Abstract
Anaplasma and Ehrlichia are tick-borne bacterial pathogens that cause anaplasmoses and ehrlichioses in humans and animals. In this study, we examined the prevalence of Anaplasma and Ehrlichia species in ticks and domesticated animals in Suizhou County, Hubei Province in the central China. We used PCR amplification and DNA sequencing of the 16S rRNA, groEL, and gltA genes to analyze. We collected 1900 ticks, including 1981 Haemaphysalis longicornis and 9 Rhipicephalus microplus, 159 blood samples of goats (n = 152), cattle (n = 4), and dogs (n = 3) from May to August of 2023. PCR products demonstrated that Anaplasma bovis, Anaplasma capra, and an Ehrlichia species were detected in the H. longicornis with the minimum infection rates (MIR) of 1.11%, 1.32%, and 0.05%, respectively; A. bovis, A. capra, and unnamed Anaplasma sp. were detected in goats with an infection rate of 26.31%, 1.31% and 1.97%, respectively. Anaplasma and Ehrlichia species were not detected from cattle, dogs and R. microplus ticks. The genetic differences in the groEL gene sequences of the Anaplasma in the current study were large, whereas the 16S rRNA and gltA gene sequences were less disparate. This study shows that ticks and goats in Suizhou County, Hubei Province carry multiple Anaplasma species and an Ehrlichia species, with relatively higher infection rate of A. bovis in goats. Our study indicates that multiple Anaplasma and Ehrlichia species exist in ticks and goats in the central China with potential to cause human infection.
Similar content being viewed by others
Introduction
Anaplasma and Ehrlichia are tick-borne bacterial pathogens causing anaplasmoses and ehrlichioses respectively in humans and animals1,2,3. The pathogens have a high degree of biodiversity and a wide geographical distribution, posing a serious threat to humans and domesticated animals worldwide. At present, the genus Anaplasma comprises A. phagocytophilum, A. bovis, A. capra, A. centrale, A. marginale, A. ovis, and A. platys4.
Anaplasma phagocytophilum was first identified in 1932 as a tick-borne pathogen, which can infect the neutrophils of rodents and ruminants, such as cattle and sheep, with fever, malaise, headache, myalgia, leukopenia and thrombocytopenia5. Among healthy populations, Africa has the highest seropositivity rate for A. phagocytophilum (21.7%), followed by Asia (3.30%–27.08%), Europe (0%–16.28%), and the Americas (0%–7.75%)6. Anaplasma bovis, which normally parasitizes mononuclear cells, was first detected in cattle in Brazil in 1936. Its primary host is cows, buffaloes, goats, and sheep. Recent studies have shown the presence of A. bovis in the blood and lung tissue of horses7,8. Anaplasma capra, recently discovered in China, can infect a wide range of ruminants, including goats, sheep, and deer, as well as humans9.
Ehrlichia spp. are intracellular bacteria found in the cytoplasmic vesicles of monocytes, granulocytes, or platelets in humans and animals, causing fever, leukopenia, and thrombocytopenia10. In the genus Ehrlichia, E. ruminantium and E. minasensis can infect cattle. Ehrlichia ruminantium can also infect other ruminants like sheep, goats, and buffalo; E. chaffeensis, E. ewingii, E. muris, and E. canis have been reported to infect humans11.
Anaplasma and Ehrlichia are a group of zoonotic pathogens, and ticks play a very important role in transmitting the pathogens. Ticks can transmit the pathogens to a wide range of domesticated animals such as dogs, goats, sheep and bovines and also to humans, resulting in anaplasmoses and ehrlichioses respectively. Haemaphysalis longicornis, a hard body tick, is distributed throughout China and can infest on a wide range of host animals such as dogs, goats, sheep, and cattle1,12,13,14. Anaplasma capra, A. bovis, A. ovis, A. phagocytophilum, and A. marginale have all been reported in H. longicornis in China4,15,16. In Hubei Province, China, H. longicornis have been found to transmit Ehrlichia sp., A. marginale and A. bovis. Rhipicephalus microplus have been found to transmit E. canis, A. marginale, A. capra and A. platys from Wuhan and Huangshi cities of Hubei Province17.
The aim of this study was to investigate the prevalence and genetic diversity of Anaplasma and Ehrlichia in ticks and domesticated animals collected from Suizhou County, Hubei Province.
