Abstract
Hypertrophic cardiomyopathy (HCM) remains the most common cardiomyopathy in humans and cats with few preclinical pharmacologic interventional studies. Small-molecule sarcomere inhibitors are promising novel therapeutics for the management of obstructive HCM (oHCM) patients and have shown efficacy in left ventricular outflow tract obstruction (LVOTO) relief. The objective of this study was to explore the 6-, 24-, and 48-hour (h) pharmacodynamic effects of the cardiac myosin inhibitor, CK-586, in six purpose-bred cats with naturally occurring oHCM. A blinded, randomized, five-treatment group, crossover preclinical trial was conducted to assess the pharmacodynamic effects of CK-586 in this oHCM model. Dose assessments and select echocardiographic variables were assessed five times over a 48-h period. Treatment with oral CK-586 safely ameliorated LVOTO in oHCM cats. CK-586 treatment dose-dependently eliminated obstruction (reduced LVOTOmaxPG), increased measures of systolic chamber size (LVIDs Sx), and decreased select measures of heart function (LV FS% and LV EF%) in the absence of impact on heart rate. At all tested doses, a single oral CK-586 dose resulted in improved or resolved LVOTO with well-tolerated, dose-dependent, reductions in LV systolic function. The results from this study pave the way for the potential use of CK-586 in both the veterinary and human clinical setting.
Similar content being viewed by others
Introduction
Hypertrophic cardiomyopathy (HCM) remains the most common heritable cardiomyopathy of humans affecting approximately 1 in every 500 people1. The disease is largely considered genetic, most commonly caused by mutations in genes encoding sarcomeric proteins with a resultant pathogenic effect of sarcomeric hypercontractility2. This aberrant dynamic change predominantly affects the left ventricular (LV) chamber of the heart and leads to concentric hypertrophy, myocardial fibrosis, and myofiber disarray3. In the case of severe LV hypertrophy or asymmetric septal hypertrophy (ASH), hyperdynamic LV contractility causes systolic anterior motion of the mitral valve resulting in obstruction to LV outflow and LV pressure overload (obstructive HCM [oHCM])4. Obstruction at rest (without provocation) is observed in ~ 20–33% of human HCM patients and is associated with increased disease morbidity (e.g., exercise intolerance, syncope, angina, and fatigue) and severe disease sequalae (i.e., left-side congestive heart failure [CHF], thromboembolic disease, and sudden cardiac death) inherently decreasing patient quality-of-life5,6,7,8,9,10.
Hypertrophic cardiomyopathy is seen at an even greater prevalence in cats, affecting up to 14% of the general cat population11,12,13,14. Like humans, the etiology of the disease has a genetic basis; however, all previously reported feline HCM-causing mutations remain breed-specific (i.e., Maine Coons, Ragdolls, and Sphynx) and explain only a small portion of total feline HCM cases15,16,17. Left ventricular outflow tract obstruction (LVOTO) is observed in ~ 33–63% of HCM-affected cats; yet, unlike oHCM-affected human patients, the presence of LVOTO has not yet been linked to increased disease morbidity in cats13,14,18,19. Despite the aforementioned clinical differences in disease manifestations, cats closely recapitulate the genetic, pathophysiologic, and hemodynamic aspects of human HCM. As such, genetic feline models of HCM and oHCM provide a unique opportunity for the study of novel therapeutics aimed to treat features of HCM, including LVOTO20.
Small-molecule inhibitors that modulate the sarcomere are promising novel therapeutics for LVOTO management in oHCM patients. However, the availability of such pharmaceuticals is limited in both human and veterinary patients. In addition to the use of surgical or interventional procedures (e.g., surgery and alcohol septal ablation), where incomplete response and risk of in-hospital morbidity and mortality is relatively high21,22,23, mavacamten is currently the only FDA-approved cardiac sarcomere inhibitor treatment option for symptomatic oHCM. The drug’s mechanism of action is to bind to the cardiac sarcomere myosin heads and reduce sarcomeric ATPase activity, consequently decreasing the myocardial hypercontractility that leads to outflow tract pressure gradients and LVOTO24. The novel compound, aficamten, is in Phase-III human clinical trial investigation at the time of this writing. Neither mavacamten nor aficamten are FDA-approved for use in animals. In feline HCM or oHCM, pharmaceutical management of LVOTO is limited to the use of beta-blockers (e.g., atenolol) which have not been shown to improve feline quality-of-life or lessen mortality25,26. Collectively, this represents a dire need for novel pharmaceuticals for the treatment of HCM and oHCM in human and veterinary patients alike.
