Abstract
Studies on the association between coffee consumption and risk of lung cancer have been conflicting. The aim of this study was to systematically review the current evidence on the association between coffee consumption and risk of lung cancer and to quantify this association by performing a meta-analysis. A comprehensive systematic search was performed on online databases up to July 2023 investigating the association between coffee consumption and risk of lung cancer. All prospective cohort studies reporting odds ratios (ORs), rate or risk ratios (RRs), or hazard ratios (HRs) and 95% confidence intervals (CIs) in this context were included. The overall effect size was calculated using the random-effects model and statistical between-studies heterogeneity was examined using Cochrane’s Q test and I2. A total of 14 prospective cohort studies were included in this systematic review and meta-analysis. We found a significant positive association between coffee consumption and risk of lung cancer (RR: 1.28; 95% CI: 1.12, 1.47). This association remained significant when we included a pooled analysis paper and excluded 5 cohort studies (RR: 1.37; 95% CI: 1.12, 1.66). We observed no proof of significant publication bias using Egger’s test (P = 0.58). Moreover, dose–response analysis showed that each one cup/day increase in coffee consumption was related with a 6% higher lung cancer risk (RR: 1.06; 95% CI: 1.03, 1.09). In conclusion, we found a significant positive association between coffee consumption and risk of lung cancer.
Similar content being viewed by others
Introduction
Lung cancer is the second commonly diagnosed cancer and one of the leading causes of cancer mortality by its high fatality rate1. Smoking is a well-known modifiable risk factor for lung cancer followed by carcinogen exposures such as asbestos, heavy metals, and polycyclic aromatic hydrocarbons and etc2,3,4. Dietary intakes have also been shown to contribute to this type of cancer. Earlier studies suggested an inverse association between healthy dietary pattern and risk of this cancer, while high consumption of red and processed meat and total and saturated fats have been associated with elevated risk5,6,7.
Coffee is one of the most widely consumed beverages throughout the world next to water and tea. It contains mixtures of biochemically active ingredients such as antimutagenic and antioxidant or cancer-promoting agents including caffeine, acrylamide, melanoidins, chlorogenic acid, diterpenes, and trigonelline, which might be important in cancer development or prevention8,9. Previous investigations have indicated that coffee may have a protective role in type 2 diabetes, stroke, dementia, and cardiovascular diseases; however, data about cancer is conflicting10,11. While coffee drinking was associated with a lower risk of liver, oral, endometrial, and esophageal cancers, it was associated with an elevated risk of bladder cancer and leukemia12. The role of coffee intake in lung cancer has also been extensively examined, but the findings were conflicting.
Findings from a meta-analysis published in 2010 revealed a significant positive association between coffee consumption and risk of lung cancer in cohort studies. However, only five cohort studies were included in that meta-analysis13. Another meta-analysis on coffee consumption and risk of lung cancer, published in 2016, reached no significant association among non-smokers14. However, that meta-analysis included 8 cohort studies and two cohort studies were missed15,16. In addition, they combined results from case–control and prospective cohort studies which is not a correct method. After release of the latest meta-analysis in 2016, data from 6 large prospective cohort studies appeared17,18,19,20,21,22. Alternatively, some methodological concerns in earlier meta-analyses might limit their interpretation. For instance, both previous meta-analyses have included the study by Khan et al.23 in their analysis, while Khan et al. investigated the association between coffee consumption and risk of mortality from lung cancer, not lung cancer incidence per se. This also applies to Chow et al. study24. Therefore, we aimed to perform an updated comprehensive meta-analysis by including recently published studies. The aim of the present study, therefore, was to systematically review the current evidence on the association of coffee consumption and risk of lung cancer.
Methods and materials
Search strategy
Online databases including PubMed/Medline, Scopus, and ISI Web of Science were systematically searched up to July 2023 using following keywords: (coffee OR caffeine OR drink OR beverage OR “caffeinated beverages” OR “coffee consumption” OR “coffee intake” OR “coffee drinking” OR “caffeine consumption”) AND (“pulmonary neoplasm” OR “lung neoplasm” OR “pulmonary cancer” OR “lung cancer” OR “pulmonary tumor” OR “lung tumor”). No time of publication limitation was taken into account. However, only studies in English were included in the current study. We also performed a manual search of related articles’ references list to avoid missing any relevant published papers. Two reviewers (MJ and ASM) independently screened the output of the search to identify potentially eligible studies. Any disagreements between the two reviewers were solved by consultation with the principal investigator (AE). In addition, no attempt was made to include unreported studies.
