Abstract
Extracellular fluid (ECF) excess is common in patients with chronic kidney disease (CKD). This study (involving 284 patients with CKD) explored the association between choroidal vascularity index (CVI) and ECF excess. We categorised patients into three groups based on extracellular water/total body water: normal, mildly overhydrated, and severely overhydrated. The more severe ECF status was associated with a lower CVI after adjustment (B = − 0.902, p = 0.001). In non-diabetic patients, both vascular luminal (LA, p < 0.001) and stromal areas (SA, p = 0.003) were significantly reduced in patients with severe ECF excess compared to others, whereas diabetic patients showed no significant differences in LA (p = 0.96) and SA (p = 0.86) based on ECF excess status. These findings suggest that ECF status may influence CVI in patients with CKD, underscoring the need for further research to clarify its direct impact on choroidal changes.
Similar content being viewed by others
Introduction
The choroid is a dense network of blood vessels and pigmented stroma between the retina and the sclera. It plays a crucial role in supplying oxygen and nutrients to the outer retina, light absorption, and thermoregulation1. The choroidal circulation (responsible for 85% of eye blood flow and is mainly controlled by sympathetic innervation) lacks autoregulation2. Although structural analysis of the choroidal vasculature has historically been challenging due to its inaccessibility, enhanced depth imaging (EDI)—optical coherence tomography (OCT) has enabled high-resolution visualisation of subtle vascular changes in choroidal structures3.
Choroidal Vascularity Index (CVI) was recently proposed as a novel marker, complementing subfoveal choroidal thickness (SFCT) in assessing choroidal changes using OCT4. It evaluates the choroidal vascular system by quantifying both its vascular (luminal) and stromal components. Notably, CVI measurements are a more stable parameter than SFCT, with reduced inter-assay variability and lower dependence on physiological factors5.
Chronic kidney disease (CKD), affecting over 10% of the global population, has emerged as one of the leading causes of mortality worldwide6. Some studies have investigated the impact of CKD on the choroid. Patients with CKD exhibit choroidal thinning compared to those with normal kidney function, and this thinning is correlated with the level of systemic inflammation and the degree of renal dysfunction7,8. A reduction in CVI has also been observed in patients with CKD, both in those with hypertension and diabetes mellitus (DM)5,9. These findings suggest that choroidal alterations observed in individuals with CKD might indicate broader systemic microvascular damage and potentially reflect underlying kidney injury.
Extracellular fluid (ECF) excess is common in individuals with CKD. ECF excess increases as the estimated glomerular filtration rate (eGFR) declines and has been identified as an independent factor influencing structural cardiac and vascular alterations in the course of CKD10. Chronic fluid overload has been observed even in the early stages of CKD, and this condition has been linked to both mortality and cardiovascular morbidity11,12. Some studies have suggested changes in SFCT and CVI before and after haemodialysis in patients with kidney failure13 who have ECF excess14,15. However, the associations between ECF excess and choroidal morphology have not been reported in an independent context. This study aimed to explore the association between the CVI and the state of ECF excess in individuals with CKD, along with related factors.
Results
Baseline characteristics
The average age was 61.72 years, and 58.8% were male. The mean extracellular water (ECW)/total body water (TBW) ratio was 0.391, and the ECF excess status was distributed as follows: 43.3% for normal, 31.7% for mildly overhydrated, and 25% for severely overhydrated status. The mean SFCT was 259.43 μm, and the mean CVI was 65.87%.
We analysed baseline characteristics by categorising the participants into three groups based on ECF excess status (Table 1). Concerning OCT parameters, the luminal area (LA) (p = 0.050) and stromal areas (SA) (p = 0.25) did not exhibit significant differences among the groups, whereas the CVI showed a significant difference (p < 0.001). The severely overhydrated status group had a significantly lower CVI compared to the normal (p < 0.001) and mildly overhydrated groups (p = 0.006). Furthermore, ECW/TBW exhibited a negative correlation with CVI, with a Pearson correlation coefficient of − 0.190 (p = 0.001).
Association of CVI with ECF excess status
In the univariate linear regression analysis, the presence of DM (p = 0.002), systolic blood pressure (SBP) (p = 0.003), eGFR (p < 0.001), and the ECF excess status (p < 0.001) were associated with CVI. In the multivariate analysis, after adjusting for age (p = 0.99), sex (p = 0.09), presence of DM (B = − 1.149, p = 0.011), SBP (p = 0.34), eGFR (B = 0.036, p < 0.001), low-density lipoprotein (LDL)-cholesterol (p = 0.76) and Haemoglobin A1c (HbA1c) (p = 0.96), a significant association between more severely overhydrated status and lower CVI was observed (B = − 0.902, p = 0.001) (Table 2).