Materials and methods
Ethical statement
The collection of ticks and animals’ blood for microbiological studies was approved by the Ethics Committee of the Medical School, Wuhan University (2020YF0051), and all efforts were made to minimize discomfort to the animals. We confirm that all methods were carried out in accordance with Laboratory animal—Guideline for ethical review of animal welfare.
Sample collection and processing
From May to August, 2023, ticks and blood samples of domesticated animals were collected from Suizhou County (113.82°E, 31.61°N), Hubei Province in central China (Fig. 1). Suizhou County is located in the northern part of Hubei Province, which is a hilly area with low mountains. It belongs to subtropical monsoon climate, with warm and humid climate, average annual rainfall of 865–1040 millimeters, and average annual temperature of 15.5 °C. There are 37 towns in Suizhou County, eight towns were randomly selected as sampling sites through simple random sampling method, and three grazing areas were randomly selected as sampling areas in each selected town. Blood (1–2 mL) was collected from the jugular vein of goats and cattle and from the cephalic vein of dogs with a 5 mL syringe and stored in EDTA anticoagulant tube. The blood samples were placed on ice and transported to the laboratory. Tick species were identified morphologically under a stereo microscope (Phenix, Shangrao, China) with a taxonomic key18, and representative tick species were confirmed with PCR amplification and sequencing the 16S rRNA gene (rrs) with primers listed in Table 1. The amplified sequences were compared with tick sequences in the GenBank with BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to obtain tick species.
A total of 1900 ticks were collected in this study. Haemaphysalis longicornis (n = 1891, 99.53%) and R. microplus (n = 9, 0.47%) were identified by morphology and molecular biology (Fig. 2). Ticks were collected from grassland (n = 932, 49.05%) and from animals’ bodies (n = 968, 50.95%). The ticks from animals were further categorized according to the degree of blood sucking as fully engorged ticks (n = 57, 3.00%), partially engorged ticks (n = 298, 15.68%), and unfed ticks (n = 1,545, 81.32%). A total of 152 goats’ blood samples, 4 cattle blood samples, and 3 dogs’ blood samples were collected.
DNA extraction and PCR amplification of tick-borne bacteria
In order to facilitate sample processing and reduce the number of samples for DNA extraction, ticks were pooled together according to their life stage and feeding status. Each group consisted of 20 larvae, 20 nymph ticks, 10 non-engorged adult ticks, or 1 engorged tick. Ticks were rinsed three times with 70% ethanol and distilled water for 5 min each time, and air-dried. After freezing with liquid nitrogen, ticks were homogenized with metal beads using Restsch MM400 mixer mill (Retsch, Haan, Germany). Tick DNA and animal whole blood genomic DNA (gDNA) were extracted using the Trelief™ Animal Genomic DNA kit (Tsingke, Beijing, China) following the manufacturer’s instructions. The DNA concentration and quality were assessed using QIAxpert (Qiagen, Hilden, Germany). DNA samples were stored at –40 °C.
16S rRNA, groEL and gltA gene fragments of Anaplasma and Ehrlichia were amplified with primers described in Table 1. Negative controls were set in each PCR assay with nuclease-free water as template. The amplification was performed as follows: 95 °C for 3m, 35 cycles of 95 °C for 30 s, annealing for 30 s, and 72 °C for 30–60 s, and a final extension at 72 °C for 10m. Annealing temperatures are presented in Table 1. The PCR condition was applied to all genes’ amplification in this study. PCR products were analyzed with a 1–1.5% agarose gel, stained with GoldviewTM nuclear staining dyes (GL biotech, Shanghai, China), and visualized under UV light. DNA extraction, fragment amplification, and agarose gel electrophoresis were performed in separate rooms to prevent PCR contamination. The expected PCR bands were excised from gel and purified using DNA Gel Extraction Kit (Tsingke, Beijing, China). The gel-purified DNA was ligated into the pMD 19-T vector (Takara Bio, Dalian, China) and transformed into DH5α competent cells of Escherichia coli for cloning. At least three positive clones were sequenced with universal primers M13–47/M13–48 for each sample. DNA sequencing was performed by Sangon Biotech Company (Shanghai, China). The company used an Applied Biosystems™ 3730XL sequencer from Thermo Fisher.