Study selection
Articles’ title and abstract were reviewed to find relevant publications by two independent reviewers (MJ and ASM). Publications were fully reviewed if the abstract stated that coffee consumption had been examined in relation to risk of lung cancer. Studies were eligible for inclusion in the current systematic review and meta-analysis if they met the following criteria: (1) all prospective cohort studies performed on adults ≥ 18 years of age; (2) considered coffee as the exposure variable and lung cancer as the main outcome variable or as one of the outcomes; and (3) publications in which odds ratios (ORs), rate or risk ratios (RRs), or hazard ratios (HRs) were reported as effect size.
Data extraction
Two reviewers (MJ and ASM) independently extracted the following data from eligible studies: first author’s last name, year of publication, cohort name/country, mean age or age range (years), sex, number of subjects, number of cases, follow up duration (years), exposure assessment, outcome assessment, comparison, fully adjusted effect size (ORs, RRs, or HRs) with the corresponding 95% CIs, adjustments, and study quality score. Characteristics of included studies in this systematic review and dose–response meta-analysis are provided in Table 1.
Quality assessment
The quality of studies included in this systematic review and meta-analysis was examined by the Newcastle–Ottawa Scale (NOS). Based on this method, a maximum of nine scores can be awarded to each study. In our analysis, we considered scores of ≥ 6 as high-quality studies, otherwise, the study was deemed to have low quality. Table 1 indicates the results of the quality assessment of the eligible cohorts.
Statistical analysis
All reported ORs, RRs, and HRs and their 95% CIs for risk of lung cancer were used to calculate the log RRs and their SEs. The overall effect size was calculated using the random-effects model, which incorporates between-study heterogeneity. Statistical between-studies heterogeneity was examined using Cochrane’s Q test and I-squared (I2). Publication bias was assessed by visual inspection of funnel plots. Formal statistical assessment of funnel plot asymmetry was carried out with Egger’s regression asymmetry tests. Sensitivity analysis was used to explore the extent to which inferences might depend on a particular study or group of studies. Statistical analyses were made with Stata MP, version 14. P-values < 0.05 were considered statistically significant.
To perform a dose–response analysis, we used studies that reported sufficient information. Studies were considered eligible if they reported the range or median/mean dose of coffee consumption (cups per day), the numbers of cases and participants/person-years, adjusted RRs and their 95% CIs across categories of coffee consumption. We divided the total number of cases, participants, and person-years by the number of categories if a study had not reported the sufficient information in each category. The linear dose–response association was measured using generalized least squares trend estimation, based upon the work of Greenland and colleagues25,26. The RR was calculated for a daily increase of one cup of coffee intake in each study. To pool the results of each study, a random-effects model was used. Restricted cubic splines with 3 knots according to Harrell’s recommended percentiles of distribution (10th, 50th, and 90th) were used to examine the potential nonlinear association27. The null hypothesis was tested by calculating a P-value for non-linearity of the meta-analysis. The test was conducted to check if the coefficient of the second spline was equal to 0.
Results
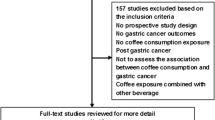
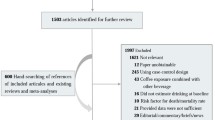
Letters, reviews, meta-analyses, comments, animal studies, and ecological studies were excluded in the current systematic review and meta-analysis. Following our initial search, 19,389 articles were identified. After removing 1293 duplicates, 18,096 reports remained for further assessment. After title and abstract careful checking and review, 18,076 articles were excluded and 20 publications remained for full-text assessment. Five studies were excluded due to the following reasons: two studies had reported lung cancer mortality23,24. In addition, one thesis28 and two Mendelian studies29,30 were also excluded. Finally, a total of 14 prospective cohort studies15,16,17,18,19,20,21,22,31,32,33,34,35,36 and one pooled analysis37 were included in this systematic review and meta-analysis. Figure 1 illustrates the study selection process.
