30% and 40% for the full Two-Shot period (Fig. 4C; 30%: F(1,18) = 7.910, p = 0.0115, R2 = 0.3053; 40%: F(1,18) = 8.897, p = 0.0080, R2 = 0.3308), and for Shot-1 alone (Fig. 4D; 30%: F(1,18) = 5.130, p = 0.0361, R2 = 0.2218; 40%: F(1,18) = 12.22, p = 0.0026, R2 = 0.4043). Male 20% intake did not correlate with 50% consumption levels (Two-Shot: F(1,18) = 2.552, p = 0.1276, R2 = 0.1242), although it was significant without one outlier (F(1,17) = 5.477, p = 0.0317, R2 = 0.2437; Shot1: F(1,18) = 1.547, p = 0.2295, R2 = 0.0792). In addition, Shot-2 20% intake did not correlate with Shot-2 drinking of any other alcohol concentrations in either sex (Fig. S1A,B), and Shot-1 and Shot-2 intake were not correlated for any alcohol concentration for either sex (Fig. S1C,D). These results suggest that, even with briefer intake periods, rats titrated the amount of alcohol consumed during Two-Shot and Shot-1, regardless of alcohol concentration.
Relation of 20% alcohol intake to intake of other alcohol concentrations. (A,B) Relation of female intake level for 20% alcohol to higher alcohol concentrations, for (A) Two-Shot and (B) Shot-1. (C,D) Relation of male intake level for 20% alcohol to higher alcohol concentrations for (C) Two-Shot and (D) Shot-1 alone. *p < 0.05; **p < 0.01; ***p < 0.001.
Female drinking was inhibited by lower concentrations of adrenergic receptor blockers relative to males
Next, to pharmacologically validate that the Two-Shot model was impacted by a compound that reduces human drinking, we first tested naltrexone, a drug that can be used to treat AUD22,34. Thus, we administered 1 mg/kg naltrexone, a dose that reduces alcohol intake in rodents22. In addition, we examined the effect of prazosin, antagonist of α1 adrenergic receptor, and propranolol, the β adrenergic receptor antagonist, as we24,39 and others40,41 have examined in previous preclinical studies.
First, we compared the impact of 5 mg/kg propranolol and 1 mg/kg naltrexone versus saline vehicle in 18 females and 15 males using a within-rat, randomized design. In females, there was a significant effect by one-way repeated-measures ANOVA (Fig. 5B,C; F(1.932,32.84) = 28.23, p < 0.0001), with post-hoc differences between saline and naltrexone (p < 0.0001) and between saline and 5 mg/kg propranolol (p = 0.0206). In males, there was a significant effect by one-way repeated-measures ANOVA (Fig. 5D,E; F(1.702,22.12) = 22.21, p < 0.0001), with post-hoc differences between saline and naltrexone (p = 0.0005) but not between saline and 5 mg/kg propranolol (p = 0.5471). Thus, naltrexone reduced Two-Shot intake in both sexes, providing a pharmacological validation that Two-Shot drinking is reduced by a compound that can decrease human AUD drinking, perhaps suggesting utility of this novel BD model. However, 5 mg/kg propranolol only inhibited drinking in the Two-Shot model in female rats. In a second set of experiments in the same rats, we increased the dose of propranolol to 10 mg/kg to verify if we could also decrease drinking in males39,40, compared to vehicle. This higher dose significantly reduced BD in females (Fig. 5F,G; t(17) = 4.546, p = 0.0003) and males (Fig. 5H,I; t(14) = 4.107, p = 0.0011) (both paired t-test saline vs 10 mg/kg within-rat). Thus, the higher tested dose of propranolol reduced alcohol drinking in both sexes, but the lower propranolol dose tested only impacted female binge intake.
Two-Shot intake was inhibited by naltrexone in both sexes, and females drinking was inhibited by lower concentrations of propranolol. (A) Cartoon of injection timing. (B–E) Impact of 1 mg/kg naltrexone or 5 mg/kg propranolol in (B,C) female and (D,E) male rats. (F–I) Impact of 10 mg/kg propranolol in (F,G) female and (H,I) male rats. *p < 0.05; **p < 0.01; ***p < 0.001, vs group control.