Phylogenetic analyses
DNA sequences from this study were first analyzed using Seqman Pro (www.dnastar.com) (DNASTAR, Madison, WI)24 and then were compared to the sequences in the GenBank with the BLAST program. After removing the primers regions, sequences from this study, together with the reference sequences from the GenBank were imported into MEGA 11.0 software (https://www.megasoftware.net), and aligned with ClustalW (Selecting the default parameters). Only high-quality sequences with a single peak distribution of all bases and with overlapping forward and reverse sequences were used for phylogenetic analysis. Phylogenetic analysis was performed using the Maximum Likelihood method, Kimura 2-parameter model25 with 1000 bootstraps in MEGA 11.0 (other parameters are default). The phylogenetic results were exported to Microsoft Office for phylogenetic tree landscaping and editing.
Results
Prevalence of Anaplasma and Ehrlichia in ticks and animals
The DNA samples were first amplified with Anaplasma and Ehrlichia groEL universal primers. The DNA sequences of the PCR products were analyzed with BLAST to determine which species the DNA sequences belonged to. The infection rate of Anaplasma in H. longicornis was 2.43% (46/1891) including 1.11% A. bovis (21/1891), 1.32% A. capra (25/1891), and the infection rate of Ehrlichia in H. longicornis was 0.05% (1/1891). Neither Anaplasma nor Ehrlichia was detected in R. microplus (0%, 0/9). The infection rate of Anaplasma in goats was 29.60% (45/152), including 26.31% A. bovis (40/152), 1.31% A. capra (2/152), and 1.97% an unnamed Anaplasma sp. (3/152) (Table 2). No goat was positive to Ehrlichia; neither cattle nor dogs were positive to Anaplasma or Ehrlichia.
The groEL PCR-positive tick DNA was further amplified with Anaplasma and Ehrlichia rrs primers and A. bovis and A. capra gltA species-specific primers. Of the 47 groEL positive H. longicornis samples, 33 samples were positive with rrs, including A. bovis (10), A. capra (22) and an unnamed Ehrlichia sp. (1) respectively; and 32 samples were also positive with gltA, including A. bovis (10), A. capra (22) respectively. Of the 45 groEL positive goat samples, 43 samples were positive with rrs, including A. bovis (39), A. capra (1) and an unnamed Anaplasma sp. (3), respectively; and 40 samples were positive with gltA, including A. bovis (39), A. capra (1) respectively.
Phylogenetic analyses of Anaplasma and Ehrlichia species
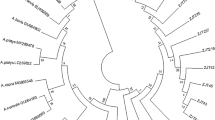
A total of 76 rrs sequences were obtained from H. longicornis and goats. BLAST indicated that they belonged to A. bovis (n = 49), A. capra (n = 23), an unnamed Anaplasma species (n = 3), and an unnamed Ehrlichia species (Additional file 1: Table S1). Representative strains of each species from this study were used for phylogenetic analysis. Phylogenetic analysis indicated that the sequences from goats in this study were most closely related to A. bovis (represented by G25, G31, G37, G49, G51, G61, G91 and G99) and that a sequence from ticks were clustered together with E. chaffeensis (T85) (Fig. 3).
The phylogeny of these strains were further analyzed with groEL gene, which indicated that Anaplasma strains from goats belonged to 3 clusters (the gray background) (Fig. 4). Strain (G51) was in the clade with an unnamed Anaplasma sp. (AB454079) from deer in Japan. The strains of A. bovis were divided into 3 clusters ((I, II and III) and the strains of A. bovis from this study were dispersed in first 2 genotypes (I and II). Genotype I included strains from this study (G25, G61, G91, and G99) and previous reported A. bovis sequences from China (MH255906 cattle) and (ON245124 H. longicornis); and Genotype II included strains from this study (G31, G37, and G49) and previous reported sequences from goats in China (MH255905 and OQ263274). The third group included only the A. bovis strain isolated from patients in the United States (OQ693619)22. The groEL sequence homology among the first two genotypes of A. bovis ranged from 92.28 to 93.75%, which is much low than the sequence homology within each group from 98.02 to 99.26%. The groEL phylogeny indicated that two sequences from ticks belonged to A. capra (T76) and E. chaffeensis (T85), respectively.