A recent pooled analysis by Zhu et al.37 included 17 cohort studies; however, 12 of them were unpublished data with no available full-texts. Therefore, we decided to analyze data once by including the study by Zhu et al.37 and excluding the 5 studies17,20,21,31,32 that overlapped with the Zhu et al., and once again by adding the 5 studies and excluding the study of Zhu et al. for better understanding of the association.
Narita et al.17 had reported effect sizes separately for men and women, however, we combined these two effect sizes and then, included in our analysis. Three studies had not reported the 95% CIs for the association between coffee consumption and risk of lung cancer34,35,36. Therefore, we derived relevant data for these studies from the previous meta-analysis14.
Findings from the systematic review
Study characteristics
Overall, 14 cohort studies15,16,17,18,19,20,21,22,31,32,33,34,35,36 and one pooled analysis37 were included in the present systematic review (Table 1). These studies were reported from 1986 to 2020; four were from the United States18,20,31,32, three from Norway22,34,36, three from Japan15,17,35, one each from Thailand19, Italy16, Singapore21, and Korea33. The median follow-up duration ranged from 10 to 17.7 years. For the exposure assessment, 10 studies had used food frequency questionnaire15,16,17,18,20,21,22,32,33,34, 1 had collected data based on diet history questionnaire31 and one had used a structured questionnaire19. Others had reported using a questionnaire36 and 24-h dietary recall history35. For the outcome assessment, all, but four, of the included studies had used cancer registries. Outcome assessment in the PLCO study31 was self-reported and Nomura et al. had used histologic examination35. Based on the NOS, all included studies were of high quality.
Findings from the meta-analysis
First, we examined the association by including data from 9 cohort studies not included in the pooled analysis paper of Zhu et al. along with the findings from the pooled analysis. The overall effect size based on these 10 studies15,16,18,19,22,33,34,35,36,37 revealed a statistically significant association between coffee consumption and risk of lung cancer (RR: 1.37; 95% CI: 1.12, 1.66; Fig. 2).
We also found an evidence of statistically significant between-study heterogeneity (I2 = 76.9%, P < 0.001). No evidence of publication bias was seen (P = 0.93).
In a further analysis, we excluded the study of Zhu et al. and included 14 cohort studies in the analysis. Combining 14 effect sizes from 14 studies15,16,17,18,19,20,21,22,31,32,33,34,35,36, we observed a statistically significant positive association between coffee consumption and risk of lung cancer (RR: 1.28; 95% CI: 1.12, 1.47; Fig. 3).
However, a significant between-study heterogeneity was found (I2 = 69.5%, P < 0.001). A sensitivity analysis showed that no particular study had a significant influence on the summary effects. In addition, we observed no proof of significant publication bias using Egger’s test (P = 0.58). (Funnel plot has provided as Supplementary Fig. 1).
To find sources of heterogeneity, we performed subgroup analyses based on fixed-effects model. In the subgroup analyses, we found that sex, follow-up duration, and country might explain between-study heterogeneity (Table 2).
A significant positive association between coffee consumption and risk of lung cancer was seen in women (RR: 2.01; 95% CI: 1.47, 2.75), men (RR: 1.67; 95% CI: 1.05, 2.68), and both sexes (RR: 1.21; 95% CI: 1.13, 1.29). In addition, we observed a significant positive association between coffee consumption and risk of lung cancer in studies with < 15-year duration of follow-up (RR: 1.33; 95% CI: 1.22, 1.44), as well as those with ≥ 15-year of follow-up (RR: 1.13; 95% CI: 1.01, 1.26), those conducted in USA (RR: 1.17; 95% CI: 1.09, 1.27), and those conducted in non-USA countries (RR: 1.42; 95% CI: 1.26, 1.59). In addition, there was a significant positive association between coffee consumption and risk of lung cancer among studies that adjusted analysis for smoking status (RR: 1.26; 95% CI: 1.17, 1.36) and those that did not adjust for smoking status (RR: 1.19; 95% CI: 1.06, 1.35).
A total of 3 studies were excluded from dose–response analysis as they did not provide sufficient information even after receiving two email requests19,20,35. Therefore, 11 studies remained for further analyses. Results from 8 studies including Zhu et al. study demonstrated that each one cup/day increase in coffee consumption was associated with a 6% higher risk of lung cancer (RR: 1.06; 95% CI: 1.03, 1.09; Fig. 4).
