Abstract
Hemoglobin A1c (HbA1c) plays a crucial role in diabetes management. We aimed to evaluate the analytical performance of a new enzymatic method kit for HbA1c measurement. The performance of the enzymatic method, including precision, accuracy, and linearity, was evaluated. Moreover, the interference effect from conventional interferents, Hb derivatives, Hb variants, and common drugs were assessed. In addition, the agreement of HbA1c results was compared between enzymatic methods, cation-exchange high-performance liquid chromatography (HPLC), and immunoassays. The intra-assay, between-assay, and total precision of HbA1c were all lower than 2%. HbA1c showed good linearity within the range of 3.96–20.23%. The enzymatic assay yielded results consistent with the external quality control samples, with a bias of less than ± 6% from the target values. The enzymatic method showed no interference from bilirubin, intralipid, vitamin C, Hb derivatives, common Hb variants, as well as antipyretic analgesics and hypoglycemic drugs. The HbA1c results of the enzymatic assay showed good agreement and accuracy compared to those obtained from the HPLC method and the immunoassay. The enzymatic method kit performed on the BS-600M chemistry analyzer is a reliable and robust method for measuring HbA1c. It is suitable for routine practice in clinical chemistry laboratories.
Similar content being viewed by others
Introduction
Hemoglobin A1c (HbA1c) is a modified hemoglobin with a stable adduct of glucose (covalently linked) to the N-terminal valine of the β chain. The formation of HbA1c is a nonenzymatic process that occurs continuously in vivo, starting with glucose in the open-chain form bound to the N-terminus to form aldimines (Schiff bases), which then undergo an Amadori rearrangement to form the more stable ketamine. HbA1c exhibits minimal biological variability and reflects the average blood glucose level over the lifespan of red blood cells1. It is strongly associated with the progression of diabetic complications2,3. The American Diabetes Association (ADA) and other major organizations have recommended HbA1c for diagnosing diabetes and have adopted a threshold of 6.5% (48 mmol/mol)4,5. Therefore, accurate and reliable HbA1c results are important for screening and diagnosing diabetes, monitoring the effectiveness of diabetes treatment, and predicting the risk of diabetes and its complications.
In China, the National Center for Clinical Laboratories (NCCLS) showed that over 70% of laboratories use cation-exchange high-performance liquid chromatography (HPLC) methods, followed by immunoassay, boronate affinity method, capillary electrophoresis (CE), and enzymatic method. These methods make use of the difference in charge between HbA1c and HbA0 or the structural differences between glycated and non-glycated forms of hemoglobin6. Each method exhibits distinct characteristics and performance and may be affected by factors such as lipemia, anemia, drugs, or hemoglobin variants7,8,9,10. Therefore, it is necessary to conduct a comprehensive performance evaluation of HbA1c assays prior to their use in the clinical laboratory.
The BS-600M (Mindray, Shenzhen, China) is a newly developed, fully automated biochemistry analyzer that allows for the direct loading of whole blood samples for HbA1c testing using Hemoglobin A1c Kit (Enzymatic Method) at a rate of 120 tests per hour. This study evaluated the analytical performance of a new enzymatic kit conducted on the BS-600M to determine if it meets the requirements of clinical laboratories for HbA1c testing.
Materials and methods
Samples
Performance was evaluated using 350 residual whole blood samples in ethylenediaminetetraacetic acid (EDTA)-containing tubes collected from the Laboratory Department of Peking University Shenzhen Hospital. Several small aliquots were made from each fresh sample and stored at − 80 °C prior to analysis. Method comparisons were conducted at Wuhan Asian General Hospital between enzymatic and immunoassay (Roche c501), and at Shenzhen Integrative Medicine Hospital between enzymatic and HPLC (Tosoh G8). The study received approval from the Ethics Committee of Peking University Shenzhen Hospital, and informed consent was waived because there was no information that could help identify individuals. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
HbA1c measurement
HbA1c was measured using the BS-600M automatic biochemistry analyzer (enzymatic assay), the HLC-723 G8 HbA1c analyzer (HPLC method), and the Roche c501 (Immunoassay). All samples were used in the accompanying glycated hemoglobin assay kits.
