Abstract
Angiotensin-converting enzyme (ACE) is closely related to cardiometabolic risk factors and atherosclerosis. This study aims to investigate whether the insertion/deletion (I/D) variant of ACE gene impacts cardiometabolic risk factors, premature coronary artery disease (PCAD), and severity of coronary lesions. PubMed, Cochrane Library, Central, CINAHL, and ClinicalTrials.gov were searched until December 22, 2023. 94,270 individuals were included for the analysis. Carriers of DD genotype had higher levels of triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), systolic blood pressure (SBP), diastolic blood pressure (DBP), body mass index (BMI), and waist circumference (WC) than carriers of II or ID genotypes. In addition, carriers of DD genotype were at high risk of PCAD and multiple vessel lesions. The impacts of ACE I/D variant on lipid levels were significant in American individuals but stronger in male individuals. In contrast, the impacts of ACE I/D variant on PCAD and severity of coronary lesions were primarily significant in Caucasian individuals. This study indicates that the ACE I/D variant has a slight but significant impact on cardiometabolic risk factors, PCAD, and severity of coronary lesions. Angiotensin-converting enzyme inhibitors (ACEI) may benefit high-risk populations with ACE DD genotype to prevent PCAD and multiple vessel lesions.
PROSPERO registration number: CRD42023426732
Similar content being viewed by others
Introduction
Clinical and experimental studies demonstrated that the activation of the renin-angiotensin system (RAS) is pivotal in coronary artery disease (CAD) pathogenesis1,2. Angiotensin-converting enzyme (ACE), a major component of RAS, converts angiotensin I to angiotensin II (a vasoconstrictor) and degrades bradykinin (a vasodilator). Thus, ACE is critical in regulating the vasomotor tone of arteries. However, chronic exposure to high circulating and tissue levels of ACE predisposes to vascular wall thickening and atherosclerosis3.
The human ACE gene is located on chromosome 17q23.3 and contains 26 exons and 25 introns. The insertion/deletion variant of the ACE gene is characterized by the absence (deletion, D) rather than the presence (insertion, I) of a 287-base pair Alu repeat sequence within intron 164, which results in 3 genotypes: II, ID, and DD. The DD and II genotypes encode high and low activity of ACE5, respectively. Individuals of the DD genotype had twofold higher serum ACE levels6 than individuals of the II genotype.
Serum ACE levels are closely related to cardiometabolic parameters. For instance, high levels of ACE were associated with high levels of lipid, fasting plasma glucose (FPG), and blood pressure7. Using angiotensin-converting enzyme inhibitors (ACEI) improved cardiometabolic parameters (i.e. lipid, FPG, and blood pressure)8,9,10, and attenuated hypercholesterolemia-induced atherosclerotic lesions in rabbits11,12 and minipigs13. In addition, knockout of the ACE gene14 inhibited atherosclerosis development in mice subjected to a high-cholesterol diet.
A series of studies5,6,15 revealed that serum ACE levels are determined by the ACE I/D variant. Since high ACE levels were related to dyslipidemia, dysglycemia, and hypertension7,8,9,10,11,12,13,14, it is tempting to speculate that the ACE I/D variant may induce dyslipidemia, dysglycemia, and hypertension. According to the newest management strategies for premature coronary artery disease (PCAD)16, severe dyslipidemia, diabetes with diabetes-specific risk-enhancing factors, hypertension, or multiple other risk-enhancing factors [e.g. body mass index (BMI) and waist circumference (WC)] were recognized as the primary risk factors for PCAD. In addition to the risk factors in PCAD patients (e.g. dyslipidemia, dysglycemia, and hypertension)16 were replicated in those patients with multiple vessel lesions17. It is tempting to speculate that the I/D variant of the ACE gene may influence the risk of PCAD and the severity of coronary lesions. Here, we conducted this study to investigate this hypothesis.