The risk of lung cancer increased linearly with coffee consumption of approximately 1–5 cups per day in a nonlinear dose–response analysis (P nonlinearity: 0.94; P dose–response: 0.001; Fig. 5).
Such associations were observed when we excluded the study of Zhu et al. and included 11 cohort studies in the linear dose–response analysis (RR: 1.06; 95% CI: 1.03, 1.08, Pnonlinearity = 0.65; Fig. 6).
There was also a linear association between coffee consumption and risk of lung cancer (P nonlinearity = 0.94, P dose–response: 0.001; Fig. 7).
Discussion
This systematic review and meta-analysis on 14 prospective cohort studies and a pooled analysis indicated a significant positive association between coffee consumption and risk of lung cancer. It was also found that an increase of one cup of coffee per day was linked to a higher risk of lung cancer, according to the dose–response analysis. To the best of our knowledge, this is the most comprehensive and updated meta-analysis about coffee consumption and risk of lung cancer.
Lung cancer imposes a great burden on the health care system. Although smoking is a well-established risk factor for this condition, dietary factors also play an important role. Fruit and vegetables consumption was inversely associated with risk of lung cancer in earlier studies38. In addition, consumption of American/Western dietary pattern has been associated with 45% elevated risk of lung cancer39. Coffee is a regular drink in most parts of the world and evaluating its contribution to human health is of high importance. We found that coffee consumption was associated with a greater risk of lung cancer. Such findings were also reported from a meta-analysis on 5 cohort studies in 201013 and a meta-analysis on 8 cohort studies in 201614. Another previous meta-analysis conducted in 2016 also indicated a significant positive association between coffee consumption and risk of lung cancer11. Data from a prospective cohort study on Women’s Health Initiative (WHI) observational study reported a significant elevated risk of lung cancer for regular, decaffeinated, and total coffee consumption28. In contrast to our findings, a meta-analysis on 8 case–control studies revealed no significant association between coffee consumption and risk of lung cancer13. In addition, a case–control study reported a significant inverse association between weekly compared to never coffee consumption and risk of lung cancer40. In the meta-analysis published in 2016, when the authors combined prospective cohort and case–control studies, without controlling for smoking, a significant association was seen between coffee consumption and risk of lung cancer; however, after restricting the analysis to studies that adjusted for smoking, no significant association was observed14. The discrepant findings can be explained by the difference in the number of studies included in different meta-analyses. In addition, combining effect sizes from case–control studies with those from prospective cohort studies would result in misleading findings. We included a total of 14 cohort studies in the current analysis with a total population of 1,061,854 people and 19,643 incident cases of lung cancer15,16,17,18,19,20,21,22,31,32,33,34,35,36. Comparing these figures with the numbers in previous meta-analyses, it is clear that we had a larger number of people and incident cases in the current analysis, which make our findings more valid and reliable.
The mechanisms through which coffee consumption might affect the risk of lung cancer still remain to be identified. Some biochemically active components of coffee might influence cancer risk. Coffee can be an important dietary source of acrylamide which is a genotoxic agent. Roasting process helps increasing acrylamide content of coffee41. Acrylamide can cause DNA damage in mammalian tissues and induce oxidative stress and thus trigger cancer cell formation42. Caffeine is a widely known substance in coffee which might have mutagenic effect on cancer development43. However, some studies have also reported anti-cancer properties for caffeine44. Despite these contents of coffee, it might also have cancer-protective effects. Cafestol and Kahweol may potentially inhibit tumor growth by blocking or diminishing neoangiogenesis, however, they also increase cardiovascular risk by raising the concentration of serum lipids45. Overall, it seems that coffee with its ingredients might be beneficial or detrimental to different cancers and further studies are needed to elucidate the relevant mechanisms.