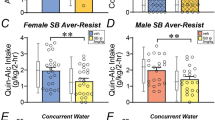
We next tested if the α1 adrenergic inverse agonist, prazosin could impact 20% alcohol consumption in the Two-Shot model. For this, we compared 0.75 mg/kg and 1.5 mg/kg prazosin versus saline vehicle in 16 females and 12 males, in randomized order within and across rats. In females, there was a significant effect by one-way repeated-measures ANOVA (Fig. 6B,C; F(1.972,29.57) = 11.13, p = 0.0003), with post-hoc significant effects of both 1.5 mg/kg (p = 0.0036) and 0.75 mg/kg (p = 0.0024) prazosin. In males, there was a significant effect by one-way repeated-measures ANOVA (Fig. 6D,E; F(1.460,14.60) = 6.581, p = 0.0141), with post-hoc significant effects of 1.5 mg/kg prazosin (Fig. 6F; p = 0.0026) but not for 0.75 mg/kg prazosin (Fig. 6G; p = 0.3326). Thus, the higher dose of prazosin tested reduced drinking in both sexes, while the lower dose of prazosin tested only decreased binge alcohol consumption in females.
Female drinking was inhibited by lower concentrations of prazosin relative to males. (A) Cartoon of injection timing. (B–E) Impact of 0.75 mg/kg or 1.5 mg/kg prazosin on (B,C) female and (D,E) male rat Two-Shot drinking. (F,G) Relationship between basal intake levels and impact of (F) 0.75 mg/kg prazosin or (G) 5 mg/kg propranolol on drinking (change in drinking determined as drug/vehicle intake and expressed as percent). *p < 0.05; **p < 0.01.
Relation of basal intake levels to effectiveness of lower dose prazosin and propranolol.
Taken together, the results presented above suggest that prazosin and propranolol were effective at reducing excessive alcohol consumption in females at lower doses than those that were effective in males. However, since females drank more alcohol than males, another possibility is that these lower tested doses of prazosin and propranolol reduced intake only in higher drinkers. For 0.75 mg/kg prazosin, higher drinkers did show a greater prazosin reduction in drinking, which was significant in females (Fig. 7A; F(1,14) = 7.592, p = 0.0155, R2 = 0.352) with a similar trend but non-significant in males (Fig. 7A; F(1,9) = 1.140, p = 0.3134, R2 = 0.113). For 5 mg/kg propranolol, basal intake did not relate to propranolol reduction in drinking in females (Fig. 7B; F(1,16) = 2.323, p = 0.1470, R2 = 0.127) or males (Fig. 7B; F(1,14) = 2.175, p = 0.1660, R2 = 0.154), even though the lower propranolol dose was overall significantly more effective in females versus males. Thus, lower concentrations of propranolol might reflect a more sex-selected target to reduce female drinking, while lower doses of prazosin might impact higher drinkers more generally.
Relation of basal intake levels to effectiveness of lower dose prazosin and propranolol. Relationship between basal consumption levels of 20% alcohol under Two-Shot, and impact of (A) 0.75 mg/kg prazosin or (B) 5 mg/kg propranolol on drinking (change in drinking determined as drug/vehicle intake and expressed as percent). *p < 0.05.
Discussion
Since most rat alcohol models with voluntary intake do not result in binge-level intake, we sought to develop a novel, restricted-access paradigm. Thus. after 3+ months of IA2BC, rats switched to drinking twice per week in a Two-Shot model, with two 5-min intake periods, separated by 10 min break, in each drinking session. To test BACs, rats were briefly food-restricted to reduce the impact of food in gut and to give the maximum possible BAC. Most animals reached ≥ 80 mg% BAC levels during Two-Shot, providing a novel model to assess BD mechanisms. Since our rats rapidly front-load, we also reasoned that increasing alcohol concentrations (from 20 to 30%, 40%, or 50%) might lead to even higher intake levels. Surprisingly, rats titrated intake of different alcohol concentrations to similar g/kg drinking levels in the first 5-min intake period (Shot-1), suggesting that they rapidly sensed some aspect of alcohol effects even when front-loading. Nonetheless, to pharmacologically validate that the Two-Shot model was impacted by a compound that reduces human drinking, we found that intake was reduced in both sexes by naltrexone, a human therapeutic agent for problem drinking. In addition, blocking β adrenergic receptors (with propranolol) or α1 adrenergic receptors (with prazosin) reduced drinking in a sex-specific manner according with the dose used. Thus, our results provide a novel model for BD in outbred rats, and suggest that female binge intake is more sensitive to adrenergic modulation than males.