Subgroup analysis: non-DM vs. DM patients
Since the regression analysis indicated that the presence of DM influenced the association between ECF excess status and CVI, the subjects were divided into two groups: a non-DM group and a DM group for further analysis. Of the 284 subjects, 112 were in the non-DM group, and 172 were in the DM group. Patients in the DM group were older (p < 0.001) and had a higher prevalence of hypertension (p = 0.029) than those in the non-DM group. In addition, body mass index (BMI) (p = 0.001), SBP (p = 0.002), CKD stage (p < 0.001), and HbA1c levels (p < 0.001) were higher in the DM group. LDL-cholesterol (p = 0.010) and CVI (p = 0.004) were lower in the DM group than in the non-DM group (Supplemental Table 1).
In the non-DM and DM groups, severe excess of ECF was significantly associated with lower CVI (Table 3). In the non-DM group, even after adjusting for age, sex, SBP, eGFR and LDL-cholesterol, there remained an association of ECF excess status with lower CVI (B = − 1.500, p = 0.007). In the DM group, after further adjustment for HbA1c level and diabetic retinopathy (DR) grade, this association remained significant (B = − 0.658, p = 0.025).
We analysed how the components contributing to CVI, specifically the LA and SA, differed according to the ECF excess status (Table 4). In the non-DM group, the severely overhydrated group had significantly lower values for both the LA and SA compared to the normal (p < 0.001 for LA, p = 0.005 for SA) and mildly overhydrated groups (p = 0.002 for LA, p = 0.013 for SA). In contrast, in the DM group, there were no significant differences in the LA (p = 0.96) and SA (p = 0.86) based on the ECF excess status.
Discussion
The current study demonstrated a significant association between ECF excess and reduced CVI in patients with CKD. This finding was consistent across both the non-DM and DM groups. In the non-DM group, individuals with severe ECF excess exhibited notably lower LA and SA than those with normal or mild ECF excess; however, in DM patients, there was no significant difference in LA and SA based on ECF excess status.
Several studies have explored the effect of renal function on the choroid in patients with CKD. Thinner choroids have been observed in patients with CKD compared to those with normal renal function7,8, and a reduction in CVI has also been documented in patients with CKD5,9. Chorioretinal thinning in CKD is associated with a lower eGFR and increased circulating levels of inflammatory markers, such as C-reactive protein, interleukin-6, asymmetric dimethylarginine, and endothelin-1. These findings suggest that choroidal changes in patients with CKD are correlated with systemic inflammation, endothelial dysfunction, and vasoconstriction16. In the present study, multivariate regression analysis revealed an association between reduced renal function (lower eGFR) and decreased CVI, which is consistent with previous findings.
ECF excess is prevalent in patients with CKD, increasing as eGFR declines10,11. This chronic fluid overload is evident even in the early CKD stages and has been identified as a significant independent factor contributing to structural cardiac and vascular changes throughout the disease course10. The clinical assessment of the ECF status is relatively difficult because the physical signs of oedema are of limited value in the diagnosis of ECF excess17. In this study, we used bioelectrical impedance analysis (BIA) to assess the ECF status by measuring the impedance of the body to applied electric currents of different frequencies18, and showed that ECF excess is independently linked to decreased CVI in patients with CKD after adjusting for multiple confounding factors.
The association between ECF excess and lower CVI can be attributed to several potential mechanisms. Diminished renal function leads to reduced urinary excretion of sodium and fluid when ECF excess is present, prompting the activation of the intrarenal renin–angiotensin–aldosterone system, which exacerbates ECF excess. Angiotensin II acts as a powerful vasoconstrictor and promotes sympathetic overactivity through the regulation of central sympathetic outflow19,20. This interaction could potentially contribute to the decrease in the CVI. Furthermore, the persistent ECF excess results in pathologic mechanical stimuli on vascular endothelial and smooth muscle cells11. ECF overload is significantly linked to microinflammation and markers of endothelial dysfunction21, contributing to increased superoxide production and reduced nitric oxide (NO) bioavailability in patients with CKD22. Accelerated atherosclerosis, vasoconstriction, endothelial dysfunction, and proliferation of smooth muscle cells in vessel walls potentially lead to choroidal changes in the context of ECF excess. Further research is warranted to comprehensively understand the mechanisms underlying the relationship between ECF excess and CVI in patients with CKD.