Our study has several strengths. Restricting the analysis to prospective cohort studies as well as large number of included studies and participants compared to previous ones are among several strengths. In addition, findings from a recent pooled analysis were also used carefully without overlapping the included studies. This has been resulted to include a large number of individuals in the analysis, in particular from cohort studies for which there are no original report about coffee consumption and lung cancer. However, some limitations must be noted when interpreting our results. This systematic review and meta-analysis was performed based on observational studies with their inherent limitations. Therefore, it is difficult to make a conclusive decision about the causal association between coffee consumption and risk of lung cancer. In addition, for most included studies, coffee consumption was assessed using a food frequency questionnaire. Therefore, measurement error and misclassification of study participants in terms of exposure were unavoidable. Both non-differential misclassification and measurement errors attenuate the relative risk. Furthermore, the present systematic review and meta-analysis included studies that had enrolled subjects from different countries with different dietary habits and racial factors, which could be associated with different risks of lung cancer. Despite adjustment for several potential confounders in primary studies, the possibility of residual confounding cannot be ignored. The quality of the included studies and generalizability of the results should also be noted. Finally, we were unable to examine the association between different types of coffee and risk of lung cancer, because included studies had not reported such information separately.
In conclusion, this systematic review and meta-analysis indicated a significant positive association between coffee consumption and risk of lung cancer. Further studies, especially with prospective design, are required to expand our knowledge on the association between coffee consumption and risk of lung cancer.
Data availability
The dataset used and analyzed during the current study is available from the corresponding author on a reasonable request.
Abbreviations
- CIs:
-
Confidence intervals
- HRs:
-
Hazard ratios
- NOS:
-
Newcastle–Ottawa Scale
- ORs:
-
Odds ratios
- RRs:
-
Rate or risk ratios
- WHI:
-
Women’s Health Initiative
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249. https://doi.org/10.3322/caac.21660 (2021).
Chen, C.-Y., Peng, H.-C., Chen, Y.-Y., Chan, C.-C. & Yu, C.-J. Association of environmental heavy metals exposure and lung cancer incidence and prognosis. Eur. Respir. J. 48(suppl 60), PA2805. https://doi.org/10.1183/13993003.congress-2016.PA2805 (2016).
de Groot, P. & Munden, R. F. Lung cancer epidemiology, risk factors, and prevention. Radiol. Clin. North Am. 50(5), 863–876. https://doi.org/10.1016/j.rcl.2012.06.006 (2012).
Singh, A. et al. PAH exposure-associated lung cancer: An updated meta-analysis. Occup. Med. 68(4), 255–261. https://doi.org/10.1093/occmed/kqy049 (2018).
Sun, Y., Li, Z., Li, J., Li, Z. & Han, J. A healthy dietary pattern reduces lung cancer risk: A systematic review and meta-analysis. Nutrients 8(3), 134. https://doi.org/10.3390/nu8030134 (2016).
Xue, X.-J. et al. Red and processed meat consumption and the risk of lung cancer: A dose-response meta-analysis of 33 published studies. Int. J. Clin. Exp. Med. 7(6), 1542–1553 (2014).
Yang, J. J. et al. Dietary fat intake and lung cancer risk: A pooled analysis. J. Clin. Oncol. 35(26), 3055–3064. https://doi.org/10.1200/jco.2017.73.3329 (2017).
Nuhu, A. A. Bioactive micronutrients in coffee: Recent analytical approaches for characterization and quantification. ISRN Nutr. 2014, 384230. https://doi.org/10.1155/2014/384230 (2014).
Yu, X., Bao, Z., Zou, J. & Dong, J. Coffee consumption and risk of cancers: A meta-analysis of cohort studies. BMC Cancer 11, 96. https://doi.org/10.1186/1471-2407-11-96 (2011).
Wachamo, H. L. Review on health benefit and risk of coffee consumption. Med. Arom. Plants. https://doi.org/10.4172/2155-9821.1000301 (2017).
Xie, Y. et al. Coffee consumption and the risk of lung cancer: An updated meta-analysis of epidemiological studies. Eur. J. Clin. Nutr. 70(2), 199–206. https://doi.org/10.1038/ejcn.2015.96 (2016).
Zhao, L.-G. et al. Coffee drinking and cancer risk: An umbrella review of meta-analyses of observational studies. BMC Cancer 20(1), 101. https://doi.org/10.1186/s12885-020-6561-9 (2020).