PCAD is defined as the first onset of CAD in males less than 55 and females less than 65 years of age18. Elevated fibrinogen levels were found to be associated with a greater extent of coronary atherosclerosis in women with PCAD but not in men19. It indicates that the magnitude of the pathological pathways contributing to PCAD differs in women and men19. Despite the already known differences19, we lack sufficiently high-quality studies to assess the differences in PCAD formation and progression in different sexes. Here, this study was conducted to clarify this point. Some previous studies have already investigated the impact of the ACE I/D variant on PCAD. For instance, Abd El-Aziz et al.20 and Berdeli et al.21 found that the DD genotype increased the risk of PCAD in the Egyptian and Turkish populations. Niemiec et al.22 found that the DD genotype was more frequent in patients with stenoses in at least four coronary vessels or critical arterial occlusions. While Kretowski et al.23 reported that patients with type 1 diabetes who had the TT-ID-AA/AC genotype were at high risk of PCAD.
Results
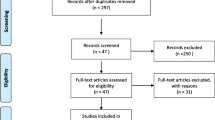
Study selection and characteristics
The details of the study selection are summarized in Fig. 1. The present study included 91 studies in a total of 94,270 individuals (Table S1), including 3195 patients with PCAD, and 2395 patients with multiple vessel lesions (Table S1).
Impact of the ACE I/D variant on lipid levels
All the results stated below were the data that excluded heterogeneity. The DD genotype was linked to higher levels of triglycerides (TG) (Fig. S1), total cholesterol (TC) (Fig. S2), and low-density lipoprotein cholesterol (LDL-C) (Fig. 2). Subgroup analysis by race indicated that the impacts of the ACE I/D variant on TG, TC, LDL-C, and high-density lipoprotein cholesterol (HDL-C) levels were consistently significant in American individuals (Table 1). In addition, subgroup analysis by sex indicated that the impacts of the ACE I/D variant on TC and LDL-C levels were significant in male individuals (Table 1).
Impacts of the ACE variant on other cardiometabolic risk factors
The DD genotype was associated with elevated systolic blood pressure (SBP) (Fig. 3), diastolic blood pressure (DBP) (Fig. S3), BMI (Fig. S4), and WC (Fig. S5). Subgroup analysis indicated that the impact of the ACE I/D variant on SBP was significant in Caucasian individuals, American individuals, male individuals, patients with CAD, and patients with type 2 diabetes mellitus (T2DM) (Table 1). In contrast, the impact of the ACE I/D variant on DBP was significant in male individuals and patients with cardiovascular disease (CVD) (Table 1). In addition, the impacts of the ACE I/D variant on BMI and WC were significant in other ethnicities individuals (Table 1). However, the impact of the ACE I/D variant on FPG levels did not show statistically significant (Table 1).
Impact of the ACE I/D variant on the risk of PCAD
The DD genotype increased the risk of PCAD compared with II or ID genotypes (Fig. 4). Subgroup analysis indicated that the impact of the ACE I/D variant on PCAD risk was primarily significant in Caucasian and male individuals (Table 2).
Impact of the ACE I/D variant on the severity of coronary lesions
The DD genotype increased the risk of multiple vessel lesions compared with II or ID genotypes (Fig. 5). Subgroup analysis indicated that the impact of the ACE I/D variant on multiple vessel lesions was significant in Caucasian and other ethnicities individuals (Table 3).
Publication bias test
Begg funnel plot was used to evaluate publication bias among the included studies. This meta-analysis had no publication bias, which was confirmed by the Egger linear regression test.
Discussion
The present study indicated that the DD genotype of the ACE I/D variant increased the risk of PCAD (Table 2) and multiple vessel lesions (Table 3), as well as elevated TG, TC, LDL-C, SBP, DBP, BMI, and WC (Table 1). Since dyslipidemia, hypertension, high BMI, and large WC were considered the primary risk factors for PCAD and multiple vessel lesions16,17, it indicated that the increased risk of PCAD (Table 2) and multiple vessel lesions (Table 3) associated with DD genotype was attributed, at least partly, by the impacts of the ACE I/D variant on TG, TC, LDL-C, SBP, DBP, BMI, and WC (Table 1).
Subgroup analyses by sex revealed that the impact of the ACE I/D variant on PCAD was significant in male individuals (Table 2). Since the impacts of the ACE I/D variant on LDL-C, TC, SBP, and DBP were significant in male individuals (Table 1), it indicated that the impact of the ACE I/D variant on PCAD in male individuals (Table 2) was at least partly mediated by the elevated LDL-C, TC, SBP, and DBP (Table 1). In addition, subgroup analyses by race showed that the impacts of the ACE I/D variant on PCAD and multiple vessel lesions were significant in Caucasian individuals (Tables 2, 3). Since the impact of the ACE I/D variant on SBP was significant in Caucasian individuals (Table 1), it indicated that the impacts of the ACE I/D variant on PCAD and CAD severity in Caucasian individuals (Tables 2, 3) were partially mediated by the elevated SBP (Table 1).