We previously demonstrated successful acute dose-dependent (0.30- and 1.0 mg/kg) reductions in LV systolic function, left ventricular outflow tract maximum pressure gradient (LVOTmaxPG), and isovolumetric relaxation times (IVRT) after of a single oral gavage dose of aficamten in cats affected by asymptomatic oHCM harboring at least one copy of the feline A31P myosin-binding protein C3 (MYBPC3) mutation27,28. Aficamten was well tolerated by all cats receiving treatment and successfully improved LVOTO. In this study, we embarked on the first-ever examination of the 6-, 24-, and 48-hour (h) pharmacodynamic effects of an oral formulation of a novel cardiac myosin inhibitor, CK-4021586 (CK-586), in six purpose-bred cats with naturally occurring feline oHCM. We hypothesized that oral administration of CK-586 in cats afflicted by oHCM will result in dose-dependent relief of LVOTO, and that the drug will be safe and well tolerated. The results of this study highlight the promising pharmacodynamic effects of CK-586 for alleviation of obstruction in feline HCM.
Material and methods
Ethics statement
All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California-Davis (Davis, CA, USA) and carried out in accordance with guidelines and regulations (protocol #22376). Additionally, this study was executed in compliance with the ARRIVE 2.0 guidelines, the Animal Welfare Act, and the Institute for Laboratory Animal Research Guide for the Care and Use of Laboratory Animals29,30,31.
Pharmacokinetic study dose determinations and sample analyses
Eight months prior to the echocardiography study, 12 apparently healthy cats, free of any cardiac and metabolic disease confirmed by board certified veterinary cardiologists (J.L.K. and J.A.S.), were selected to assess the pharmacokinetic profile of CK-586 and determine doses for subsequent pharmacodynamic investigations of CK-586. Eight cats in this cohort were equally randomized and dosed with either a low- (3 mg/kg) or high-dose (10 mg/kg) of capsular CK-586 as a single oral dose. Four additional dose levels (4.8-, 10.5-, 15.9-, and 16.2 mg/kg) of CK-586 were given to four additional cats to assess the drug profiles at altered concentrations. All cats were sedated with a combination of 2- and 0.30 mg/kg of alfaxalone and midazolam, respectively, for placement of a jugular sampling catheter. Cats were monitored and allowed to fully recover from conscious sedation (a period of approximately 1.5 h); subsequently, a single randomized oral dose of CK-586 was administered. Cat blood was obtained at 1-, 1.5-, 4-, 8-, and 24 h after dosing from the jugular sampling catheter via standard methodology into 2 mL EDTA tubes (Fig. 1). Whole-blood EDTA samples were processed to plasma via centrifugation (1000 × g for 15 min) and stored in − 80 °C for subsequent drug profiling.
Total CK-586 plasma concentrations were measured in a single batch by routine liquid chromatography-tandem mass spectrometry (LC-MS/MS) methodology as previously described28. Briefly, a 40 µL aliquot of each plasma was mixed with 120 µL of acetonitrile that contained N1-(butylcarbamoyl)-sulfanamide (0.1 µM) as the internal standard (retention time = 1.29 min). The mixture was then vortexed and centrifuged. The resulting supernatant was transferred and filtered through a membrane (Pall Corporation AcroprepAdv 96-well Filter Plate, 0.2 µm WWPTFE membrane), then diluted 1:1 with HPLC grade water. Ten µL of the resulting solution was injected onto a reverse-phase C18 column, and the resultant peaks detected on a SCIEX API 4000 LC-MS/MS equipped with a turbo ion spray ionization source. Mobile Phase A was 0.1% formic acid in water and Phase B was 0.1% formic acid in acetonitrile. The lower limit of quantification and retention time for CK-586 was 1 ng/mL and 1.48 min, respectively.
Pharmacodynamics study animals and design
The pharmacodynamic effects of CK-586 delivered as an active pharmaceutical ingredient (API) in capsule form was assessed as a blinded, randomized, five-treatment group, crossover preclinical trial in a purpose-bred feline model of oHCM20. A total of six ACVIM Stage B1 HCM-confirmed cats (i.e., subclinical LV hypertrophy without left atrial [LA] enlargement) with concomitant LVOTO, diagnosed by a board-certified veterinary cardiologist (J.A.S.), were initially randomized into one of three treatment groups (Vehicle, 5-, and 10 mg/kg). Following interim data analysis, cats were later again randomized to a lower (2 mg/kg) and higher (15 mg/kg) dose group. Cat Stage B1 HCM-affection status was confirmed by an echocardiographic diagnosis following ACVIM consensus guideline inclusion criteria (i.e., 2D or M-mode diastolic diameter of the interventricular septum or LV posterior wall > 6 mm, excluding insertion sites of moderator bands, with normal LA size confirmed by a left atrial-to-aortic root ratio [LA:Ao] measurement < 1.5) with concomitant LVOTO as evidenced by a left ventricular outflow tract (LVOT) velocity > 1.9 m/s (LVOTmaxPG ≥ 14.5 mmHg)12,32,33,34. HCM-affected cats between one and 10 years-of-age, with no evidence of CHF, and deemed to be otherwise healthy (i.e., clear from metabolic disease and systemic hypertension as a component of routine cat colony protocol by ensuring a Doppler blood pressure < 160 mmHg, normal fundic examination, and normal serum biochemical profile confirming absence of renal disease), were included in the study. Baseline primary data and sample collections were obtained one day prior to CK-586 administration. Cats received a single dose of assigned treatment and additional data was collected 6-, 24-, and 48 h post-treatment (Fig. 1). In addition to standard timepoint evaluation, intravenous (IV) challenge with a 10 mcg/kg/min dose of dobutamine was performed for all cats at the 6 h timepoint to increase heart rate (HR) and contractility, provoking LVOTO (‘6 h Challenge’). All cats had an average washout period of 18.5 days between treatment groups (7-day minimum and 35-day maximum), and subsequently re-randomized until all six cats had successfully received treatments at all doses previously described. With the exception of the treatment dispenser (C.E.J.), all study participants were blinded for all aspects of the study. Demographic information for all cats used in this study are presented in Supplemental Table 1.