The principle of the enzymatic assay kit
The principle of the enzymatic assay kit is as follows: Firstly, red blood cells are lysed in a hypotonic environment. Then sodium nitrite oxidizes Fe2+ ions in hemoglobin to Fe3+ ions, resulting in the formation of methemoglobin. In the presence of sodium azide, methemoglobin azide is formed, and the absorbance value is measured at 505/800 nm to determine the concentration of total hemoglobin. Protease cleaves the connection between leucine and histidine at the N-terminal end of the β-chain, resulting in the formation of a glycated dipeptide fragment. This glycated dipeptide then undergoes a reaction with fructosyl peptide oxidase, resulting in the production of hydrogen peroxide. Subsequently, under the action of peroxidase, hydrogen peroxide reacts with a colorant, resulting in color shades that are directly proportional to the HbA1c content. The HbA1c content and its percentage in total hemoglobin can be calculated by measuring the absorbance at 660/800 nm.
Analytical performance
Precision
Intra-assay, inter-assay, and total precision were evaluated using high and low-level HbA1c quality control (QC) and fresh whole blood samples. Total precision was evaluated according to the Clinical and Laboratory Standards Institute (CLSI) EP05-A2 guidelines. These samples were mixed and assayed on the BS-600M instrument for 20 consecutive days, with 2 analytical batches per day, 2 tests per analytical batch.
Linearity
Linearity was assessed according to the CLSI EP06-A2 guidelines. One sample with a high value (20.23% HbA1c) and one sample with a low value (2.96% HbA1c) were mixed at different proportions (0%, 6.25%, 12.5%, 18.75%, 25%, 37.5%, 50%, 62.5%, 75%, 87.5%, and 100%) to obtain 11 concentration gradient samples. Each sample was measured on the BS-600M for three times consecutively to obtain measured values and compare them with expected values. The expected HbA1c values were calculated according the following formula: Expected value = (%HbA1c × VL × HbL + %HbA1c × VH × HbH)/(VL × HbL + VH × HbH). VL/H: volume of the sample with low/high HbA1c value; HbL/H: Hb concentration of the sample with low/high HbA1c value. Hb concentration was measured by a Sysmex XN9000 hematology analyzer (Sysmex Co., Japan).
Accuracy
Accuracy was evaluated according to CLSI EP9A2 guidelines by analyzing External quality control (QC) from the International Federation of Clinical Chemistry and laboratory medicine (IFCC, 8 samples) and the National Glycohemoglobin Standardization Program (NGSP, 40 samples). The assay results were compared to the target values and the bias between the two was calculated. The acceptable bias was defined as ± 6.0%. The sigma value for HbA1c was calculated using the formula σ = (TEa − |bias|)/CV.
Method comparison
A total of 197 fresh whole blood samples were collected from the Laboratory Department of Shenzhen Integrative Medicine Hospital for the method comparison of BS-600M and Tosoh G8, and 112 fresh whole blood samples were collected from the Laboratory Department of Wuhan Asian General Hospital for the method comparison of BS-600M and Roche c501. The data was analyzed using Passing-Bablok regression analysis and a Bland–Altman plot. Clinical significant bias was defined as a difference exceeding ± 6% at 6% or 9% HbA1c11.
Interference
Lipemia, Bilirubin, and Vitamin C
The potential error caused by the interfering substance is compared to the allowable error as stated in the CLSI EP7-A2 guideline. Interference was determined by measuring HbA1c in samples containing increasing proportions of lipemia (up to 500 mg/dL), bilirubin (up to 15 mg/dL), and vitamin C (up to 3 mg/dL).
Hemoglobin derivatives and hemoglobin variants
Glucose (up to 10,000 mg/dL), aspirin (up to 500 mg/dL), and potassium cyanate (up to 1 mmol/L) were added to whole blood samples. After incubation at 37 °C for 2 h, these samples were assayed to determine the effect of labile A1c (LA1c), acetylated hemoglobin, and carbamylated hemoglobin on enzymatic HbA1c determination, respectively. We analyzed samples containing the most common Hb variants (HbS, HbC, HbD, and HbE) to determine the potential bias effect of these variants on HbA1c measurement.