Phylogenetic analysis with gltA sequences showed that the strains from goats including G25, G31, G37, G49, G51, G61, G91 and G99 were all in the same clade with A. bovis. The tick strain T76 is in the same clade with A. capra (KM206274) from humans in northern China (Fig. 5).
To get accurate phylogenetic analysis, the rrs, groEL and gltA gene sequences of each strain was concatenated to do phylogeny (Fig. 6). Strain G51 was clustered together with an unnamed Anaplasma species (AB454079) from deer in Japan. Two tick strains from this study were classified as A. capra (T76) and Ehrlichia species (T85).
Discussion
Suizhou County is characterized by dense scrub forests and a wide variety of wildlife species, which are suitable for ticks to reproduce, making it a landform with a high incidence of zoonotic diseases. The H. longicornis is one of the dominant tick species in East Asian countries. It has been reported in all provinces (except for Hong Kong and Macao Special Administrative Regions) in China26. This study shows that H. longicornis is the dominant species in Suizhou County (99.53%). Haemaphysalis longicornis also transmits the largest number of pathogens, with up to 44 known pathogen species, which is of great significance for the study of tick-borne pathogens26,27,28. The biological carriers of Anaplasma are hard ticks, among which H. longicornis plays a crucial role as a carrier in the transmission of Anaplasma pathogens.
Anaplasma bovis has a wide host range, including H. longicornis, Rhipicephalus appendiculatus, and H. qinghaiensis, and the MIR of A. bovis in H. longicornis in this study was 1.11%. Related studies have been reported in South Korea (0.1%), Shandong (1.6%), Jilin and Heilongjiang (0.3%), and Henan (9.0%) provinces of China29,30,31,32. Domesticated ruminants infected with A. bovis are mainly found in African and Asian countries33. The results of the current study showed that goats in Suizhou County were mainly infected with A. bovis, which is consistent with the results of Liu et al. in central and southern China14. The average prevalence of A. bovis infection was found to be 16.00% in goats collected in four provinces of China in 2010, with the highest prevalence up to 20.30%14. In Guizhou Province, the prevalence of A. bovis infection in goats was 30.80%34. The prevalence of A. bovis infection in goats, sheep, cattle, and deer ranged from 4.10 to 24.40%35,36,37,38, and even 45.71% in yak38. In contrast, the infection rate of A. bovis in ticks in China mostly ranged from 0.10 to 11.10%35,39,40,41. In this study, the infection rate of A. bovis in goats was 26.31% and in H. longicornis was 1.11%. It has been noted that A. ovis are persistently infected in sheep for up to 4–6 years; Anaplasma capra are also persistently or chronically infected in deer42. Because of the high infection rate of A. bovis in goats’ whole blood samples in this study, we hypothesized that persistent infection with A. bovis may exist in goats in this region.
Anaplama bovis was only considered as human pathogen until recently. A total of 7 cases of human infection with A. bovis have been reported worldwide, with 2 cases reported in Jiangxi, China in 2013 and 1 case reported in Anhui, China in 2021. In addition, 4 cases of A. bovis-like infection were detected in the United States from 2015 to 201722,43,44. Most animals infected with A. bovis are asymptomatic, but human infections with A. bovis can present with fever (up to 39 °C), rigor, headache, myalgia, anorexia, rash, chill, diarrhea, thrombocytopenia and lymphadenopathy. Therefore, the A. bovis should be considered to be pathogenic to humans.
Anaplasma capra was first identified in goats and subsequently recognized as an emerging human pathogen in China9. In this study, for the first time, Anaplasma capra were found in H. longicornis collected from Hubei Province. In the present study, both H. longicornis (1.32%) and goats (1.31%) were infected with A. capra. The primary host of A. capra is small ruminants, and A. capra has been detected in 14%-18% of small ruminants45. Anaplasma capra has been found in H. longicornis in Shandong Province, in H. qinghaiensis in Gansu Province, and in a variety of tick species in South Korea46. Anaplasma capra has also been detected in R. microplus in the Hubei Province47. Sequence homology between the A. capra sequences in this study and those of A. capra isolated from patients from northern China was 100% on both groEL and gltA. Patients infected with A. capra usually present with nonspecific febrile symptoms such as fever, malaise, headache, dizziness, myalgia, and chills, and some patients present with severe symptoms such as encephalitis. It is reported that a more frequent symptom in patients infected with A. capra than in human granulocytic anaplasmosis cases is a rash9.