ECF excess status was associated with a decrease in CVI in the DM and non-DM groups. However, when the SA and LA were analysed separately, the DM and non-DM groups exhibited different patterns. In the non-DM group, individuals with severe ECF excess exhibited significantly reduced LA and SA compared to those with normal or mild ECF excess. There was a more prominent decrease in the LA, which might have led to a secondary reduction in the SA, potentially leading to a decrease in the CVI. In DM patients, no significant differentiation was observed in the LA and SA based on ECF excess status. Despite the lack of statistical significance, patients with severe ECF excess in the DM group exhibited larger LA and SA than those with a normal ECF status or mild excess. The significant decrease in CVI within the ECF excess group in the DM group may be explained by the relatively modest increase in the LA compared to the greater increase in the SA. Given the differences in baseline characteristics between the DM and non-DM groups, it is challenging to interpret the results as a direct comparison; however, these findings suggest the possibility of distinct choroidal changes in response to ECF excess status between the non-DM and DM groups.
This study had some limitations. First, the causative relationship could not be determined due to the cross-sectional study design. However, longitudinal studies are required to confirm these conclusions. Second, although patients taking diuretics or steroids were excluded from the analysis, the influence of other medications (for example, angiotensin-converting enzyme inhibitors, beta-blockers, and statins) could potentially affect the results. Third, information regarding the ocular factors that could affect the choroid, such as intraocular pressure or refractive error, was not available. However, CVI is less affected by ocular factors than SFCT4,5. Despite these limitations, we demonstrated that the non-traditional factor of ECF excess independently affects the choroid in patients with CKD. Larger studies focusing on specific patient groups are necessary to better understand the longitudinal changes during the progression of CKD. In addition, considering the known association between ECF excess and cardiovascular morbidity and mortality11,12, exploring whether CVI could serve as a biomarker reflecting systemic vascular status would be an interesting avenue of research.
In summary, this study demonstrated a significant and independent association between ECF status, influenced by renal function, and the CVI in patients with CKD. These findings imply that ECF status could play a role in influencing CVI in patients with CKD, prompting the need for further research to elucidate the direct impact of ECF status on choroidal changes.
Methods
The study protocol was approved by the Institutional Review Board of the Yonsei University Health System Clinical Trial Centre. The study was conducted according to the principles of the Declaration of Helsinki. All the patients provided written informed consent before participating in the study (IRB No. 4–2013-0661). All the methods were carried out in accordance with relevant guidelines and regulations.
Study populations
The participants in this study were selected from the Cardiovascular and Metabolic Diseases Etiology Research Center High-Risk Cohort (CMERC-HI, https://clinicaltrials.gov/NCT02003781) study. This cohort consisted of individuals at a high risk of developing cardiovascular disease (CVD) who did not exhibit symptomatic CVD at baseline, and the participants were enrolled from 2013 to 2018. The study protocol will be reported in detail elsewhere23. Subjects were deemed to have cardiovascular risk factors and were enrolled in the CMERC-HI cohort study if they had any of the following: high-risk hypertension (eGFR > 60 mL/min/1.73 m2 with damage to at least one target organ or an eGFR ≤ 60 mL/min/1.73 m2); DM with albuminuria; anuric kidney failure requiring dialysis; a relative who sustained an acute myocardial infarction when aged younger than 55 years (if male) or 65 years (if female); asymptomatic atherosclerotic disease (abdominal aorta diameter ≥ 3 cm or ankle-brachial index > 0.9, carotid plaque or carotid intima-media thickness ≥ 0.9 mm, an asymptomatic old cerebrovascular accident, or > 30% stenosis in at least one major coronary artery); rheumatoid arthritis, age > 40 years, and taking methotrexate and steroids; atrial fibrillation with a CHA2DS-VASc score ≥ 1; and a history of kidney transplantation more than 3 months beforehand. Subjects aged > 20 years who met at least one inclusion criterion were enrolled in the study. Subjects with any of the following were excluded: acute myocardial infarction, acute coronary syndrome, or symptomatic coronary artery disease, or a history of either of these diseases; symptomatic peripheral artery disease or heart failure, or a history of either of these diseases; expected survival of less than 6 months for a non-cardiovascular reason (e.g., cancer or sepsis); history of contrast allergy or side effects related to contrast materials; and current pregnancy or lactation.