Drugs
The effect of medication on the enzyme-based HbA1c assay was analyzed by adding various medications to whole blood specimens. The drug doses were as follows: Paracetamol (0 mg/dL, 50 mg/dL, 100 mg/dL, 150 mg/dL, 200 mg/dL), Ibuprofen (0 mg/dL, 12.5 mg/dL, 25 mg/dL, 37.5 mg/dL, 50 mg/dL), Aspirin (0 mg/dL, 12.5 mg/dL, 25 mg/dL, 37.5 mg/dL, 50 mg/dL), Metformin (0 mg/dL, 1.25 mg/dL, 2.50 mg/dL, 3.75 mg/dL, 5.0 mg/dL), and Acarbose (0 mg/dL, 12.5 mg/dL, 25 mg/dL, 37.5 mg/dL, 50 mg/dL).
Hemoglobin concentration
Samples with a hemoglobin concentration range of 30–180 g/L were prepared by mixing erythrocytes and plasma in various proportions. All samples were assayed on the BS-600M and compared to the original HbA1c result.
Data analysis
Data were analyzed and plotted using Analyze-it statistical software (version 6.15) and Microsoft Excel 2019. The Kolmogorov–Smirnov test was used to assess the normal distribution of continuous variables. Data that followed a normal distribution were reported as mean and standard deviation (SD). A t-test was used to compare between two groups. Data for variables with skewed distributions were expressed as the median and interquartile range (IQR values). Comparisons between two groups were conducted using the Wilcoxon rank sum test. Categorical variables were expressed as frequencies. A two-sided p-value of lower than 0.05 was considered statistically significant.
Results
Analytical performance
Precision
When the results of HbA1c were calculated in IFCC units, the coefficients of variation for within-batch precision for the two levels of patient samples were 0.37% and 0.33%, and the coefficients of variation for total precision were 0.67% and 0.40%, respectively, and the coefficients of variation for within-batch precision for the two levels of QC samples were 0.65% and 0.21%, and the coefficients of variation for total precision were 0.87% and 0.35%, respectively. When calculated in NGSP units, the coefficients of variation for within-lot precision for the two levels of patient samples were 0.25% and 0.26%, with total precision coefficients of variation of 0.46% and 0.31%, respectively, and for the two levels of quality-control samples, the coefficients of variation for within-lot precision were 0.38% and 0.17%, with total precision coefficients of variation of 0.51% and 0.27%, respectively (Table 1).
Linearity
The relationship between HbA1c and its concentration exhibited excellent linearity across the range of 2.96–20.23%. The regression equation between the theoretical and measured values is Y = 17.15*X + 2.788 (R2 = 0.9991), where X represents the theoretical value and Y represents the measured value.
Accuracy
A relative bias of ± 6.0% was used as the total allowable error for HbA1c11. The results of enzymatic assay of whole blood samples of glycosylated hemoglobin assigned by IFCC calibrators and NGSP were correlated with their target values with slopes of 0.9882 and 1.0496 and intercepts of 0.0408 and − 0,3259, respectively, and the relative biases were all within ± 6%. Sigma-metric values for different concentration points were calculated using total precision (CV%) and relative bias at the precision sample mean (Bias%) (Table 2).
Method comparison
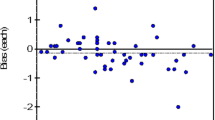
The regression equation for BS-600M VS G8 was y = 0.979x + 0.005, with a correlation coefficient of 0.997 (Fig. 1A). The biases at the 6.0% and 9.0% levels were − 2.00% and − 2.03%, respectively (Fig. 1B); The regression equation for the BS-600M VS Roche c501 was y = 0.934x + 0.317, with a correlation coefficient of 0.992 (Fig. 1C). The biases at the 6.0% and 9.0% levels were − 1.34% (− 1.85% to − 0.71%) and − 3.10% (− 4.36% to − 1.91%), respectively (Fig. 1D).
Interference
Lipemia, Bilirubin, and Vitamin C
The HbA1c (6.1% and 8.5%) biases caused by the Bilirubin (0–15 mg/dL), Vitamin C (0–3 mg/dL), and Intralipid (0–500 mg/dL) were − 3.49% (95% CI − 5.16% to − 2.12%), 0.32% (95% CI − 0.83% to 1.13%), and 0.80% (95% CI − 3.87% to 1.02%), respectively (Fig. 2A).
Interferences of HbA1c measured by enzymatic assay on the BS-600M. (A) Lipemia, Bilirubin, and Vitamin C; (B) LA1c, acetylated hemoglobin, and carbamylated hemoglobin; (C) Hb S, Hb C, Hb D, and Hb E; (D) Metformin. (E) Paracetamol. (F) Acarbose. (G) Ibuprofen. (H) Aspirin. Level 1, HbA1c = 6.5%; alevel 2, HbA1c = 9.0%.