In this study, the prevalence of II genotype in Caucasian individuals, American individuals, Asian individuals, and other ethnicities individuals was 0.40 (range from 0.08 to 0.72), 0.43 (range from 0.22 to 0.64), 0.50 (range from 0.22 to 0.78), and 0.535 (range from 0.22 to 0.85), respectively. In contrast, the prevalence of DD genotype in Caucasian individuals, American individuals, Asian individuals, and other ethnicities individuals was 0.325 (range from 0.15 to 0.50), 0.275 (range from 0.18 to 0.37), 0.23 (range from 0 to 0.46), and 0.17 (range from 0.04 to 0.30), respectively. In addition, the prevalence of ID genotype in Caucasian individuals, American individuals, Asian individuals, and other ethnicities individuals was 0.275 (range from 0.22 to 0.33), 0.295 (range from 0.18 to 0.41), 0.27 (range from 0.205 to 0.335), and 0.295 (range from 0.15 to 0.44), respectively.
The impacts of the ACE I/D variant on PCAD and the severity of coronary lesions were primarily significant in Caucasian individuals, but not in Asian individuals and other ethnicities individuals (Tables 2, 3). One particular reason could be proposed to interpret this phenomenon. That is, Caucasian individuals had a higher carrying rate of DD than Asian and other ethnicities individuals (Caucasian individuals vs. Asian individuals vs. Other ethnicities individuals = 0.325 vs. 0.23 vs. 0.17). Similarly, since the prevalence rate of DD was relatively higher in American individuals than in Asian individuals and other ethnicities individuals (American individuals vs. Asian individuals vs. other ethnicities individuals = 0.275 vs. 0.23 vs. 0.17), it is plausible to observe that the impacts of the ACE I/D variant on cardiometabolic parameters (Table 1) were primarily significant in American individuals.
According to the 2018 American College of Cardiology (ACC)/American Heart Association (AHA)24, the 2019 European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS)25, and the Adult Treatment Panel III (ATP III) cholesterol guidelines26, LDL-C was considered the major cause of CAD and treated as the primary target for therapy, while other lipids were used as the secondary or supplementary therapeutic targets. In this study, the strongest impact of the ACE I/D variant on LDL-C levels was detected in male individuals (SMD = 0.30, 95% CI 0.15 to 0.45, P = 0.03) (Table 1). It indicated that male individuals with DD were at a very high risk of CAD. In addition, the impact of the ACE I/D variant on LDL-C levels was also significant in American individuals (Table 1), suggesting that American individuals with DD were at high risk of CAD.
A small sample case–control study conducted by Winkelmann and colleagues27 included 209 male patients with CAD and 92 male controls and found that the ACE I/D variant was not associated with an increased risk for CAD or myocardial infarction (MI). In contrast, a moderate-scale case–control study conducted by Cambien and colleagues28 included 610 patients with MI and 733 controls and found that the ACE I/D variant was a potent risk factor for CAD in subjects formerly considered to be at low risk according to common criteria. In addition, Miao et al.29, Zhou et al.30, and Zhang et al.31 respectively conducted a meta-analysis (sample size: 1241 cases and 3452 controls for Miao et al. 5215 cases and 4782 controls for Zhou et al. 5619 cases and 4865 controls for Zhang et al.) to investigate the association between ACE I/D variant and the risk of CAD. Intriguingly, all three mete-analyses demonstrated that there was a significant association between ACE I/D variant and CAD [odds ratio (OR) = 1.92 for Miao et al., OR range from 1.19 to 2.40 for Zhou et al., OR = 1.95 for Zhang et al.]. In line with previous clinical trials21,22,32,33,34,35,36,37,38,39,40. Taken together, the correlation between the ACE I/D variant and CAD appears to be related to the sample size of studies. In which, studies with small samples are unlikely to detect the true impact of the ACE I/D variant on CAD, whilst studies with relatively larger samples may detect the true association between the ACE I/D variant and CAD due to adequate statistical power.