Echocardiographic evaluations
On the days of echocardiographic evaluation, a single 100 mg dose of oral gabapentin was given to all study participants an hour prior to complete echocardiographic assessment. All cats were sedated with a combination of 2- and 0.30 mg/kg of alfaxalone and midazolam, respectively, for all study echocardiographic examinations. Routine 2D, M-mode, Color and Spectral Doppler echocardiography was performed to acquire measurements of chamber size, LV wall thickness, and systolic function parameters from the right parasternal long-axis (RPLA) imaging window. Using the left-apical imaging window, indices of diastolic function and LVOTmaxPG were interrogated. Briefly, the following selected measures were recorded using previously described methodology and an off-cart analysis software (Syngo Dynamic Workplace, Siemens Medical Solutions, Malvern, PA, USA): HR, maximal right parasternal long-axis left atrial diameter (RPLA LA), short-axis diastolic left ventricular internal diameter (LVIDd Sx), short-axis systolic left ventricular internal diameter (LVIDs Sx), percent left ventricular fractional shortening (LV FS%), percent left ventricular ejection fraction (LV EF%), LVOTmaxPG, mitral valve passive filling/active filling ratio (MV E/A), isovolumetric relaxation time (IVRT), and left auricular flow velocity (Lau)33. Measurements were recorded as an average of three consecutive cardiac cycles when possible, avoiding any cycles during or immediately following cardiac arrhythmias. Cats were monitored for a period of at least three hours after each echocardiographic examination and returned to the colony following confirmation of full recovery from conscious sedation.
A concomitant lead-II electrocardiogram (ECG) was acquired throughout echocardiography to ensure measurements obtained avoided arrhythmic cycles and no arrythmias requiring therapy were present. All echocardiograms were performed and measured by a single, treatment and subject-blinded, board-certified veterinary cardiologist (J.A.S.).
Statistical analyses
Pharmacokinetic parameters for CK-586 were examined following oral dosing and were estimated by noncompartmental analysis using the commercially available software program Phoenix® WinNonlin® (v8.0, Certara Inc., Princeton, NJ, USA). Pharmacokinetic parameters are reported as mean with standard deviation. Plasma CK-586 concentration–time graphs were generated using Prism Software (GraphPad, San Diego, CA, USA).
D’Agostino-Pearson, Anderson–Darling, and Kolmogorov–Smirnov normality testing was performed for all echocardiographic variables; all echocardiographic variables in this study were treated as non-normally distributed. To determine treatment-related changes within each dose group in HR, measures of chamber size (RPLA LA, LVIDd Sx, LVIDs Sx), and indices of heart function (LV FS%, LV EF%, LVOTmaxPG, MV E/A, LV IVRT, and Lau) at each dose (Vehicle, 2-, 5-, 10-, and 15 mg/kg), a Friedman’s analysis of variance (ANOVA) was employed with Dunn’s multiple comparisons testing, where the mean rank of each timepoint was compared to the mean rank of Baseline values. For each variable with a significant Friedman’s P-value, a two-way repeated measures ANOVA to fit a full model between dose groups (column effect: dose; row effect: timepoint; and column/row interactions were evaluated) with Geisser-Greenhouse correction and Dunnett’s multiple comparisons test was performed. This testing compared means of treatment (2-, 5-, 10-, and 15 mg/kg) with control means (Vehicle). A 2 × 2 contingency table was constructed and Fisher’s exact test was executed to assess differences between the observed incidence of LVOTO at every timepoint where cats were receiving treatment as opposed to the number of times obstruction was evidenced when cats were given Vehicle. Echocardiographic data was analyzed using Prism software (GraphPad, San Diego, CA, USA) where results were considered significant at a P-value < 0.05.
Results
Safety and tolerability
CK-586 (2–15 mg/kg) was safe and well tolerated in cats with oHCM. No adverse events along with no evidence of critically reduced LV EF% across all doses were recorded throughout all aspects of the study.
Pharmacokinetics/dose-finding
At the timepoints evaluated, the mean maximum concentration (Cmax) for total plasma CK-586 was 1.21 µM and 0.28 µM in the 10- and 3 mg/kg dose groups, respectively (Fig. 2 and Supplemental Table 2). The average time-to-maximum plasma concentration (Tmax) across all cats is estimated to be 6.6 h (± 6.0) (Supplemental Table 2). Using this pharmacokinetic data from the 12 previously mentioned healthy cats and ex-ante pharmacodynamic data in healthy rats (Supplemental Fig. 1), it was determined that an initial 5- and 10 mg/kg dose of CK-586 would be used to assess the pharmacodynamic effects of the drug on LVOTO amelioration followed by interim analysis and subsequent dose selection of higher and lower dose groups.