We note that we did not test BACs without food deprivation, and thus BACs under ab libitum conditions would be somewhat lower. However, it is likely that rats by chance would drink with minimal food in gut on some days, and thus could come to learn the association between intake and intoxication. In addition, we surprisingly found that varying the concentration of alcohol consumed, across 20% to 50%, did not alter the g/kg level of intake. This was unexpected, since our rats have strong, rapid initial intake (front-loading)28,29,30, and dialysis studies have found that, with front loading, alcohol takes several minutes to enter the blood stream in significant levels36,37. Thus, we predicted a priori that rats would consume similar volumes of alcohol, before strong bodily and intoxication effects would occur. Rats might also change intake levels based on alcohol taste, although our other studies find that rats exhibit aversion-resistant intake (continued consumption even with the bitter quinine in the alcohol)24,28,29,30,33,39. Instead, we found that drinking levels were similar across the different alcohol concentrations tested (20%, 30%, 40%, 50%), although females had higher intake than males for each concentration. This suggests that rats have some, as yet unidentified, mechanism for sensing their alcohol intake level. Considerable future studies will be needed to understand these findings. We also note that rats self-administering different higher concentrations of alcohol under operant methods also titrate to similar drinking levels38. While such operant methods have slower rates of intake, relative to bottle-based drinking, our findings agree with previous studies suggesting that responding for alcohol reflects “motivation to obtain a specific pharmacological effect of ethanol”38.
Previous work has put considerable effort into developing rat models which lead to binge-level intake. Certainly C57BL/6 mice are widely observed to reach binge-level BACs42, and thus are a valuable model, but rats have generally been considered to afford easier training in a wider range of behavioral paradigms. Alcohol dependence, through use of repeated alcohol vapor exposure or liquid diet, can lead to high alcohol intake in rats (reviewed in5). Other studies have utilized rat lines genetically selected for high alcohol preference or intake43. The intermittent access model we and others use (IA2BC) can lead to withdrawal signs in rats, although moderate26,27. However, some studies have highlighted how many problem drinkers do not exhibit dependence7. Our goal was to develop a BD model in outbred rats to facilitate the identification of individual and sex differences in drive for alcohol. It is important to note that others have developed useful binge operant intake models in rats5, which can have sex differences in their impact19,20. While there is some consideration of which rat strain might be optimal for BD studies44, as discussed by Sauton and colleagues44, rats of a given strain can differ among vendors, complicating identification of an widely usable optimal model. Indeed, having several BD models in outbred rat lines will likely accelerate and better validate our understanding of BD mechanisms.
Here, we addressed Two-Shot drinking after a 3-month history of intermittent access intake. We did this in part because previous studies22,23,24,25,26,27,28,29,30,31,32, noted in Introduction, have found that longer-term intermittent intake leads to development of some features that could be considered to reflect human AUD, including compulsion-like intake31,32. However, it would be interesting to determine whether Two-Shot access from the onset of drinking (i.e., without pre-exposure to intermittent access) would lead to higher drinking levels. We note that outbred rats show escalation of drinking levels across the first 5–6 weeks of intermittent access22, which could suggest that rats require somewhat substantial and protracted access to drink alcohol, each day and across weeks, perhaps so that they can learn the effects of alcohol; indeed, escalation of intake is a component of development of addiction in humans23. Even so, it would be interesting in future studies to determine whether Two-Shot or related limited-access schedules alone can lead to excessive drinking in outbred rats.