The present meta-analysis included 3195 patients with PCAD and 2395 patients with multiple vessel lesions and found that the DD genotype significantly increased the risk of PCAD and multiple vessel lesions by 9% and 4%, respectively, suggesting that the ACE I/D variant was slightly but significantly associated with PCAD and multiple vessel lesions (Tables 2, 3). In line with these findings, this meta-analysis also indicated that the DD genotype significantly increased TG, TC, LDL-C, SBP, DBP, BMI, and WC (Table 1). It indicated that the impacts of the ACE I/D variant on PCAD and the severity of coronary lesions (Tables 2, 3) were at least partly mediated by the impacts of the ACE I/D variant on cardiometabolic risk factors (Table 1).
The impacts of the ACE I/D variant on cardiometabolic risk factors, PCAD, and severity of coronary lesions (Tables 1, 2 and 3) were essentially attributed to high levels of ACE7,8,9,10,11,12,13,14,16,17. Since ACEI/angiotensin receptor blockers (ARBs) were prominent inhibitors of ACE that had been widely used in clinical practice41, it is tempting to hypothesize that preventive use of ACEI/ARBs may benefit high-risk populations with the DD genotype to prevent cardiometabolic disorder, PCAD, and multiple vessel lesions. Further randomized controlled trials (RCTs) are needed to verify this hypothesis and detail the dose, frequency, and duration of administration. Since the DD genotype was closely linked to cardiometabolic disorder (Table 1), PCAD (Table 2), and multiple vessel lesions (Table 3), the genetic screening of the ACE I/D variant may benefit the early prevention or control of PCAD and multiple vessel lesions.
The connection between genotype and phenotype is intricate. For instance, the genotypes may generate complex clinical phenotypes, which can be further modified by external factors (e.g. environmental factors and lifestyles). In other words, the clinical phenotypes of individual organisms are jointly determined by multiple factors (e.g. genetic factors, environmental factors, and personal lifestyles). Additionally, there are often many genotypes that produce the same phenotype, adding a layer of complexity in establishing valid genotype–phenotype relationships42. Therefore, prediction of trait heritability via genotype–phenotype association remains a major challenge in modern genetics. How to reasonably regulate the trait inheritance association with heterogeneous genotype–phenotype is becoming an important field of biological research and application, and it is also the prerequisite and foundation to execute gene editing and gene therapy.
The present meta-analysis has several strengths (1) all results are recalculated after excluding the studies with heterogeneity (Table 1), which advances the preciseness of conclusions drawn in this paper; (2) the conclusions will no doubt benefit clinicians to make the optimum management strategies for high-risk population with the DD genotype to prevent PCAD or multiple vessel lesions; (3) genetic screening of the DD genotype is critical for the high-risk population to prevent PCAD or multiple vessel lesions. In addition, several limitations should be noted (1) there is no information regarding the moment of measurement (on treatment or without treatment); (2) CAD is a multifactorial disease, and the major risk factors include genetic factors, environmental influences, and personal lifestyles. To explain the role of specific mutation (e.g. ACE I/D variant), all relevant risk factors (i.e. genetic factors, environmental influences, and personal lifestyles) should be explored. However, the impacts of environmental factors and personal lifestyles associated with the ACE I/D variant on PCAD and CAD severity, as well as the interactions of the ACE I/D variant with environmental factors and personal lifestyles on PCAD and CAD severity have yet to be investigated in the present meta-analysis due to the lack of original data from the included studies. In other words, more precise results could have been gained if more detailed individual data were available or the stratification analyses based on environmental factors and personal lifestyles, such as diet, exercise, smoking, alcohol consumption, etc., were performed; (3) there was no study investigated the impact of ACE I/D variant on PCAD in Asian individuals, American individuals, and female individuals (Table 2). More comprehensive and diversified results would be available if more studies on specific populations (e.g. Asian individuals, American individuals, and female individuals) were carried out.
Materials and methods
The current meta-analysis follows the Preferred Reporting Items for Systematic Reviews and Meta-analyses (see Table S2 for more details)43.