Plasma [CK-586] pharmacokinetic/dose-finding analysis. Mean total plasma [CK-586] values across four cats in the 3- and in the 10 mg/kg dose groups, as well as a single cat in the 4.8-, 10.5-, 15.9-, and 16.2 mg/kg dose groups spanning a 24 h period immediately following a single oral dose delivered as an API in capsule are illustrated. h hour, API active pharmaceutical ingredient.
Heart rate
Comparisons within treatment groups to baseline: After dobutamine challenge, all dose groups showed a notable increase in HR with medians exceeding 200 beats per minute (bpm) for all groups. Heart rate values were significantly higher in the Vehicle and 10 mg/kg dose group after dobutamine challenge (Padjusted = 0.0139 and Padjusted = 0.0423, respectively; Table 1).
Comparisons between treatment groups to vehicle: Vehicle and all other dose group HR values were not different from each other at any timepoint. The overall ANOVA P-value for HR was 0.9368 (Table 2).
Chamber size
LA diameter in right parasternal long-axis
Comparisons within treatment groups to baseline: In the 5- and 10 mg/kg treated cats, LA chamber size values significantly increased from Baseline at the 6 h Challenge timepoint (Padjusted = 0.0076 and Padjusted = 0.0002, respectively). 6 h Post Drug RPLA LA values were significantly higher compared to Baseline in the 10 mg/kg group (Padjusted = 0.0247; Table 1).
Comparisons between treatment groups to vehicle: There was no difference between vehicle and all other dose group RPLA LA values at any timepoint. The overall ANOVA P-value for RPLA LA was 0.8430 (Table 2).
Diastolic LV internal diameter in short-axis
There was no difference in LVIDd Sx values between Baseline and any other timepoint across dose groups (Table 1). Therefore, a two-way repeated measures ANOVA test was not performed for this variable.
Systolic LV internal diameter in short-axis
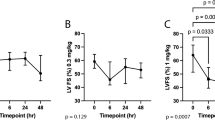
Comparisons within treatment groups to baseline: Drug treatment resulted in significantly increased LVIDs Sx values in the 15 mg/kg dose group (Padjusted = 0.0325; Fig. 3 and Table 1). No other dose group had significant differences in LVIDs Sx values between Baseline and any other timepoint.
Changes in measures of LV systolic chamber size across timepoints. Median LVIDs Sx values in six cats across Vehicle and four CK-586 oral doses (2-, 5-, 10-, and 15 mg/kg) spanning a 48 h time period are illustrated. Dashed line represents lower-bound cut-off for normal feline LVIDs Sx measurement (3.7 mm)36. LVIDs Sx systolic left ventricular internal diameter in short-axis, h hour.
Comparisons between treatment groups to vehicle: At the 6 h Dobutamine Challenge timepoint, values for LVIDs Sx were significantly greater in the 2- and 15 mg/kg dose group when compared to Vehicle (P = 0.0369 and P = 0.005, respectively). The overall ANOVA P-value for LVIDs Sx was 0.0786 (Table 2).
LV systolic function
LV fractional shortening
Comparisons within treatment groups to baseline: In Vehicle-treated cats, dobutamine challenge resulted in significantly increased LV FS% values (Padjusted = 0.0423). Administration of 2-, 5-, 10-, and 15 mg/kg CK-586 resulted in resolution of dobutamine-induced (6 h Challenge) increases in LV FS% seen in cats administered Vehicle. Additionally, at 15 mg/kg, LV FS% was significantly reduced at the 6 h Post Drug timepoint (Padjusted = 0.0423; Fig. 4A and Table 1).
Changes in measures of LV function and LV pressure gradient across timepoints. Median LV FS% (A), LV EF% (B), LVOTmaxPG (C), and LV IVRT (D) values in six cats across Vehicle and four CK-586 oral doses (2-, 5-, 10-, and 15 mg/kg) spanning a 48 h time period are illustrated. Dashed line represents the lower-bound cut-off for normal feline LV FS% measurements (35%) and the upper-bound cut-off LVOTmaxPG (14.5 mmHg)12,14,32. LV left ventricular, FS% percent fractional shortening, EF% percent ejection fraction, LVOTmaxPG left ventricular outflow tract maximum pressure gradient, IVRT isovolumetric relaxation time, h hour.
Comparisons between treatment groups to vehicle: Values for LV FS% were significantly higher when cats were given Vehicle as opposed to 15 mg/kg of CK-586 at the 6 h Post Drug and 6 h Challenge timepoints (P = 0.0111 and P = 0.0004, respectively). Vehicle LV FS% values were also statistically greater at the 24 h Post Drug timepoint than that of the 10 mg/kg dose group (P = 0.0282). The overall ANOVA P-value for LV FS% was 0.0024 (Table 2).