We also examined the pharmacological sensitivity of female and male binge-level alcohol intake. There is a critical need for better therapies, especially drugs that are already FDA approved and could be quickly repurposed34,45. Prazosin and related compounds have been of particular interest since prazosin reduces drinking and craving in humans with drinking problems25. The involvement of β AdRs for human problem drinking has received limited study in recent times45,46, although early clinical studies found that β AR antagonists can reduce symptoms related to alcohol withdrawal and cravings47,48,49,50. Here, we found that lower tested doses of prazosin and propranolol reduced BD in females but not males. Prazosin decreases intake in dependent rats at lower doses (0.5–1.0 mg/kg) than are effective in non-dependent rats (1.5–2.0 mg/kg)41, while 5 and 10 mg/kg propranolol decrease operant responding for alcohol in dependent male rats, with only 10 mg/kg affecting non-dependent rats40, and dependence produces changes in β AdR function that promote intake51. Thus, our findings support the possibility that female drinking may be preferentially impacted by AdR modulation. Indeed, prazosin is more effective in human drinkers with greater withdrawal anxiety25, and women more often report drinking alcohol to cope with stress and anxiety17,52.
This study has several important limitations. First, we did not examine estrous cycle: once an addiction-related state is established, alcohol intake level is often unrelated to estrous stage53,54,55, including in our studies30. In addition, we did not test the impact of prazosin and propranolol on sweet fluid intake, to control for potential non-specific effects. However, prazosin does not impact saccharin intake or locomotion in our rats24, similar to the lack of prazosin effects on sugar56 or water41,57 intake, or locomotion41,57,58, and the lack of effect of propranolol on saccharin59 or sugar60 intake. Even so, it would be interesting and important for future studies to examine sucrose intake in this novel Two-Shot paradigm, including the effects of naltrexone, prazosin, and propranolol on such sucrose Two-Shot intake. Indeed, limited access paradigms can lead to what could be considered addiction-like consumption of sweet fluids61,62,63 and Two-Shot might represent such a model.
In summary, our findings showed that alcohol intake in Wistar rats under the Two-Shot paradigm can lead to BAC levels ≥ 80%, which is reduced by the human AUD drug naltrexone. Moreover, propranolol and prazosin reduced drinking in females at lower doses than in males, suggesting that female BD is more sensitive to adrenergic modulation than males.
Materials and methods
Animals and alcohol drinking methods
All experimental procedures were conducted in strict accordance with the Guide for Care and Use of Laboratory Animals provided by the National Institutes of Health and approved by the Institutional Animal Care and Use Committee of Indiana University. Additionally, all methods and results were described following Animal Research: Reporting In Vivo Experiments Arrive. A total of 30 female and 33 male Wistar rats arrived at 45–50 days old and were singly housed, with both sexes in the same housing room in a reverse dark–light cycle (lights off 11 am-11 pm). All behavioral tests happened in a dark cycle. After 2wk acclimation, rats began access to alcohol (20% v/v diluted in water) in the intermittent two-bottle choice paradigm (IA2BC), which involves intake of 20% alcohol vs water-only for 18–24 h starting on Monday, Wednesday, and Friday at ~ 1 h into the dark cycle22, as we used before24,28,29,30,33,39. After 3+ months IA2BC, we switched rats to drink twice per week in the Two-Shot model (Fig. 1), which occurred 1–3 h into the dark cycle. In each Two-Shot session, rats had access to drink for 5 min (Shot-1), followed by 10 min of break and, then a second 5-min drinking (Shot-2). Rats were allowed 2–3 months of Two-Shot drinking before experiments began.
For comparison of drinking levels across 20%, 30%, 40%, and 50% alcohol concentrations (Figs. 3, 4), 20 rats of each sex were tested; for most rats, two drinking sessions for each alcohol concentration were assessed in randomized order across rats and alcohol concentrations. Data were then averaged for each alcohol concentration for each rat, as we have done before to reduce variability in drinking values24,33,39. These studies were in a different cohort of rats from those tested with pharmacological agents.