Literature search
A comprehensive literature search was performed from January 18, 2021 to December 22, 2023 using PubMed, Cochrane Library, Central, CINAHL, and ClinicalTrials.gov. The following keywords were used in the search: [“angiotensin-converting enzyme,” “renin-angiotensin system”] AND [“variant,” “polymorphism,” “mutation,” “variation,” “mutant,” “SNP”] OR [“single nucleotide polymorphism”] AND [“lipid,” “circulating lipids,” “blood lipids,” “plasma lipids,” “serum lipids,” “lipid profile”] OR [“triglycerides,” “total cholesterol,” “low-density lipoprotein cholesterol,” “high-density lipoprotein cholesterol”] OR [“fasting plasma glucose,” “fasting blood glucose”] OR [“blood pressure,” “systolic blood pressure,” “diastolic blood pressure”] OR [“body mass index”] OR [“waist circumference”] OR [“premature coronary artery disease,” “early-onset coronary artery disease”] AND/OR [“coronary artery disease severity,” “triple-vessel lesions,” “multiple-vessel lesions”]. Additionally, the reference lists of all eligible studies were manually retrieved to search for additional literature.
Inclusion criteria
The inclusion criteria for the impacts of the ACE I/D variant on PCAD and CAD severity include (1) case–control design; (2) CAD cases were angiographically defined; (3) studies provided the number of individual genotypes in cases and controls for the ACE I/D variant; (4) studies provided the number of individual genotypes in multiple (≥ 3) and non-multiple (< 3) lesions for the ACE I/D variant. The inclusion criteria for the impacts of the ACE I/D variant on cardiometabolic risk factors include (1) studies investigated the association of ACE I/D variant with lipid [including four lipid parameters, i.e. TG, TC, LDL-C, and HDL-C, rather than other lipoproteins, e.g. apolipoprotein B (APOB), apolipoprotein E (APOE), and apolipoprotein A1 (APOA1), etc.; expressed as mean with standard deviation (SD) or standard errors (SE), but not other forms, such as, mean with 95% confidence intervals (CI) and median with interquartile range (IQR); data of dyslipidemia and normal lipid levels were available]; (2) studies investigated the association of ACE I/D variant with blood pressure (including SBP and DBP; expressed as mean with SD or SE; data of hypertension and normal blood pressure levels were available); (3) studies investigated the association of the ACE I/D variant with FPG (rather than postprandial plasma glucose; expressed as mean with SD or SE; data of dysglycemia and normal plasma glucose were available); (4) studies investigated the association of ACE I/D variant with BMI [expressed as mean with SD or SE; data of underweight (< 18.5 kg/m2), overweight (25–29.9 kg/m2), obesity (> 30 kg/m2), and normal range (18.5–24.9 kg/m2) were available]; (5) studies investigated the association of ACE I/D variant with WC (expressed as mean with SD or SE); (6) studies provided the number of individual genotypes for the ACE I/D variant; (7) the language of eligible studies was restricted to English. The exclusion criteria of this meta-analysis include (1) studies that were not related to the ACE I/D variant; (2) studies that were not related to cardiometabolic risk factors; (3) studies that were not related to PCAD or CAD severity; (4) studies not presenting genotype count in controls, or the genotype distribution of controls deviate from the Hardy–Weinberg equilibrium (HWE); (5) studies having invalid data; (6) studies having incomplete data; (7) pedigree studies; (8) overlapping studies; and (9) abstract, review, case report, meta-analysis, and animal studies.
Data extraction
The data extraction was conducted by ZL. From each included study, the following was extracted: the last name of the first author; publication time; country, race, sex, age, case and control counts, genotype count, study design, study period, and mean lipid, blood pressure, FPG, BMI, and WC with SD or SE by the genotype of the ACE I/D variant (i.e. II, ID, and DD).