The G51 strain in this study and the Anaplasma strain from Japan (AB454079) are very different from other Anaplasma strains and should be a new species. Ticks and domesticated animals infected with Ehrlichia were low in this study. The unnamed Ehrlichia species identified in this study was reported to be prevalent in H. longicornis in Hebei Province (1.2%)48. Anaplasma and Ehrlichia were not detected in R. microplus, cattle and dogs, probably due to small sample sizes. Consequently, the Anaplasma and Ehrlichia species in ticks and animals need to be further investigated in other areas in Hubei Province.
In Suizhou County, patients with unknown fever and a history of tick bites are often diagnosed and local hospitals and the local Center for Disease Control and Prevention (CDC) had difficulty to make accurate diagnoses due to lack of pathogen information. This sentinel and pioneer study in analysis of the tick borne-pathogens in the region provide knowledge of the tick-borne pathogens for physicians and health-care workers for correct diagnosis of the patients with potential tick-borne diseases in the area.
Conclusions
Our study shows that ticks in Hubei Province carry multiple Anaplasma species and an Ehrlichia species, and the infection rate of A. bovis is relatively high in goats. Anaplama bovis was only considered as animal pathogen before, but recent studies showed that it is an emerging human pathogen. Therefore, human infection of Anaplasma and Ehrlichia in central China should be monitored.
Data availability
The sequences of this study are available in the GenBank under the accession numbers: OR647343-OR647344, OR751938-OR751939 (16S rRNA), OR671916-OR671922, OR757467-OR757469 (groEL), OR671915, OR727627-OR727634 (gltA). (https://www.ncbi.nlm.nih.gov/nuccore).
References
Yan, Y. et al. Molecular detection and phylogeny of Anaplasma spp. closely related to Anaplasma phagocytophilum in small ruminants from China. Ticks Tick Borne Dis. 13, 101992. https://doi.org/10.1016/j.ttbdis.2022.101992 (2022).
Li, Y. et al. Molecular survey of Anaplasma and Ehrlichia of red deer and sika deer in Gansu, China in 2013. Transbound. Emerg. Dis. 63, e228–e236. https://doi.org/10.1111/tbed.12335 (2016).
Woldehiwet, Z. Tick-borne fever: A review. Vet. Res. Commun. 6, 163–175 (1983).
Yan, Y. et al. Molecular detection and phylogenetic analyses of Anaplasma spp. in Haemaphysalis longicornis from goats in four provinces of China. Sci. Rep. 11, 14155. https://doi.org/10.1038/s41598-021-93629-3 (2021).
Dumler, J. S. et al. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 11, 1828. https://doi.org/10.3201/eid1112.050898 (2005).
Wang, F. et al. The seroprevalence of Anaplasma phagocytophilum in global human populations: A systematic review and meta-analysis. Transbound. Emerg. Dis. https://doi.org/10.1111/tbed.13548 (2020).
Seo, M. G., Ouh, I. O. & Kwak, D. Detection and genotypic analysis of Anaplasma bovis and A. phagocytophilum in horse blood and lung tissue. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24043239 (2023).
Seo, M.-G., Kwon, O.-D. & Kwak, D. Anaplasma bovis infection in a horse: First clinical report and molecular analysis. Vet. Microbiol. 233, 47–51 (2019).
Li, H. et al. Human infection with a novel tick-borne Anaplasma species in China: A surveillance study. Lancet Infect. Dis. 15, 663–670 (2015).
Wen, B., Cao, W. & Pan, H. Ehrlichiae and ehrlichial diseases in China. Ann. N. Y. Acad. Sci. 990, 45–53. https://doi.org/10.1111/j.1749-6632.2003.tb07335.x (2003).
Dumler, J. S. & Bakken, J. S. Ehrlichial diseases of humans: Emerging tick-borne infections. Clin. Infect. Dis. 20, 1102–1110. https://doi.org/10.1093/clinids/20.5.1102 (1995).
Niu, Q. et al. Genetic diversity and molecular characterization of Babesia motasi-like in small ruminants and Ixodid ticks from China. Infect. Genet. Evol. 41, 8–15. https://doi.org/10.1016/j.meegid.2016.03.007 (2016).