LV ejection fraction
Comparisons within treatment groups to baseline: Similar to LV FS%, dobutamine challenge resulted in significantly increased LV EF% values in the Vehicle group (Padjusted = 0.0423). Administration of 2-, 5-, 10-, and 15 mg/kg CK-586 resulted in resolution of dobutamine-induced (6 h Challenge) increases in LV EF%. 6 h Post Drug LV EF% values were statistically decreased compared to Baseline values in the 15 mg/kg dose group (Padjusted = 0.0423; Fig. 4B and Table 1).
Comparisons between treatment groups to vehicle: Vehicle versus 15 mg/kg LV EF% values were statistically significant at the 6 h Post Drug and 6 h Challenge timepoint (P = 0.0106 and P = 0.0154, respectively), with 15 mg/kg resulting in reduced LV EF%. LV EF% values between the Vehicle and 10 mg/kg group were also statistically reduced in the 10 mg/kg group at the 24 h Post Drug timepoint (P = 0.0209). The overall ANOVA P-value for LV EF% was 0.0034 (Table 2).
LVOT maximum pressure gradient
Comparisons within treatment groups to baseline: Dobutamine challenge significantly increased LVOTmaxPG values when compared to Baseline values in the Vehicle-, 2 mg/kg-, and 5 mg/kg-treated cats (Padjusted = 0.0021, Padjusted = 0.0423, and Padjusted = 0.0139, respectively). LVOTmaxPG was blunted when challenged with dobutamine in the setting of 10- and 15 mg/kg of CK-586. Treatment with a single 15 mg/kg dose of CK-586 successfully decreased LVOTmaxPG values 6 h post-treatment (Padjusted = 0.0139; Fig. 4C and Table 1).
Comparisons between treatment groups to vehicle: No comparisons between Vehicle and 2-, 5-, 10-, and 15 mg/kg LVOTmaxPG values were statistically significant from each other at any timepoint. The overall ANOVA P-value for LVOTmaxPG was 0.1307 (Table 2).
Mitral valve E/A, LV isovolumetric relaxation times, and left auricular flow
Within-dose differences in MV E/A, LV IVRT, and/or Lau values between Baseline and other study timepoints were not observed via Dunn’s multiple comparisons testing in the Vehicle, 2-, 5-, 10-, and 15 mg/kg groups. However, the LV IVRT variable resulted in an overall statistically significant Friedman P-values across dose groups (Table 1 and Fig. 4D). Thus, to assess between-timepoint LV IVRT value differences between Vehicle and treatment doses that would otherwise not be captured via Friedman and Dunn’s testing, a two-way repeated measures ANOVA test was performed for only this variable. No comparisons between Vehicle and 2-, 5-, 10-, and 15 mg/kg LV IVRT values were statistically significant from each other at any timepoint; the overall ANOVA P-value for LV IVRT was 0.9206 (Table 2).
Incidence of obstruction
A decrease in the incidence of obstruction with an increase in CK-586 dose was observed at each timepoint (Fig. 5); however, the distribution of the observed incidence of obstruction between different timepoints was not statistically different across any of the doses (Table 3).
Observed incidence of LVOTO across timepoints. The total number of observed LVOTO incidences defined by an LVOT velocity > 1.9 m/s (LVOTmaxPG ≥ 14.5 mmHg)12,32 in six cats across Vehicle and four doses of CK-586 (2-, 5-, 10-, and 15 mg/kg). LVOTO left ventricular outflow tract obstruction, LVOT left ventricular outflow tract, LVOTmaxPG left ventricular outflow tract maximum pressure gradient, h hour.
Discussion
Treatment with oral CK-586, a novel cardiac myosin inhibitor, safely ameliorated LVOTO in oHCM cats. In this study, we report the beneficial effects of CK-586 treatment at eliminating obstruction (reducing LVOTOmaxPG), increasing measures of systolic chamber size (LVIDs Sx), and thus, decreasing select measures of heart function (LV FS% and LV EF%) in the absence of impact on HR. Dose-dependent effects were observed for the aforementioned echocardiographic changes after drug administration in spite of dobutamine challenge. The greatest effects were observed at the 6 h Post Drug timepoint for the LV FS%, LV EF%, and LVOTmaxPG variables. Pharmacodynamic similarities were observed between the 10- and 15 mg/kg doses for LVIDS Sx, LV FS%, and LVOTOmaxPG values. Similar to other cardiac myosin inhibitors, dose dependency of hemodynamic functional responses were observed. When dobutamine was administered at the 6 h timepoint, protection against obstruction was apparent in the 10- and 15 mg/kg dose groups. Median LVOTmaxPG values remained below the obstruction threshold (14.5 mmHg) in the 5-, 10-, and 15 mg/kg dose groups up until the 48 h Post Drug timepoint; in the Vehicle and 2 mg/kg dose group, values approaching this cut-off were noted. The Vehicle and 2 mg/kg group exhibited similar pharmacodynamic characteristics, particularly for median LVOTmaxPG and LV EF% values. Collectively, this data suggests that the > 5 and < 15 mg/kg dose range of CK-586 represents a promising target dose for the treatment of obstruction and feline subjects with oHCM. Of note, when cats received a single 15 mg/kg dose of CK-586, median 6 h Post Drug LV FS% values fell below the lower-bound cut-off point (35%) suggesting this level of dose escalation in cats is not desirable.