Data analysis
The units of TG, TC, LDL-C, HDL-C, and FPG were converted into mmol/L. The unit of blood pressure was converted into mmHg. The unit of BMI was converted into kg/m2. The unit of WC was converted into cm. All extracted data were expressed as mean ± SD. Risk ratios (RR) and corresponding 95% CI were used to evaluate the strength of the ACE I/D variant in PCAD and CAD severity. Standardized mean difference (SMD) with 95% CI was used to evaluate the differences in cardiometabolic parameters (i.e. TG, TC, LDL-C, HDL-C, SBP, DBP, FPG, BMI, and WC) between carriers of DD and carriers of II or ID. The pooled RR was performed in the allelic model (D vs. I), additive model (DD vs. II), heterozygote model (ID vs. II), dominant model (ID + DD vs. II), recessive model (DD vs. II + ID), and overdominant model (ID vs. II + DD). Since most publications compared cardiometabolic parameters between carriers of DD and carriers of II or ID, a recessive genetic model (DD vs. II + ID) was adopted to analyze the strength of the ACE I/D variant in cardiometabolic parameters. All statistical tests were conducted with the Cochrane Collaboration meta-analysis software (Review Manager 5.4). P < 0.05 was recognized as statistically significant.
Subgroup analysis
Subgroup analysis was performed by race, sex, and health status. The race was divided into Caucasian, Asian, American, and other ethnicities. Healthy status was divided into CAD, T2DM, hypertension, obesity, metabolic syndrome (Mets), CVD, and healthy individuals. In some studies, subjects were divided into multiple subpopulations (e.g. patients with different diseases or individuals from different races, etc.). Each subpopulation was regarded as an independent comparison in this study.
Evaluation of heterogeneity
Heterogeneity was tested by I2 statistic and Cochran's χ2-based Q statistic. If heterogeneity was significant (I2 > 50%, P ≤ 0.05), the random-effect model (DerSimonian-Laird method) was used to calculate the results44. Otherwise, the fixed-effect model (Mantel–Haenszel method) would be adopted (I2 < 50%, P > 0.05). In addition, the Galbraith plot was employed to detect the potential sources of heterogeneity. To eliminate the impact of heterogeneity on the ultimate results, all preliminary results were recalculated after excluding the studies with heterogeneity.
Publication bias test
The Begg funnel plot and Egger linear test evaluated the probability of publication bias among the included studies45.
The summary statistics of this study
Primary results
-
1.
The differences in cardiometabolic parameters (i.e. TG, TC, LDL-C, HDL-C, FPG, SBP, DBP, BMI, and WC) between carriers of DD and carriers of II or ID in an integrated population (i.e. Caucasian, Asian, American, and other ethnicities).
-
2.
The impact of ACE I/D variant on PCAD risk in allelic model (D vs. I), additive model (DD vs. II), heterozygote model (ID vs. II), dominant model (ID + DD vs. II), recessive model (DD vs. II + ID), and overdominant model (ID vs. II + DD).
-
3.
The impact of ACE I/D variant on CAD severity in allelic model (D vs. I), additive model (DD vs. II), heterozygote model (ID vs. II), dominant model (ID + DD vs. II), recessive model (DD vs. II + ID), and overdominant model (ID vs. II + DD).
Secondary results
-
1.
The differences in cardiometabolic parameters (i.e. TG, TC, LDL-C, HDL-C, FPG, SBP, DBP, BMI, and WC) between carriers of DD and carriers of II or ID in specific populations, including Caucasian, Asian, American, and other ethnicities, males, females, CAD patients, T2DM patients, hypertension patients, obesity patients, Mets patients, CVD patients, and healthy individuals.
-
2.
The impact of ACE I/D variant on PCAD risk in Caucasians, other ethnicities, and males under different genetic models.
-
3.
The impact of ACE I/D variant on CAD severity in Caucasians, Asians, other ethnicities, males, and females under different genetic models.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
van der Net, J. B. et al. Gene-load score of the renin-angiotensin-aldosterone system is associated with coronary heart disease in familial hypercholesterolaemia. Eur. Heart J. 29, 1370–1376 (2008).
Brugts, J. J. et al. Genetic determinants of treatment benefit of the angiotensin-converting enzyme-inhibitor perindopril in patients with stable coronary artery disease. Eur. Heart J. 31, 1854–1864 (2010).
Kawamoto, R., Kohara, K., Tabara, Y. & Miki, T. An interaction between systolic blood pressure and angiotensin-converting enzyme gene polymorphism on carotid atherosclerosis. Hypertens. Res. 25, 875–880 (2002).
Rigat, B., Hubert, C., Corvol, P. & Soubrier, F. PCR detection of the insertion/deletion polymorphism of the human angiotensin converting enzyme gene (DCP1) (dipeptidyl carboxypeptidase 1). Nucleic Acids Res. 20, 1433 (1992).