Chen, Z. et al. Tick-borne pathogens and associated co-infections in ticks collected from domestic animals in central China. Parasit. Vectors 7, 237. https://doi.org/10.1186/1756-3305-7-237 (2014).
Zhang, L. et al. Anaplasma phagocytophilum infection in domestic animals in ten provinces/cities of China. Am. J. Trop. Med. Hyg. 87, 185–189. https://doi.org/10.4269/ajtmh.2012.12-0005 (2012).
Hou, J. et al. A molecular survey of Anaplasma, Ehrlichia, Bartonella and Theileria in ticks collected from southeastern China. Exp. Appl. Acarol. 79, 125–135 (2019).
Qin, X. R. et al. Anaplasma species detected in Haemaphysalis longicornis tick from China. Ticks Tick Borne Dis. 9, 840–843. https://doi.org/10.1016/j.ttbdis.2018.03.014 (2018).
Lu, M. et al. Extensive diversity of rickettsiales bacteria in ticks from Wuhan, China. Ticks Tick-borne Dis. 8, 574–580. https://doi.org/10.1016/j.ttbdis.2017.03.006 (2017).
Barker, S. C. & Walker, A. R. Ticks of Australia: The species that infest domestic animals and humans. Zootaxa 3816, 141–144 (2014).
Stanek, G., Wormser, G. P., Gray, J. & Strle, F. Lyme borreliosis. Lancet 379, 461–473 (2012).
Kurlovs, A. H., Li, J., Cheng, D. & Zhong, J. Ixodes pacificus ticks maintain embryogenesis and egg hatching after antibiotic treatment of Rickettsia endosymbiont. PLoS ONE 9, e104815 (2014).
Cicuttin, G. L. et al. Molecular characterization of Rickettsia massiliae and Anaplasma platys infecting Rhipicephalus sanguineus ticks and domestic dogs, Buenos Aires (Argentina). Ticks Tick-Borne Dis. 5, 484–488 (2014).
Karpathy, S. E. et al. Anaplasma bovis-like infections in humans, United States, 2015–2017. Emerg. Infect. Dis. 29, 1904–1907. https://doi.org/10.3201/eid2909.230559 (2023).
Wen, B., Jian, R., Zhang, Y. & Chen, R. Simultaneous detection of Anaplasma marginale and a new Ehrlichia species closely related to Ehrlichia chaffeensis by sequence analyses of 16S ribosomal DNA in Boophilus microplus ticks from Tibet. J. Clin. Microbiol. 40, 3286–3290. https://doi.org/10.1128/JCM.40.9.3286-3290.2002 (2002).
Cannon, G. C. Sequence analysis on microcomputers. Science 238, 97–103 (1987).
Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16, 111–120 (1980).
Zhao, L. et al. Distribution of Haemaphysalis longicornis and associated pathogens: Analysis of pooled data from a China field survey and global published data. Lancet Planet Health 4, e320–e329. https://doi.org/10.1016/S2542-5196(20)30145-5 (2020).
Zhao, G. P. et al. Mapping ticks and tick-borne pathogens in China. Nat. Commun. 12, 1075. https://doi.org/10.1038/s41467-021-21375-1 (2021).
Luo, L. M. et al. Haemaphysalis longicornis ticks as reservoir and vector of severe fever with thrombocytopenia syndrome virus in China. Emerg. Infect. Dis. 21, 1770–1776. https://doi.org/10.3201/eid2110.150126 (2015).
Seo, M. G. et al. Tick populations and molecular analysis of Anaplasma species in ticks from the Republic of Korea. Microorganisms https://doi.org/10.3390/microorganisms11040820 (2023).
Qin, X.-R. et al. Anaplasma species detected in Haemaphysalis longicornis tick from China. Ticks Tick-Borne Dis. 9, 840–843 (2018).
Wei, F. et al. Molecular detection and characterization of zoonotic and veterinary pathogens in ticks from northeastern China. Front. Microbiol. 7, 1913. https://doi.org/10.3389/fmicb.2016.01913 (2016).
Zhuang, L. et al. Identification of tick-borne pathogen diversity by metagenomic analysis in Haemaphysalis longicornis from Xinyang, China. Infect. Dis. Poverty 7, 45. https://doi.org/10.1186/s40249-018-0417-4 (2018).