Cardiac myosin inhibitors, mavacamten and aficamten, have previously been evaluated in the same cat model27,28,35. Because each study was conducted under different experimental conditions, it is difficult to make direct comparisons between these three cardiac myosin inhibitors in this model. For example, mavacamten was administered by IV infusion, echocardiographic assessment was performed in fully anesthetized (alfaxalone and midazolam induction with isoflurane and oxygen maintenance) cats, and isoproterenol was administered to elevate HR and induce previously observed LVOTO to pre-anesthetized values35. In the current study, CK-586 was administered orally and LVOTO was induced by dobutamine. Overall, like mavacamten and aficamten, CK-586 demonstrated dose- and exposure-related reductions in measures of systolic contractility and LVOTO and further provides support for cardiac myosin inhibitors as a potential therapeutic approach for cats with oHCM.
The present study only included cats in the B1 stage of their disease; therefore, the results from this study are only applicable to cats that have yet to progress into advanced subclinical (i.e., stage B2 [subclinical LV hypertrophy with evidence of left atrial enlargement]) and/or symptomatic disease states (i.e., left-sided CHF). Whether CK-586 has the propensity to mitigate obstruction at different disease stages, particularly in patients that have progressed onto CHF, remains unknown and warrants further investigation. However, results from human cardiac myosin inhibitors trials suggest that symptom treatment is feasible. While echocardiographic assessment of systolic function remained normal throughout this study, invasive hemodynamic assessments and measurements of blood pressure were not performed.
This is a hypothesis-driven, non-GLP, exploratory study assessing the pharmacodynamic effects of CK-586 in six cats serving as their own controls across five different study groups. Although this design helps to limit the challenges of a smaller sample size, the possibility of type-II errors cannot be excluded; this may be evidenced by the trends in study variables that did not meet statistical significance. The effect sizes in this repeated-measures study appear biologically meaningful, however, this study did not specifically evaluate intra-day or inter-day precision or accuracy. This study highlights the promising short-term effects of CK-586 dosing in asymptomatic oHCM cats. As such, long-term and disease outcomes data cannot be extrapolated from the results presented here. Further studies investigating the extent to which CK-586 administration may benefit cats with oHCM is needed. Lastly, the cats in this study exhibited oHCM at rest but were sedated for the evaluations of this study, necessitating the use of a dobutamine challenge to provoke reliable obstruction. Ideally, future chronic dosing studies would assess drug impact in the resting and provoked state without the variable of sedation.
Data availability
Data is available upon reasonable request from the corresponding author.
Abbreviations
- ANOVA:
-
Analysis of covariance
- API:
-
Active pharmaceutical ingredient
- AUC:
-
Area under the curve
- ASH:
-
Asymmetric septal hypertrophy
- CHF:
-
Congestive heart failure
- Cmax:
-
Maximum concentration
- CmaxD:
-
Dose-normalized maximum concentration
- ECG:
-
Electrocardiogram
- EF%:
-
Percent ejection fraction
- FS%:
-
Percent fractional shortening
- HCM:
-
Hypertrophic cardiomyopathy
- HR:
-
Heart rate
- h:
-
Hour
- IACUC:
-
Institutional Animal Care and Use Committee
- IV:
-
Intravenous
- IVRT:
-
Isovolumetric relaxation time
- Kel:
-
Elimination rate constant
- LA:
-
Left atrium/atrial
- Lau:
-
Left auricular flow velocity
- LC-MS/MS:
-
Liquid chromatography-tandem mass spectrometry
- LV:
-
Left ventricle/ventricular
- LVIDd Sx:
-
Short-axis diastolic left ventricular internal diameter
- LVIDs Sx:
-
Short-axis systolic left ventricular internal diameter
- LVOT:
-
Left ventricular outflow tract
- LVOTmaxPG:
-
Left ventricular outflow tract maximum pressure gradient
- LVOTO:
-
Left ventricular outflow tract obstruction
- MYBPC3:
-
Myosin-binding protein C3
- MV E/A:
-
Mitral valve passive filling/active filling ratio
- oHCM:
-
Obstructive hypertrophic cardiomyopathy
- RPLA:
-
Right parasternal long-axis
- RPLA LA:
-
Maximal right parasternal long-axis left atrial diameter
- Tmax:
-
Time-to-maximum plasma concentration
References
Maron, B. J. et al. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA study. Coronary artery risk development in (Young) adults. Circulation 92(4), 785–789. https://doi.org/10.1161/01.cir.92.4.785 (1995).
Maron, B. J., Maron, M. S. & Semsarian, C. Genetics of hypertrophic cardiomyopathy after 20 years: Clinical perspectives. J. Am. Coll. Cardiol. 60(8), 705–715. https://doi.org/10.1016/j.jacc.2012.02.068 (2012).