Danser, A. H. et al. Angiotensin-converting enzyme in the human heart. Effect of the deletion/insertion polymorphism. Circulation 92, 1387–1388 (1995).
Tiret, L. et al. Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am. J. Hum. Genet. 51, 197–205 (1992).
Nagi, D. K. et al. Angiotensin-1-converting enzyme (ACE) gene polymorphism, plasma ACE levels, and their association with the metabolic syndrome and electrocardiographic coronary artery disease in Pima Indians. Metabolism 47, 622–626 (1998).
Matchar, D. B. et al. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann. Intern. Med. 148, 16–29 (2008).
Keilani, T., Schlueter, W. A., Levin, M. L. & Batlle, D. C. Improvement of lipid abnormalities associated with proteinuria using fosinopril, an angiotensin-converting enzyme inhibitor. Ann. Intern. Med. 118, 246–254 (1993).
Pollare, T., Lithell, H. & Berne, C. A comparison of the effects of hydrochlorothiazide and captopril on glucose and lipid metabolism in patients with hypertension. N. Engl. J. Med. 321, 868–873 (1989).
Schuh, J. R., Blehm, D. J., Frierdich, G. E., McMahon, E. G. & Blaine, E. H. Differential effects of renin-angiotensin system blockade on atherogenesis in cholesterol-fed rabbits. J. Clin. Investig. 91, 1453–1458 (1993).
Chobanian, A. V., Haudenschild, C. C., Nickerson, C. & Hope, S. Trandolapril inhibits atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Hypertension 20, 473–477 (1992).
Jacobsson, L. S. et al. Antiatherosclerotic effects of the angiotensin-converting enzyme inhibitors captopril and fosinopril in hypercholesterolemic minipigs. J. Cardiovasc. Pharmacol. 24, 670–677 (1994).
Hayek, T. et al. Tissue angiotensin-converting-enzyme (ACE) deficiency leads to a reduction in oxidative stress and in atherosclerosis: Studies in ACE-knockout mice type 2. Arterioscler. Thromb. Vasc. Biol. 23, 2090–2096 (2003).
Rigat, B. et al. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J. Clin. Investig. 86, 1343–1346 (1990).
Stone, N. J. et al. Managing atherosclerotic cardiovascular risk in young adults: JACC state-of-the-art review. J. Am. Coll. Cardiol. 79, 819–836 (2022).
Wang, J., Huang, X., Fu, C., Sheng, Q. & Liu, P. Association between triglyceride glucose index, coronary artery calcification and multivessel coronary disease in Chinese patients with acute coronary syndrome. Cardiovasc. Diabetol. 21, 187 (2022).
De Sutter, J. et al. Screening of family members of patients with premature coronary heart disease; results from the EUROASPIRE II family survey. Eur. Heart J. 24, 249–257 (2003).
Kryczka, K. E., Kruk, M., Demkow, M. & Lubiszewska, B. Fibrinogen and a triad of thrombosis, inflammation, and the renin-angiotensin system in premature coronary artery disease in women: A new insight into sex-related differences in the pathogenesis of the disease. Biomolecules 11, 1036 (2021).
Abd El-Aziz, T. A., Hussein, Y. M., Mohamed, R. H. & Shalaby, S. M. Renin-angiotensin system genes polymorphism in Egyptians with premature coronary artery disease. Gene 498, 270–275 (2012).
Berdeli, A. et al. Association between the eNOS (Glu298Asp) and the RAS genes polymorphisms and premature coronary artery disease in a Turkish population. Clin. Chim. Acta 351, 87–94 (2005).
Niemiec, P., Zak, I. & Wita, K. The D allele of angiotensin I-converting enzyme gene insertion/deletion polymorphism is associated with the severity of atherosclerosis. Clin. Chem. Lab. Med. 46, 446–452 (2008).
Kretowski, A. et al. Polymorphisms of the renin-angiotensin system genes predict progression of subclinical coronary atherosclerosis. Diabetes 56, 863–871 (2007).
Grundy, S. M. et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 139, e1082–e1143 (2019).
Mach, F. et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 41, 111–188 (2020).
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation, 106, 3143–3421 (2002).