Blood alcohol concentration
After testing intake with different alcohol concentrations, these same rats were used to determine BACs. One week before starting blood collection, rats were gently handled by the experimenter, once a day, for ~ 5 min. BAC was determined in rats that were previously used to test drinking levels under different alcohol concentrations (20%-50%), then were returned to 20% alcohol intake and BAC levels determined. This time point for BAC testing was chosen to prevent potential stress effects from impacting subsequent drinking level experiments. BAC was collected from the saphenous vein 20 min after the second Shot of 20% alcohol. Rats were food-restricted for 3 h before drinking, to allow determination of maximum possible BACs. For all other studies, rats were not food restricted, and thus had ad-libitum access to food. BAC concentrations were determined, relative to alcohol standards, by gas chromatography as described in22,36.
Since most alcohol intake occurred in Shot-1 (Fig. 3), it might be possible that intake within Shot-1 would be sufficient to reach BACs > 80 mg%, without need for a Shot-2. From data in Fig. 2, we estimated a BAC of ~ 57 mg% for 1 g/kg alcohol for females and ~ 60 mg% for 1 g/kg for males. Shot-1 intake was ~ 1.5 g/kg for females, predicting ~ 85 mg%, while male Shot-1 was ~ 1 g/kg alcohol, predicting ~ 60 mg%. Thus, both Shot-1 and Shot-2 would likely be required for males to reach > 80 mg% BAC, but further studies would be required to address this possible modification.
Drugs
Propranolol hydrochloride was from Tocris. Prazosin hydrochloride and naltrexone hydrochloride were from Sigma-Aldrich (USA). All drugs were dissolved in sterile saline (0.9%), except prazosin which was dissolved in sterile water (as we did before24). All drugs were prepared the same day they were used for experiments. All drugs were injected intraperitoneally (i.p.). Drug concentrations in vehicle were titrated so that a 1 kg rat would receive a 1 ml injection. E.g., the lower-dose prazosin was made up at 0.75 mg/kg per 1 ml, so that a 1 kg rat would get 1 ml of this cocktail, a 500 g rat would get 0.5 ml of this cocktail, etc.
Effect of pharmacological agents on Two-Shot drinking
Rats were exposed to each pharmacological treatment in a within-subject design. Groups were randomized across animals before the beginning of the test sessions, and all conditions were balanced to make sure that all groups were tested on the same test day. Before test sessions, rats were handled by the experimenter, with 1–3 sessions of habituation to vehicle injection, to avoid stress reactions during the injection procedure. Since propranolol and naltrexone had the same vehicle (saline), we ran both treatments in the same cohort to reduce the number of animals. Thus, propranolol 5 mg/kg (n = 18 females; Fig. 5B, and 15 males; Fig. 5D) was injected 20 min before the beginning of the Two-Shot session, while naltrexone 1 mg/kg was injected 30 min before drinking (Fig. 5D); all vehicle injections for this experiment were 30 min before drinking (Fig. 5A). Afterwards, the same rat cohort was tested with propranolol 10 mg/kg vs vehicle (Fig. 5F,H), injected 20 min before Two-Shot session Times for administration and doses of propranolol was chosen by our previous work39 and naltrexone dose was from Simms and colleagues22.
After propranolol/naltrexone testing, these same rats were tested with prazosin. These rats were given ~ 1 month of Two-Shot drinking in case of disruptions in drinking due to i.p. injections, and in this time two male and two female rats died. Prazosin was tested at the doses of 0.75 mg/kg or 1.5 mg/kg, or vehicle (Figs. 6B–E, 7A), injected 30 min before the beginning of the Two-Shot session (Fig. 6A). The doses of prazosin were chosen by our previous work24.
Data analysis
Alcohol consumption was determined through changes in bottle weight before and after a drinking session and converted to grams alcohol/kilograms body weight. Statistical comparisons were primarily performed in a within-subject design. Data were mostly analyzed by one- or two-way ANOVA with repeated measures followed by the Bonferroni test, while some comparisons used paired t-tests. Statistical analysis was performed using GraphPad Prism. All data are shown as mean ± SEM.
References
Esser, M. B. et al. Estimated deaths attributable to excessive alcohol use among US adults aged 20 to 64 years, 2015 to 2019. JAMA Netw. Open. 5, e2239485 (2022).