Participants who were enrolled in this cohort between 2013 and 2016 had undergone ophthalmic examination at the time of enrolment. Among these, OCT imaging using EDI mode was conducted in 2015, and the images of corresponding subjects were analysed (n = 372). We excluded participants who (1) had medications (diuretics or steroids) or ophthalmic conditions (other than DR) that could potentially impact the choroid, such as central serous chorioretinopathy, age-related macular degeneration, retinal vessel occlusion, glaucoma, chorioretinal scarring, or myopic macular degeneration (n = 28); (2) received laser photocoagulation or anti-vascular endothelial growth factor treatment for DR (n = 29); (3) had undergone cataract surgery or vitrectomy within 3 months (n = 1); (4) exhibited significant media opacity or poor image quality (n = 3); (5) were on maintenance dialysis or had undergone kidney transplantation (n = 22); (6) had inadequately measured or missing laboratory or BIA measurements (n = 5). In total, we enrolled 284 patients in this study.
Clinical, laboratory data collection
We collected clinical and laboratory data at the time of enrolment. Participants underwent a standardised interview that covered inquiries about established diagnoses of hypertension, DM, and CKD, ongoing treatments for these conditions, and smoking history. Anthropometric measurements, including body height and weight, were recorded. HbA1c, blood glucose, lipid (LDL-cholesterol), blood urea nitrogen, and creatinine levels were measured in blood samples collected after overnight fasting. CKD stages were determined based on the eGFR. SBP and diastolic blood pressure were measured.
Ophthalmic data collection
Fundus photography (Visucam NM/FA; Carl Zeiss Meditec, Jena, Germany) and spectral domain OCT (Spectralis HRA OCT; Heidelberg Engineering GmbH, Dossenheim, Germany) were performed on the same day. The images were assessed to identify ophthalmic conditions and evaluate image quality. DR was graded as no DR, non-proliferative DR (NPDR), or proliferative DR (PDR).
Measurement of ECF status
ECF excess status was determined using a BIA (InBody 720 Body Composition Analyzer; Biospace, Seoul, Korea) at the time of CMERC-HI enrolment. This body composition analyser employs a 4-pole, 8-point tactile electrode system, performing 30 impedance measurements at six frequencies. TBW, ECW, and intracellular water were calculated.
ECF excess was determined by calculating the ratio of ECW to TBW, denoted as ECW/TBW. Based on this ratio, ECF excess status was categorised into three groups: normal (ECW/TBW < 0.390), mildly overhydrated (0.390 ≤ ECW/TBW < 0.400), and severely overhydrated status (ECW/TBW ≥ 0.400)11.
Measurement of CVI
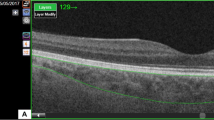
The CVI was measured using established methods with minor modifications4,24. EDI—OCT images that traversed the fovea were selected for analysis. Image binarization and segmentation were performed using the ImageJ software, version 1.47 (Bethesda, MD, USA; http://imagej.nih.gov/ij/). The total choroidal area (TCA) was determined as the chosen region within the subfoveal choroid, spanning a width of 1500 μm (with 750 μm extending both nasally and temporally from the fovea). The vascular LA was defined as the area occupied by dark pixels, whereas the SA was defined as the area occupied by light pixels. The CVI was defined as the ratio of LA to TCA (Fig. 1).
Statistical analysis
Baseline characteristics and OCT parameters were compared based on the ECF excess status using a One-way Analysis of Variance with Bonferroni's method for continuous variables and the chi-squared test for categorical variables. For comparisons between the non-DM and DM groups, independent t-test and chi-squared test were used. Pearson correlation analysis was used to evaluate the relationship between ECW/TBW and CVI. Multivariate linear regression analysis was conducted to determine the association between ECF excess status and CVI. Parameters that showed significant associations with the CVI in the univariate analysis and were considered clinically relevant were chosen as independent variables. Additionally, given the significant correlation found between the ECW/TBW, age was included as an adjusted variable in the analysis25. Statistical analysis was performed using the Statistical package for the social sciences software (SPSS statistics 22 for Windows; SPSS, Inc., IBM, Somers, NY, USA). Statistical significance was set at p < 0.05.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Nickla, D. L. & Wallman, J. The multifunctional choroid. Prog. Retin. Eye Res. 29, 144–168 (2010).
Reiner, A., Fitzgerald, M. E. C., Del Mar, N. D. & Li, C. Neural control of choroidal blood flow. Prog. Retin. Eye Res. 64, 96–130 (2018).
Spaide, R. F., Koizumi, H. & Pozzoni, M. C. Enhanced depth imaging spectral-domain optical coherence tomography. Am. J. Ophthalmol. 146, 496–500 (2008).
Agrawal, R. et al. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci. Rep. 6, 21090 (2016).
Iovino, C. et al. Choroidal vascularity index: An in-depth analysis of this novel optical coherence tomography parameter. J. Clin. Med. 9, 595 (2020).