Hemoglobin derivatives and variants
The biases in HbA1c levels (6.5% and 9.0%) caused by LA1c, acetylated hemoglobin, and carbamylated hemoglobin were − 0.71% and − 0.29%, − 1.39% and − 1.28%, and 0.87% and 0.10%, respectively (Fig. 2B). The biases caused by HbS, HbC, HbD, and HbE on the enzymatic determination of HbA1c at levels of 5.5% and 7.5% were as follows: − 0.77% and 2.11%, 1.65% and − 1.35%, 1.10% and − 1.33%, 1.39% and − 0.78%, respectively (Fig. 2C).
Drugs
The HbA1c results showed a gradual decrease (− 0.66% to − 2.46%) with increasing concentrations of paracetamol, but there was no statistically significant difference (P = 0.1022). Ibuprofen, aspirin, metformin, and acarbose did not have significant effect (P > 0.05) on the enzymatic assay results (Fig. 2D–I).
Hemoglobin concentration
When the hemoglobin concentration was ≥ 45 g/L, there was no significant change in the HbA1c results (t = 0.88, P = 0.3890). However, when the hemoglobin concentration was < 45 g/L, the HbA1c levels were significantly higher than the original HbA1c result (t = 4.46, P = 0.0005) (Fig. 3).
Discussion
With the progress of HbA1c standardization, the discrepancies in HbA1c results from various laboratories and methods around the world are gradually decreasing11,12. HbA1c values are used not only for follow-up of glycemic control in diabetic patients but also for diagnosis. Therefore, it is crucial to utilize a testing system that provides accurate HbA1c results for the diagnosis and monitoring of diabetes mellitus. In this study, we evaluated the performance of an enzymatic HbA1c assay. We found that the enzymatic assay performed on the Mindray BS-600M demonstrated good precision, linearity, and accuracy. Moreover, the results obtained with the enzymatic assay were in good agreement with those obtained with the Tosoh HLC-723 G8 and Roche Cobas c501 systems. The performance of the enzymatic method meets the requirements for sample testing in clinical laboratories.
HbA1c is a product formed when hemoglobin and glucose combine in red blood cells. Therefore, any factors that cause changes in the quantity and quality of hemoglobin, such as hemoglobinopathies, derived hemoglobin, abnormalities in the erythrocyte survival lifespan, and drugs, can interfere with the HbA1c assay13,14,15,16. Our results indicated that routine interferences such as lipemia, hemolysis, jaundice, and vitamin C did not affect the BS-600M enzymatic assay. It is also observed that modified hemoglobins (LA1c, carbamoylated hemoglobins, acetylated hemoglobins) and drugs (paracetamol, ibuprofen, aspirin, metformin, acarbose) did not have a significant impact on the enzymatic HbA1c assay. In addition, the bias of the results of four common hemoglobin variants (Hb S, Hb C, Hb D, and Hb E) did not exceed the permitted range. This demonstrates that enzymatic methods are less susceptible to interference from hemoglobin variants than ion-exchange high-performance liquid chromatography, as previously reported14.
The enzymatic assay is based on measuring the percentage of HbA1c in total hemoglobin. Therefore, the concentration of total hemoglobin directly affects the results of the assay. Previous study found that the deviation of sample HbA1c from the target value was less than 0.5% when the hemoglobin concentration was within the range of 9–21 g/dL Total Hb17. The results of this study showed that the enzymatic assay of BS-600M provided more stable HbA1c results in the range of HGB ≥ 45 g/L, suggesting that it could be applied to anemia patients (HGB ≥ 45 g/L). It should be noted that the lifespan of red blood cells may change at low Hb concentrations. Comparison of results between assay systems showed good agreement among the enzymatic assay, HPLC, and immunoassay. The expected bias at their medical decision levels was found to be within the acceptable accuracy limit of ± 6%11.
In conclusion, the BS-600M enzymatic HbA1c assay demonstrates excellent analytical performance in terms of accuracy, precision, sensitivity and specificity. Compared to HPLC and immunoturbidimetric assay, it shows consistent results and is not adversely affected by common hemoglobin derivatives, drugs and hemoglobin variants. Moreover, this assay provides a rapid detection speed, which makes it a suitable choice for routine practice in clinical chemistry laboratories.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Braga, F., Dolci, A., Mosca, A. & Panteghini, M. Biological variability of glycated hemoglobin. Chim. Clin. Acta 411, 1606–1610 (2010).
Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329, 977–986 (1993).
U.K. Prospective Diabetes Study (UKPDS) Group. Intensive bloodglucose control with sulphonyl ureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998(352), 837–853 (1998).
Sacks, D. B. et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 34(6), e61-99 (2011).
Gillett, M. J. International Expert Committee report on the role of the A1c assay in the diagnosis of diabetes. Diabetes Care. 32, 1327–1334 (2009).
Weykamp, C., John, W. G. & Mosca, A. A review of the challenge in measuring hemoglobin A1c. J. Diabetes Sci. Technol. 3, 439–445 (2009).
Song, Y. et al. Comparability of different methods of glycated hemoglobin measurement for samples of patients with variant and non-variant hemoglobin. Clin. Chim. Acta. 533, 168–174 (2022).
Little, R. R. & Roberts, W. L. A review of variant hemoglobins interfering with hemoglobin A1c measurement. J. Diabetes Sci. Technol. 3, 446–451 (2009).
Camargo, J. L., Stifft, J. & Gross, J. L. The effect of aspirin and vitamins C and E on HbA1c assays. Clin. Chim. Acta. 372, 2069 (2006).
Kaiser, P. et al. HbA1c: EQA in Germany, Belgium and the Netherlands using fresh whole blood samples with target values assigned with the IFCC reference system. Clin. Chem. Lab. Med. 54(11), 1769–1775 (2016).
Little, R. R. et al. Effects of hemoglobin (Hb) E and HbD traits on measurements of glycated Hb (HbA1c) by 23 methods. Clin. Chem. 54(8), 1277–1282 (2008).
Kang, F., Li, W., Lou, Y. & Shan, Z. Application of biological variation and sigma metrics to evaluate the performance of HbA1c in external quality assessment. Scand. J. Clin. Lab. Invest. 82(5), 398–403 (2022).
Loh, T. P. et al. Setting analytical performance specifications using HbA1c as a model measurand. Clin. Chim. Acta. 523, 407–414 (2021).
Tang, M. et al. The impact of carbamylation and anemia on HbA1c’s association with renal outcomes in patients with diabetes and chronic kidney disease. Diabetes Care. 46(1), 130–137 (2023).
Zechmeister, B. et al. Analytical interference of 33 different hemoglobin variants on HbA1c measurements comparing high-performance liquid chromatography with whole blood enzymatic assay: A multi-center study. Clin. Chim. Acta. 531, 145–151 (2022).
Jacobs, J. W., Gisriel, S. D., Iyer, K., Hauser, R. G. & El-Khoury, J. M. Unexpectedly low hemoglobin A1c in a patient with chronic lymphocytic leukemia. Clin. Chim. Acta. 531, 91–93 (2022).
Teodoro-Morrison, T. et al. Evaluation of a next generation direct whole blood enzymatic assay for hemoglobin A1c on the ARCHITECT c8000 chemistry system. Clin. Chem. Lab. Med. 53(1), 125–132 (2015).
Acknowledgements
We thank all the laboratories that participated in this study. We sincerely thank all the laboratory staff who assisted with this study.
Funding
This study was supported by Guangdong Medical Science Foundation (B2021064). The fund provided financial support for the labor fee, communication fee, transportation and Courier fee, and reagent purchasing fees required by this study. The funding agencies had no role in study conception, data analysis, publishing decision or manuscript preparation.
Author information
Authors and Affiliations
Contributions
Mingyang Li and Anping Xu has access to all data in this study and is responsible for experimental design, the integrity of the data and the accuracy of the data analysis. Weijie Xie and Yu Zeng were responsibility for the research content, and obtain the ethical approval. Xiongjun Wu, Hui Wang, and Han Chen took responsibility for data collection and data accuracy. Mingyang Li were in charge of the statistical analysis and the manuscript draft. Anping Xu, Helu Liu, Ling Ji contributed to critical revision of the manuscript. All authors reviewed and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, M., Wu, X., Xie, W. et al. Analytical performance evaluation of the Mindray enzymatic assay for hemoglobin A1c measurement. Sci Rep 14, 12289 (2024). https://doi.org/10.1038/s41598-024-63261-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-63261-y
- Springer Nature Limited