CDC. Excessive Drinking Costs U.S. $223.5 Billion. (Center for Disease Control, 2014).
Dawson, D. A., Grant, B. F. & Li, T. K. Quantifying the risks associated with exceeding recommended drinking limits. Alcohol Clin. Exp. Res. 29, 902–908 (2005).
Moos, R. H. & Moos, B. S. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction. 101, 212–222 (2006).
Jeanblanc, J. et al. Face validity of a pre-clinical model of operant binge drinking: Just a question of speed. Addict. Biol. 24, 664–675 (2019).
Rehm, J. et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 373, 2223–2233 (2009).
Esser, M. B. et al. Prevalence of alcohol dependence among US adult drinkers, 2009–2011. Am. J. Prev. Med. 11, 140329 (2014).
Gowin, J. L., Sloan, M. E., Stangl, B. L., Vatsalya, V. & Ramchandani, V. A. Vulnerability for alcohol use disorder and rate of alcohol consumption. Am. J. Psychiatry. 174, 1094–1101 (2017).
Grant, B. F. et al. Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 72, 757–766 (2015).
Grant, B. F. et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 74, 911–923 (2017).
Carvalho, A. F., Heilig, M., Perez, A., Probst, C. & Rehm, J. Alcohol use disorders. Lancet. 394, 781–792 (2019).
McKetta, S. & Keyes, K. M. Heavy and binge alcohol drinking and parenting status in the United States from 2006 to 2018: An analysis of nationally representative cross-sectional surveys. PLoS Med. 16, e1002954 (2019).
Hasin, D. S., Shmulewitz, D. & Keyes, K. Alcohol use and binge drinking among U.S. men, pregnant and non-pregnant women ages 18–44: 2002–2017. Drug Alcohol Depend. 205, 107590 (2019).
Agabio, R., Pisanu, C., Gessa, G. L. & Franconi, F. Sex differences in alcohol use disorder. Curr. Med. Chem. 24, 2661–2670 (2017).
Erol, A. & Karpyak, V. M. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug Alcohol Depend. 156, 1–13 (2015).
Becker, J. B. & Koob, G. F. Sex differences in animal models: Focus on addiction. Pharmacol. Rev. 68, 242–263 (2016).
Altman, B. R. et al. The indirect effect of negative emotionality via alcohol craving on abstinence self-efficacy among women in alcohol treatment. Addict. Behav. 132, 107347 (2022).
Stinson, F. S. et al. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 80, 105–116 (2005).
Sauton, P., Jeanblanc, J., Benzerouk, F., Gierski, F. & Naassila, M. Sex-specific decision-making impairments and striatal dopaminergic changes after binge drinking history in rats. Front. Pharmacol. 14, 1076465 (2023).
Jeanblanc, J. et al. Sex-related differences in the efficacy of Baclofen enantiomers on self-administered alcohol in a binge drinking pattern and dopamine release in the core of the nucleus accumbens. Front. Pharmacol. 14, 1146848 (2023).
Carnicella, S., Ron, D. & Barak, S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 48, 243–252 (2014).
Simms, J. A. et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin. Exp. Res. 32, 1816–1823 (2008).
Koob, G. F. & Volkow, N. D. Neurocircuitry of addiction. Neuropsychopharmacology. 35, 217–238 (2010).
De Oliveira, S. T. et al. The role of anterior insula-brainstem projections and alpha-1 noradrenergic receptors for compulsion-like and alcohol-only drinking. Neuropsychopharmacology. 35, 1751–1760 (2021).
Sinha, R. et al. Moderation of Prazosin’s efficacy by alcohol withdrawal symptoms. Am. J. Psychiatry. 178, 447–458 (2021).
Steensland, P. et al. The monoamine stabilizer (−)-OSU6162 attenuates voluntary ethanol intake and ethanol-induced dopamine output in nucleus accumbens. Biol. Psychiatry. 72, 823–831 (2012).
Li, J., Bian, W., Dave, V. & Ye, J. H. Blockade of GABA(A) receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic-pituitary-adrenocortical axis. Addict. Biol. 16, 600–614 (2011).