Yang, B. et al. Molecular evidence of coinfection of Anaplasma species in small ruminants from Anhui Province, China. Parasitol. Int. 71, 143–146. https://doi.org/10.1016/j.parint.2019.04.004 (2019).
Zhang, Y. et al. Molecular and phylogenetic analysis of Anaplasma spp. in sheep and goats from six provinces of China. J. Vet. Sci. 17, 523–529. https://doi.org/10.4142/jvs.2016.17.4.523 (2016).
Guo, W. P. et al. GroEL gene typing and genetic diversity of Anaplasma bovis in ticks in Shaanxi, China. Infect. Genet. Evol. 74, 103927. https://doi.org/10.1016/j.meegid.2019.103927 (2019).
Cui, Y. et al. First molecular evidence of mixed infections of Anaplasma species in dogs in Henan, China. Ticks Tick-Borne Dis. 8, 283–289 (2017).
Yang, J. et al. Occurrence of four Anaplasma species with veterinary and public health significance in sheep, northwestern China. Ticks Tick-Borne Dis. 9, 82–85 (2018).
Lu, M. et al. Molecular survey of vector-borne pathogens in ticks, sheep keds, and domestic animals from Ngawa, Southwest China. Pathogens 11, 606 (2022).
Han, R. et al. Molecular detection of Anaplasma infections in Ixodid ticks from the Qinghai-Tibet Plateau. Infect. Dis. Poverty 8, 83–90 (2019).
Yu, P. et al. Molecular evidence of tick-borne pathogens in Hyalomma anatolicum ticks infesting cattle in Xinjiang Uygur Autonomous Region, Northwestern China. Exp. Appl. Acarol. 73, 269–281 (2017).
Sun, J. et al. Circulation of four species of Anaplasmataceae bacteria in ticks in Harbin, northeastern China. Ticks Tick-Borne Dis. 14, 102136 (2023).
Ruiz, H. et al. Long-lasting infection with Anaplasma ovis in sheep. Vet. Res. Commun. https://doi.org/10.1007/s11259-023-10186-y (2023).
Lu, M. et al. Anaplasma bovis infection in fever and thrombocytopenia patients: Anhui Province, China, 2021. China CDC Wkly. 4, 249–253. https://doi.org/10.46234/ccdcw2022.053 (2022).
Lu, M. et al. Epidemiology and diversity of Rickettsiales bacteria in humans and animals in Jiangsu and Jiangxi provinces, China. Sci. Rep. 9, 13176 (2019).
Yang, J. et al. A novel zoonotic Anaplasma species is prevalent in small ruminants: Potential public health implications. Parasit. Vectors 10, 264. https://doi.org/10.1186/s13071-017-2182-9 (2017).
Sun, X. F., Zhao, L., Wen, H. L., Luo, L. M. & Yu, X. J. Anaplasma species in China. Lancet Infect. Dis. 15, 1263–1264. https://doi.org/10.1016/S1473-3099(15)00377-1 (2015).
Lu, M. et al. Identification of Rickettsia spp., Anaplasma spp., and an Ehrlichia canis-like agent in Rhipicephalus microplus from southwest and south-central China. Ticks Tick Borne Dis. 13, 101884. https://doi.org/10.1016/j.ttbdis.2021.101884 (2022).
Teng, Z. et al. Molecular detection of tick-borne bacterial and protozoan pathogens in Haemaphysalis longicornis (Acari: Ixodidae) ticks from free-ranging domestic sheep in Hebei Province, China. Pathogens https://doi.org/10.3390/pathogens12060763 (2023).
Funding
This study was supported by the National Natural Science Foundation of China [81971939] and Key R&D Program of Hubei Province, China [2022BCE063]. The funders had no role in the study design, data collection and analysis, decision to publish, or the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization, XJY, FG and XZJ; methodology, XJY and XHL; investigation, JX, XFZ, HYL, TMY and ZZJ; resources, FG, FZL and QJY; writing—original draft preparation, JT; writing—review and editing, JT and XJY; project administration, XJY. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, J., Xu, J., Liu, Xh. et al. Prevalence and genetic diversity of Anaplasma and Ehrlichia in ticks and domesticated animals in Suizhou County, Hubei Province, China. Sci Rep 14, 12621 (2024). https://doi.org/10.1038/s41598-024-63267-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-63267-6
- Springer Nature Limited