Marian, A. J. & Braunwald, E. Hypertrophic cardiomyopathy: Genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ. Res. 121(7), 749–770. https://doi.org/10.1161/CIRCRESAHA.117.311059 (2017).
Raj, M. A., Ranka, S. & Goyal, A. Hypertrophic obstructive cardiomyopathy. StatPearls. (2023).
Lu, D. Y. et al. Clinical outcomes in patients with nonobstructive, labile, and obstructive hypertrophic cardiomyopathy. J. Am. Heart Assoc. https://doi.org/10.1161/JAHA.117.006657 (2018).
Autore, C., Bernabo, P., Barilla, C. S., Bruzzi, P. & Spirito, P. The prognostic importance of left ventricular outflow obstruction in hypertrophic cardiomyopathy varies in relation to the severity of symptoms. J. Am. Coll. Cardiol. 45(7), 1076–1080. https://doi.org/10.1016/j.jacc.2004.12.067 (2005).
Spirito, P., Seidman, C. E., McKenna, W. J. & Maron, B. J. The management of hypertrophic cardiomyopathy. N. Engl. J. Med. 336(11), 775–785. https://doi.org/10.1056/NEJM199703133361107 (1997).
Maron, B. J. Hypertrophic cardiomyopathy: A systematic review. JAMA. 287(10), 1308–1320. https://doi.org/10.1001/jama.287.10.1308 (2002).
Maron, M. S. et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N. Engl. J. Med. 348(4), 295–303. https://doi.org/10.1056/NEJMoa021332 (2003).
Sorajja, P. Alcohol septal ablation for obstructive hypertrophic cardiomyopathy: A word of balance. J. Am. Coll. Cardiol. 70(4), 489–494. https://doi.org/10.1016/j.jacc.2017.06.011 (2017).
Kittleson, M. D. & Cote, E. The feline cardiomyopathies: 2. Hypertrophic cardiomyopathy. J. Feline Med. Surg. 23(11), 1028–1051. https://doi.org/10.1177/1098612X211020162 (2021).
Luis Fuentes, V. et al. ACVIM consensus statement guidelines for the classification, diagnosis, and management of cardiomyopathies in cats. J. Vet. Intern. Med. 34(3), 1062–1077. https://doi.org/10.1111/jvim.15745 (2020).
Payne, J. R., Brodbelt, D. C. & Luis, F. V. Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centres (the CatScan study). J. Vet. Cardiol. 17(Suppl 1), S244–S257. https://doi.org/10.1016/j.jvc.2015.03.008 (2015).
Novo Matos, J., Payne, J. R., Seo, J. & Luis, F. V. Natural history of hypertrophic cardiomyopathy in cats from rehoming centers: The CatScan II study. J. Vet. Intern. Med. 36(6), 1900–1912. https://doi.org/10.1111/jvim.16576 (2022).
Meurs, K. M., Norgard, M. M., Ederer, M. M., Hendrix, K. P. & Kittleson, M. D. A substitution mutation in the myosin binding protein C gene in ragdoll hypertrophic cardiomyopathy. Genomics 90(2), 261–264. https://doi.org/10.1016/j.ygeno.2007.04.007 (2007).
Meurs, K. M. et al. A cardiac myosin binding protein C mutation in the Maine Coon cat with familial hypertrophic cardiomyopathy. Hum. Mol. Genet. 14(23), 3587–3593. https://doi.org/10.1093/hmg/ddi386 (2005).
Meurs, K. M. et al. A deleterious mutation in the ALMS1 gene in a naturally occurring model of hypertrophic cardiomyopathy in the Sphynx cat. Orphanet. J. Rare Dis. 16(1), 108. https://doi.org/10.1186/s13023-021-01740-5 (2021).
Saito, T. et al. Comparative study of myocardial function in cases of feline hypertrophic cardiomyopathy with and without dynamic left-ventricular outflow-tract obstruction. Front. Vet. Sci. 10, 1191211. https://doi.org/10.3389/fvets.2023.1191211 (2023).
Fox, P. R. et al. International collaborative study to assess cardiovascular risk and evaluate long-term health in cats with preclinical hypertrophic cardiomyopathy and apparently healthy cats: The REVEAL study. J. Vet. Intern. Med. 32(3), 930–943. https://doi.org/10.1111/jvim.15122 (2018).
Stern, J. A. et al. Hypertrophic cardiomyopathy in purpose-bred cats with the A31P mutation in cardiac myosin binding protein-C. Sci. Rep. 13(1), 10319. https://doi.org/10.1038/s41598-023-36932-5 (2023).
Woodland, M. & Al-Horani, R. A. New Era: Mavacamten for obstructive hypertrophic cardiomyopathy. Cardiovasc. Hematol. Agents Med. Chem. 21(2), 78–83. https://doi.org/10.2174/1871525721666221019095218 (2023).
Hegde, S. M. et al. Effect of mavacamten on echocardiographic features in symptomatic patients with obstructive hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 78(25), 2518–2532. https://doi.org/10.1016/j.jacc.2021.09.1381 (2021).