Tang, N., Wu, Y., Ma, J., Wang, B. & Yu, R. Coffee consumption and risk of lung cancer: A meta-analysis. Lung Cancer (Amsterdam, Netherlands) 67(1), 17–22. https://doi.org/10.1016/j.lungcan.2009.03.012 (2010).
Galarraga, V. & Boffetta, P. Coffee drinking and risk of lung cancer—A meta-analysis. Cancer Epidemiol. Biomark. Prev. 25(6), 951–957. https://doi.org/10.1158/1055-9965.epi-15-0727 (2016).
Takezaki, T. et al. Diet and lung cancer risk from a 14-year population-based prospective study in Japan: With special reference to fish consumption. Nutr. Cancer 45(2), 160–167. https://doi.org/10.1207/s15327914nc4502_04 (2003).
Gnagnarella, P. et al. Red meat, Mediterranean diet and lung cancer risk among heavy smokers in the COSMOS screening study. Ann. Oncol. 24(10), 2606–2611. https://doi.org/10.1093/annonc/mdt302 (2013).
Narita, S. et al. Coffee consumption and lung cancer risk: The Japan Public Health Center-Based Prospective Study. J. Epidemiol. 28(4), 207–213. https://doi.org/10.2188/jea.JE20160191 (2018).
Park, S. Y. et al. Prospective study of coffee consumption and cancer incidence in non-White populations. Cancer Epidemiol. Biomark. Prev. 27(8), 928–935. https://doi.org/10.1158/1055-9965.epi-18-0093 (2018).
Kudwongsa, W., Promthet, S., Suwanrungruang, K., Phunmanee, A. & Vatanasapt, P. Coffee consumption and lung cancer risk: A prospective cohort study in Khon Kaen Thailand. Asian Pac. J. Cancer Prev. 21(8), 2367–2371. https://doi.org/10.31557/apjcp.2020.21.8.2367 (2020).
Schmit, S. L. et al. Coffee consumption and cancer risk in African Americans from the Southern Community Cohort Study. Sci. Rep. 10(1), 17907. https://doi.org/10.1038/s41598-020-72993-6 (2020).
Seow, W. J., Koh, W. P., Jin, A., Wang, R. & Yuan, J. M. Associations between tea and coffee beverage consumption and the risk of lung cancer in the Singaporean Chinese population. Eur. J. Nutr. 59(7), 3083–3091. https://doi.org/10.1007/s00394-019-02146-7 (2020).
Lukic, M. et al. Coffee consumption and the risk of cancer in the Norwegian Women and Cancer (NOWAC) Study. Eur. J. Epidemiol. 31(9), 905–916. https://doi.org/10.1007/s10654-016-0142-x (2016).
Khan, M. M. et al. Dietary habits and cancer mortality among middle aged and older Japanese living in Hokkaido, Japan by cancer site and sex. Asian Pac. J. Cancer Prev. 5(1), 58–65 (2004).
Chow, W. H. et al. A cohort study of tobacco use, diet, occupation, and lung cancer mortality. Cancer Causes Control 3(3), 247–254. https://doi.org/10.1007/bf00124258 (1992).
Orsini, N., Bellocco, R. & Greenland, S. Generalized least squares for trend estimation of summarized dose–response data. Stata J. 6(1), 40–57 (2006).
Berlin, J. A., Longnecker, M. P. & Greenland, S. Meta-analysis of epidemiologic dose-response data. Epidemiology (Cambridge, Mass) 4(3), 218–228. https://doi.org/10.1097/00001648-199305000-00005 (1993).
Frank, E. H. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis (Springer, 2015).
Santos, A. Coffee and tea consumption and the risk of lung cancer in a population of postmenopausal women. Masters Theses https://doi.org/10.7275/5463398 (2014).
Ong, J. S. et al. Association between coffee consumption and overall risk of being diagnosed with or dying from cancer among >300 000 UK Biobank participants in a large-scale Mendelian randomization study. Int. J. Epidemiol. 48(5), 1447–1456. https://doi.org/10.1093/ije/dyz144 (2019).
Carter, P. et al. Coffee consumption and cancer risk: A Mendelian randomisation study. Clin. Nutr. 41(10), 2113–2123. https://doi.org/10.1016/j.clnu.2022.08.019 (2022).