Winkelmann, B. R. et al. Deletion polymorphism of the angiotensin I-converting enzyme gene is associated with increased plasma angiotensin-converting enzyme activity but not with increased risk for myocardial infarction and coronary artery disease. Ann. Intern. Med. 125, 19–25 (1996).
Cambien, F. et al. Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature 359, 641–644 (1992).
Miao, H. W. & Gong, H. Association of ACE insertion or deletion polymorphisms with the risk of coronary restenosis after percutaneous coronary intervention: A meta-analysis. J. Renin Angiotensin Aldosterone Syst. 16, 844–850 (2015).
Zhou, L., Xi, B., Wei, Y., Shen, W. & Li, Y. Meta-analysis of the association between the insertion/deletion polymorphism in ACE gene and coronary heart disease among the Chinese population. J. Renin Angiotensin Aldosterone Syst. 13, 296–304 (2012).
Zhang, Y., Yang, T., Zhou, W. & Huang, Y. A meta-analysis on the association of genetic polymorphism of the angiotensin-converting enzyme and coronary artery disease in the Chinese population. Rev. Assoc. Med. Bras. 65, 923–929 (2019).
Alvarez, R. et al. Association between the NOS3 (-786 T/C) and the ACE (I/D) DNA genotypes and early coronary artery disease. Nitric Oxide 5, 343–348 (2001).
Vaisi-Raygani, A. et al. The angiotensin converting enzyme D allele is an independent risk factor for early onset coronary artery disease. Clin. Biochem. 43, 1189–1194 (2010).
Tran, D. C. et al. Association between ACE I/D genetic polymorphism and the severity of coronary artery disease in Vietnamese patients with acute myocardial infarction. Front. Cardiovasc. Med. 10, 1091612 (2023).
Poorzand, H. et al. Association of polymorphisms of renin angiotensin system and endothelial nitric oxide synthase genes with premature cardiovascular disease in an Iranian population. BMC Cardiovasc. Disord. 23, 254 (2023).
Miettinen, H. E., Korpela, K., Hämäläinen, L. & Kontula, K. Polymorphisms of the apolipoprotein and angiotensin converting enzyme genes in young North Karelian patients with coronary heart disease. Hum. Genet. 94, 189–192 (1994).
Ferrari, M. et al. Angiotensin-converting enzyme insertion/deletion polymorphism does not influence the restenosis rate after coronary stent implantation. Cardiology 97, 29–36 (2002).
Narne, P., Ponnaluri, K. C., Singh, S., Siraj, M. & Ishaq, M. Relationship between angiotensin-converting enzyme gene insertion/deletion polymorphism, angiographically defined coronary artery disease and myocardial infarction in patients with type 2 diabetes mellitus. J. Renin Angiotensin Aldosterone Syst. 13, 478–486 (2012).
Sigusch, H. H. et al. Angiotensin-I-converting enzyme DD genotype is a risk factor of coronary artery disease. Scand. J. Clin. Lab. Investig. 57, 127–132 (1997).
Gardemann, A. et al. ACE I/D gene polymorphism: Presence of the ACE D allele increases the risk of coronary artery disease in younger individuals. Atherosclerosis 139, 153–159 (1998).
Ramanathan, G., Ghosh, S., Elumalai, R., Periyasamy, S. & Lakkakula, B. V. Influence of angiotensin converting enzyme (ACE) gene rs4362 polymorphism on the progression of kidney failure in patients with autosomal dominant polycystic kidney disease (ADPKD). Indian J. Med. Res. 143, 748–755 (2016).
Fisch, G. S. Whither the genotype-phenotype relationship? An historical and methodological appraisal. Am. J. Med. Genet. C Semin. Med. Genet. 175, 343–353 (2017).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 339, b2700 (2009).
DerSimonian, R. & Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 28, 105–114 (2007).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994).
Author information
Authors and Affiliations
Contributions
ZL designed this study and drafted the manuscript. ZL searched literature, collected data, and performed statistical analyses. ZL was responsible for data interpretation, revision, and approval of the manuscript. ZL reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, Z. Impacts of ACE insertion/deletion variant on cardiometabolic risk factors, premature coronary artery disease, and severity of coronary lesions. Sci Rep 14, 13171 (2024). https://doi.org/10.1038/s41598-024-64003-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64003-w
- Springer Nature Limited