Darevsky, D. et al. Drinking despite adversity: Behavioral evidence for a head down and push strategy of conflict-resistant alcohol drinking in rats. Addict. Biol. 24, 426–437 (2019).
Darevsky, D. & Hopf, F. W. Behavioral indicators of succeeding and failing under higher-challenge compulsion-like alcohol drinking in rat. Behav. Brain Res. 393, 112768 (2020).
De Oliveira Sergio, T. et al. Sex- and estrous-related response patterns for alcohol depend critically on the level of compulsion-like challenge. Progr. Neuropsychopharm. Biol. Psych. (in press) (2024) https://authors.elsevier.com/sd/article/S0278-5846(24)00076-9.
Hopf, F. W. & Lesscher, H. M. Rodent models for compulsive alcohol intake. Alcohol. 48, 253–264 (2014).
De Oliveira, S. T., Frasier, R. & Hopf, F. W. Animal models of compulsion alcohol drinking: Why we love quinine-resistant intake and what we learned from it?. Front. Psychiatry. 14, 1116901 (2023).
Wegner, S. A. et al. A novel NMDA receptor-based intervention to suppress compulsion-like alcohol drinking. Neuropharmacology 157, 107681 (2019).
Kranzler, H. R. & Soyka, M. Diagnosis and pharmacotherapy of alcohol use disorder: A review. JAMA. 320, 815–824 (2018).
Sinha, R. et al. Alcohol withdrawal symptoms predict corticostriatal dysfunction that is reversed by prazosin treatment in alcohol use disorder. Addict. Biol. 27, e13116 (2022).
Doyon, W. M. et al. Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol Clin. Exp. Res. 27, 1573–1582 (2003).
Doyon, W. M., Anders, S. K., Ramachandra, V. S., Czachowski, C. L. & Gonzales, R. A. Effect of operant self-administration of 10% ethanol plus 10% sucrose on dopamine and ethanol concentrations in the nucleus accumbens. J. Neurochem. 93, 1469–1481 (2005).
Carnicella, S., Yowell, Q. V. & Ron, D. Regulation of operant oral ethanol self-administration: A dose-response curve study in rats. Alcohol Clin. Exp. Res. 35, 116–125 (2011).
De Oliveira, S. T., Wean, S. N., Katner, S. & Hopf, F. W. The role of beta- and alph-adrenergic receptors on alcohol drinking. Neuropharmacology 234, 109545 (2023).
Gilpin, N. W. & Koob, G. F. Effects of beta-adrenoceptor antagonists on alcohol drinking by alcohol-dependent rats. Psychopharmacology 212, 431–439 (2010).
Walker, B. M., Rasmussen, D. D., Raskind, M. A. & Koob, G. F. alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 42, 91–97 (2008).
Radke, A. K., Sneddon, E. A., Frasier, R. M. & Hopf, F. W. Recent perspectives on sex differences in compulsion-like and binge alcohol drinking. Int. J. Mol. Sci. 22, 3788 (2021).
Bell, R. L., Rodd, Z. A., Engleman, E. A., Toalston, J. E. & McBride, W. J. Scheduled access alcohol drinking by alcohol-preferring (P) and high-alcohol-drinking (HAD) rats: Modeling adolescent and adult binge-like drinking. Alcohol. 48, 225–234 (2014).
Sauton, P. et al. Interstrain differences in voluntary binge-like drinking behavior and in two acute ethanol injections-induced synaptic plasticity deficits in rats. Addict. Biol. 26, e12992 (2021).
Downs, A. M. & McElligott, Z. A. Noradrenergic circuits and signaling in substance use disorders. Neuropharmacology 208, 108997 (2022).
Haass-Koffler, C. L., Swift, R. M. & Leggio, L. Noradrenergic targets for the treatment of alcohol use disorder. Psychopharmacology 235, 1625–1634 (2018).
Carlsson, C. & Johansson, T. The psychological effects of propranolol in the abstinence phase of chronic alcoholics. Br. J. Psychiatry. 119, 605–606 (1971).