Hashibe, M. et al. Coffee, tea, caffeine intake, and the risk of cancer in the PLCO cohort. Br. J. Cancer 113(5), 809–816. https://doi.org/10.1038/bjc.2015.276 (2015).
Guertin, K. et al. Coffee consumption and risk of lung cancer in the NIH-AARP diet and health study. FASEB J. 29, 906–928 (2015).
Bae, J. M. et al. Pulmonary tuberculosis and lung cancer risk in current smokers: The Seoul Male Cancer Cohort Study. J. Korean Med. Sci. 28(6), 896–900. https://doi.org/10.3346/jkms.2013.28.6.896 (2013).
Stensvold, I. & Jacobsen, B. K. Coffee and cancer: A prospective study of 43,000 Norwegian men and women. Cancer Causes Control 5(5), 401–408. https://doi.org/10.1007/bf01694753 (1994).
Nomura, A., Heilbrun, L. K. & Stemmermann, G. N. Prospective study of coffee consumption and the risk of cancer. J. Natl. Cancer Inst. 76(4), 587–590. https://doi.org/10.1093/jnci/76.4.587 (1986).
Jacobsen, B. K., Bjelke, E., Kvåle, G. & Heuch, I. Coffee drinking, mortality, and cancer incidence: Results from a Norwegian prospective study. J. Natl. Cancer Inst. 76(5), 823–831 (1986).
Zhu, J. et al. Associations of coffee and tea consumption with lung cancer risk. Int. J. Cancer https://doi.org/10.1002/ijc.33445 (2020).
Wang, M., Qin, S., Zhang, T., Song, X. & Zhang, S. The effect of fruit and vegetable intake on the development of lung cancer: A meta-analysis of 32 publications and 20,414 cases. Eur. J. Clin. Nutr. 69(11), 1184–1192. https://doi.org/10.1038/ejcn.2015.64 (2015).
Tu, H. et al. Different dietary patterns and reduction of lung cancer risk: A large case-control study in the U.S. Sci. Rep. 6, 26760. https://doi.org/10.1038/srep26760 (2016).
Pasquet, R., Karp, I., Siemiatycki, J. & Koushik, A. The consumption of coffee and black tea and the risk of lung cancer. Ann. Epidemiol. 26(11), 757–632. https://doi.org/10.1016/j.annepidem.2016.09.001 (2016).
Mojska, H. & Gielecińska, I. Studies of acrylamide level in coffee and coffee substitutes: Influence of raw material and manufacturing conditions. Roczniki Panstwowego Zakladu Higieny 64(3), 173–181 (2013).
Klaunig, J. E. & Kamendulis, L. M. Mechanisms of acrylamide induced rodent carcinogenesis. Adv. Exp. Med. Biol. 561, 49–62. https://doi.org/10.1007/0-387-24980-x_4 (2005).
Deplanque, G. et al. Caffeine and the G2/M block override: A concept resulting from a misleading cell kinetic delay, independent of functional p53. Int. J. Cancer 94(3), 363–369. https://doi.org/10.1002/ijc.1478 (2001).
Enoma, D., Dokunmu, T. & Obi, P. The anticancer activity of caffeine—A review. Fortune 3, 326–342. https://doi.org/10.26502/acbr.50170077 (2019).
Pauwels, E. K. J. & Volterrani, D. Coffee consumption and cancer risk: An assessment of the health implications based on recent knowledge. Med. Princ. Pract. 30(5), 401–411. https://doi.org/10.1159/000516067 (2021).
Author information
Authors and Affiliations
Contributions
MJ contributed to the conception, literature search, interpretation of the data, and drafting of the manuscript. ASM contributed to the design, statistical analyses, interpretation of the data, and manuscript drafting. AB contributed to the statistical analyses, interpretation of the data, and manuscript drafting. BL contributed to the design, conception, and drafting of the manuscript. AE contributed to the conception, design, statistical analyses, interpretation of the data, and drafting of the manuscript. AE supervised the study. All the authors read and approved the final manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jabbari, M., Salari-Moghaddam, A., Bagheri, A. et al. A systematic review and dose–response meta-analysis of prospective cohort studies on coffee consumption and risk of lung cancer. Sci Rep 14, 14991 (2024). https://doi.org/10.1038/s41598-024-62619-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62619-6
- Springer Nature Limited