Kovesdy, C. P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2011(12), 7–11 (2022).
Vadalà, M. et al. Retinal and choroidal vasculature changes associated with chronic kidney disease. Graefes Arch. Clin. Exp. Ophthalmol. 257, 1687–1698 (2019).
Basiony, A. I., Atta, S. N., Dewidar, N. M. & Zaky, A. G. Association of chorioretinal thickness with chronic kidney disease. BMC Ophthalmol. 23, 55 (2023).
Kim, M., Ha, M. J., Choi, S. Y. & Park, Y. H. Choroidal vascularity index in Type-2 diabetes analyzed by swept-source optical coherence tomography. Sci. Rep. 8, 70 (2018).
Essig, M. et al. Cardiovascular remodelling and extracellular fluid excess in early stages of chronic kidney disease. Nephrol. Dial. Transplant 23, 239–248 (2008).
Park, S. et al. Extracellular fluid excess is significantly associated with coronary artery calcification in patients with chronic kidney disease. J. Am. Heart Assoc. 7, e008935 (2018).
Tsai, YC. et al Association of fluid overload with cardiovascular morbidity and all-cause mortality in Stages 4 and 5 CKD. Clin. J. Am. Soc. Nephrol. 10, 39–46 (2015).
Levey, A. D. et al. Nomenclature for kidney function and disease: report of a Kidney disease: Improving global outcomes (KDIGO) consensus conference. Kidney Int. 97, 1117–1129 (2020).
Shin, Y. U. et al. Evaluation of changes in choroidal thickness and the choroidal vascularity index after hemodialysis in patients with end-stage renal disease by using swept-source optical coherence tomography. Med. Baltim. 98, 15421 (2019).
Su, Z. et al. Impact of hemodialysis on subfoveal choroidal thickness measured by optical coherence tomography: A systematic review and a pooled analysis of self-controlled case series. Ophthalmol. Ther. 12, 2265–2280 (2023).
Balmforth, C. et al. Chorioretinal thinning in chronic kidney disease links to inflammation and endothelial dysfunction. JCI Insight 1, e89173 (2016).
Agarwal, R., Andersen, M. J. & Pratt, J. H. On the importance of pedal edema in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 3, 153–158 (2008).
Davies, S. J. & Davenport, A. The role of bioimpedance and biomarkers in helping to aid clinical decision-making of volume assessments in dialysis patients. Kidney Int. 86, 489–496 (2014).
Fontecave-Jallon, J. & Thomas, S. R. Implementation of a model of bodily fluids regulation. Acta Biotheor. 63, 269–282 (2015).
Schlaich, M. P. et al. Sympathetic activation in chronic renal failure. J. Am. Soc. Nephrol. 20, 933–939 (2009).
Mitsides, N. et al. Extracellular overhydration linked with endothelial dysfunction in the context of inflammation in haemodialysis dependent chronic kidney disease. PLOS ONE 12, e0183281 (2017).
Zhang, Q. et al. Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arterioscler. Thromb. Vasc. Biol. 28, 1627–1633 (2008).
Shim, J. et al. Cardiovascular and metabolic disease etiology research center (CMERC) cohort: study protocol and results of the first 3 years of enrollment. Epidemiol. Health 39, e2017016 (2017).
Sonoda, S. et al. Luminal and stromal areas of choroid determined by binarization method of optical coherence tomographic images. Am. J. Ophthalmol. 159, 1123-1131.e1 (2015).
Lopot, F., Nejedlý, B., Novotná, H., Macková, M. & Sulková, S. Age-related extracellular to total body water volume ratio (Ecv/TBW)–can it be used for “dry weight” determination in dialysis patients? Application of multifrequency bioimpedance measurement. Int. J. Artif. Organs. 25, 762–769 (2002).
Acknowledgements
This study was supported by a faculty research grant from Yonsei University College of Medicine (6-2023-0160). The authors declare that no competing interests exist with the funder. The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
Study design and conduction: M.K., J.O., M.K., S.H.B., S.S.K., and J.Y.S. Data collection, management, analysis, and interpretation: M.K., J.O., and J.Y.S. Preparation, review, and approval of the manuscript: M.K., J.O., M.K., S.H.B., S.S.K. and J.Y.S. Decision to submit the manuscript for publication: M.K., J.O., M.K., S.H.B., S.S.K., and J.Y.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, M., Oh, J., Kim, M. et al. Extracellular fluid excess linked to reduced choroidal vascularity index in patients with chronic kidney disease. Sci Rep 14, 12769 (2024). https://doi.org/10.1038/s41598-024-63444-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-63444-7
- Springer Nature Limited