Zilm, D. H., Sellers, E. M., MacLeod, S. M. & Degani, N. Letter: Propranolol effect on tremor in alcoholic withdrawal. Ann. Intern. Med. 83, 234–236 (1975).
Horwitz, R. I., Gottlieb, L. D. & Kraus, M. L. The efficacy of atenolol in the outpatient management of the alcohol withdrawal syndrome. Results of a randomized clinical trial. Arch. Intern. Med. 149, 1089–1093 (1989).
Bailly, D., Servant, D., Blandin, N., Beuscart, R. & Parquet, P. J. Effects of beta-blocking drugs in alcohol withdrawal: A double-blind comparative study with propranolol and diazepam. Biomed. Pharmacother. 46, 419–424 (1992).
Varodayan, F. P. et al. The amygdala noradrenergic system is compromised with alcohol use disorder. Biol. Psychiatry. 91, 1008–1018 (2022).
Guinle, M. I. B. & Sinha, R. The role of stress, trauma, and negative affect in alcohol misuse and alcohol use disorder in women. Alcohol. Res. 40, 05 (2020).
Fulenwider, H. D., Nennig, S. E., Price, M. E., Hafeez, H. & Schank, J. R. Sex differences in aversion-resistant ethanol intake in mice. Alcohol. Alcohol. 54, 345–352 (2019).
Randall, P. A., Stewart, R. T. & Besheer, J. Sex differences in alcohol self-administration and relapse-like behavior in Long-Evans rats. Pharmacol. Biochem. Behav. 156, 1–9 (2017).
Satta, R., Hilderbrand, E. R. & Lasek, A. W. Ovarian hormones contribute to high levels of binge-like drinking by female mice. Alcohol Clin. Exp. Res. 42, 286–294 (2017).
Verplaetse, T. L., Rasmussen, D. D., Froehlich, J. C. & Czachowski, C. L. Effects of prazosin, an alpha1-adrenergic receptor antagonist, on the seeking and intake of alcohol and sucrose in alcohol-preferring (P) rats. Alcohol Clin. Exp. Res. 36, 881–886 (2012).
Rasmussen, D. D., Alexander, L. L., Raskind, M. A. & Froehlich, J. C. The alpha1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin. Exp. Res. 33, 264–272 (2009).
Lopez, M. F. et al. Evaluation of the effect of doxasozin and zonisamide on voluntary ethanol intake in mice that experienced chronic intermittent ethanol exposure and stress. Alcohol. 89, 37–42 (2020).
Beldjoud, H. et al. Chronic administration of a norepinephrine antagonist prevents and partially reverses escalation of cocaine self-administration. Addict. Biol. 28, e13316 (2023).
Verplaetse, T. L. & Czachowski, C. L. Low-dose prazosin alone and in combination with propranolol or naltrexone: Effects on ethanol and sucrose seeking and self-administration in the P rat. Psychopharmacology 232, 2647–2657 (2015).
Freeman, C. R. et al. Impact of sugar on the body, brain, and behavior. Front. Biosci. 23, 2255–2266 (2018).
Avena, N. M., Rada, P. & Hoebel, B. G. Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci. Biobehav. Rev. 32, 20–39 (2008).
Eikelboom, R. & Hewitt, R. Intermittent access to a sucrose solution for rats causes long term increases in consumption. Physiol. Behav. 165, 77–85 (2016).
Acknowledgements
This work was supported by AA024109 and AA030710 (FWH). Raw data is available from the corresponding author upon reasonable request. All authors declare no competing interests.
Author information
Authors and Affiliations
Contributions
T.D.O.S and F.W.H conceived the experiments; T.D.O.S, R.J.S, S.E.W, E.A.E conducted the experiments; T.D.O.S and F.W.H wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Oliveira Sergio, T., Jane Smith, R., Wean, S.E. et al. Greater inhibition of female rat binge alcohol intake by adrenergic receptor blockers using a novel Two-Shot rat binge drinking model. Sci Rep 14, 14029 (2024). https://doi.org/10.1038/s41598-024-64565-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64565-9
- Springer Nature Limited