Olivotto, I. et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 396(10253), 759–769. https://doi.org/10.1016/S0140-6736(20)31792-X (2020).
Kawas, R. F. et al. A small-molecule modulator of cardiac myosin acts on multiple stages of the myosin chemomechanical cycle. J. Biol. Chem. 292(40), 16571–16577. https://doi.org/10.1074/jbc.M117.776815 (2017).
Coleman, A. E. et al. Atenolol in cats with subclinical hypertrophic cardiomyopathy: A double-blind, placebo-controlled, randomized clinical trial of effect on quality of life, activity, and cardiac biomarkers. J. Vet. Cardiol. 30, 77–91. https://doi.org/10.1016/j.jvc.2020.06.002 (2020).
Kortas, M. & Szatmari, V. Prevalence and prognosis of atenolol-responsive systolic anterior motion of the septal mitral valve leaflet in young cats with severe dynamic left ventricular outflow tract obstruction. Animals 12(24), 3509. https://doi.org/10.3390/ani12243509 (2022).
Sharpe, A. N. et al. Effects of Aficamten on cardiac contractility in a feline translational model of hypertrophic cardiomyopathy. Sci. Rep. 13(1), 32. https://doi.org/10.1038/s41598-022-26630-z (2023).
Sharpe, A. N. et al. Pharmacokinetics of a single dose of Aficamten (CK-274) on cardiac contractility in a A31P MYBPC3 hypertrophic cardiomyopathy cat model. J. Vet. Pharmacol. Ther. 46(1), 52–61. https://doi.org/10.1111/jvp.13103 (2023).
Percie du Sert, N. et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 18(7), e3000411. https://doi.org/10.1371/journal.pbio.3000411 (2020).
Animal Welfare Act as Amended. USC. 7, S2131–2159 (2013).
8 Guide for the Care and Use of Laboratory Animals (The National Academies Press) (2010).
Chetboul, V. et al. Quantitative assessment of velocities of the annulus of the left atrioventricular valve and left ventricular free wall in healthy cats by use of two-dimensional color tissue Doppler imaging. Am. J. Vet. Res. 67(2), 250–258. https://doi.org/10.2460/ajvr.67.2.250 (2006).
Oldach, M. S. et al. Cardiac effects of a single dose of pimobendan in cats with hypertrophic cardiomyopathy; a randomized, placebo-controlled, crossover study. Front. Vet. Sci. 6, 15. https://doi.org/10.3389/fvets.2019.00015 (2019).
Haggstrom, J. et al. Effect of body weight on echocardiographic measurements in 19,866 pure-bred cats with or without heart disease. J. Vet. Intern. Med. 30(5), 1601–1611. https://doi.org/10.1111/jvim.14569 (2016).
Stern, J. A. et al. A small molecule inhibitor of sarcomere contractility acutely relieves left ventricular outflow tract obstruction in feline hypertrophic cardiomyopathy. PLoS One 11(12), e0168407. https://doi.org/10.1371/journal.pone.0168407 (2016).
Adin, D. B. & Diley-Poston, L. Papillary muscle measurements in cats with normal echocardiograms and cats with concentric left ventricular hypertrophy. J. Vet. Intern. Med. 21(4), 737–741. https://doi.org/10.1111/j.1939-1676.2007.tb03015.x (2007).
Acknowledgements
The authors would like to acknowledge Jeanelle Zamora and Keith Huie for their technical assistance.
Funding
This study was funded by Cytokinetics Inc. (South San Francisco, CA, USA); industry sponsors did not have any influence into the final results or conclusions of this study. Support for V.N.R. was provided by NIH T32 HL086350 and TL1 TR001861. Support for J.R.W. was provided by NIH T34 GM136469. Support for J.L.K. was provided by NIH T32 OD011147 and Morris Animal Foundation D22FE-027. Additional support for L.A.W. was provided by NIH K01 OD026526.
Author information
Authors and Affiliations
Contributions
D.T.H. and J.A.S. conceived the experiment(s). V.N.R., A.E.C., C.E.J., J.R.W., B.S.Y., J.L.K., D.T.H., and A.N.M. conducted the experiment(s). V.N.R., J.L.K., L.A.W., D.T.H., A.N.M., and J.A.S. analyzed the results. V.N.R., L.A.W., D.T.H., and J.A.S. wrote the original manuscript. All authors reviewed and revised the final manuscript.
Corresponding author
Ethics declarations
Competing interests
D.T.H., A.N.M., B.P.M., and F.I.M. are employees of Cytokinetics Inc. (South San Francisco, CA, USA) which served as the Sponsor of this study and were compensated for their work. Industry sponsors did not have any influence over the final results or conclusions of this study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rivas, V.N., Crofton, A.E., Jauregui, C.E. et al. Cardiac myosin inhibitor, CK-586, minimally reduces systolic function and ameliorates obstruction in feline hypertrophic cardiomyopathy. Sci Rep 14, 12038 (2024). https://doi.org/10.1038/s41598-024-62840-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62840-3
- Springer Nature Limited