Abstract
Previously, we found that patients with estrogen receptor (ER)-positive, HER2-low breast cancer are resistant to neoadjuvant chemotherapy (NACT) and have worse outcomes than those who achieve pathological complete response (pCR) after NACT. This study aimed to investigate the prognosis and influencing factors in these patients. A total of 618 patients with ER-positive breast cancer who received standard thrice-weekly NACT were enrolled, including 411 patients with ER-positive, HER2-low breast cancer. Data on the clinicopathological features of these patients before and after NACT were collected. Univariate and multivariate Cox regression analyses were used to identify the independent factors affecting 5-year disease-free survival (DFS). Among the ER-positive, HER2-low patients, 49 (11.9%) achieved a pCR after NACT. A significant difference in survival was observed between patients with and without residual disease after NACT. Additionally, changes in immunohistochemical markers and tumor stages before and after NACT were found to be significant. According to univariate and multivariate analyses, cN_stage (P = 0.002), ER (P = 0.002) and Ki67 (P = 0.023) expression before NACT were significantly associated with 5-year DFS, while pT_stage (P = 0.015), pN_stage (P = 0.029), ER (P = 0.020) and Ki67 (P < 0.001) levels after NACT were related to 5-year DFS in ER-positive, HER2-low patients with residual disease. Our study suggested that high proliferation, low ER expression and advanced stage before and after NACT are associated with a poor prognosis, providing useful information for developing long-term treatment strategies for ER-positive, HER2-low breast cancer in patients with residual disease in the future.
Similar content being viewed by others
Introduction
Breast cancer, the most frequently diagnosed cancer in women, is characterized by distinct pathological subtypes based on the expression of hormone receptors (HRs) and human epidermal growth factor receptor 2 (HER2)1. ERBB2 can activate multiple tumor-related signaling pathways, leading to rapid disease progression and a poor prognosis2,3,4. In recent years, anti-HER2 agents have significantly improved outcomes for patients with HER2-enriched breast cancer5,6. Furthermore, novel antibody‒drug conjugates (ADCs) have been developed to treat HER2-low (immunohistochemistry (IHC) 1 + or IHC 2 +, fluorescence in situ hybridization (FISH) nonamplified) breast cancer7,8. Based on the analysis of clinical characteristics and chemosensitivity, it has been suggested that HER2-low breast cancer, accounting for 70% of all cases, may represent a subtype distinct from HER2-negative (IHC 0) breast cancer9,10,11,12.
Neoadjuvant chemotherapy (NACT), utilized before surgery, is primarily used for the management of locally advanced breast cancer13. Generally, among various breast cancer subtypes, estrogen receptor (ER)-positive breast cancer exhibits poor responsiveness to chemotherapy14. Additionally, a comprehensive analysis of several large clinical studies demonstrated that HER2-low status is associated with increased resistance to NACT in HR-positive breast cancer patients (13.7% of HER2-low patients vs. 19.8% of HER2-negative patients, P = 0.014)11. Consistent with these findings, our previous study revealed that compared with ER-positive, HER2-negative breast cancer, ER-positive, HER2-low breast cancer displays greater resistance to chemotherapy. The pathological complete response (pCR) rate among ER-positive, HER2-low breast cancer patients was nearly half that of ER-positive, HER2-negative breast cancer patients (P = 0.014)12. Moreover, de Nonneville et al. reported similar associations between ER-positive, HER2-low status and achieving a pCR after NACT (10% of HER2-low patients vs. 16% of HER2-negative patients, p = 0.046)10.
Compared with HER2-negative status, HER2-low status was not found to correlate with a worse long-term prognosis. However, patients with residual disease exhibited a worse prognosis than those who achieved pCR following NACT. The aim of this study was to identify the factors influencing recurrence and metastasis among patients with ER-positive, HER2-low breast cancer after completing standard treatment.
Materials and methods
Patients
Medical records of 618 ER-positive breast cancer patients treated from 1 January 2012 to 31 December 2018 were retrieved from clinical databases of the First Affiliated Hospital of Chongqing Medical University. This retrospective study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (No. 2020-202). All procedures were performed in accordance with relevant guidelines. Inclusion criteria: (I) female; (II) performed neoadjuvant chemotherapy; (III) invasive breast cancer; (IV) ER-positive status; and (V) no anti-tumor treatment before NACT. Exclusion criteria: (I) metastasis at diagnosis; (II) other primary tumors; (III) bilateral breast cancer; and (IV) incomplete data. Finally, a total of 618 eligible patients were included in this study (Fig. 1).
All histological specimens were paraffin-embedded and evaluated by two skilled pathologists. This article does not refer to the privacy of patients, so informed consent was exempted. All data were fully anonymized before we accessed them. The authors were not provided with information that could identify individual participants during or after data collection.
Clinicopathologic analysis
In order to comprehensively analyze the factors influencing the outcomes of patients, we collected the general information (medical history, age, menopausal status, NACT regimens), pre-NACT clinicopathologic information (stage_cT, stage_cN, pre-ER status, pre-progesterone receptor (pre-PgR) status, pre-Ki67 index) and post-NACT clinicopathologic information (stage_pT, stage_pN, post-ER status, post-PR status, post-Ki67 index). Clinical assessments of the breast, including pre-NACT lymph node status (stage_cN), tumor size (stage_cT) depended on MRI or breast ultrasonography. The pathological diagnosis after the operation would provide us with the data of stage_pN and stage_pT. The pre-ER, pre-PgR, HER2 and pre-Ki67 index were evaluated by IHC on the samples obtained by core needle biopsy. While the post-ER, post-PgR and post-Ki67 index were evaluated by IHC on the samples obtained by operation. The HER2-negative group consisted of the breast cancer patients with a completely negative HER2 staining (IHC score of 0), the HER2-low group consisted of the breast cancer patients with low level of HER2 expression (IHC scores of 1 + and 2 + with FISH nonamplified) and the HER2-enriched group consisted of the breast cancer patients with high level of HER2 expression (IHC scores of 3 + and 2 + with FISH amplified) (Fig. 2). Samples with more than 1% of cells positive for ER/PgR expression were identified ER-positive/PgR-positive. The Ki67 index was defined as the percentage of the total number of tumor cells (at least 1000) with nuclear staining over 10 high powered fields (× 40). RECIST criteria is the standard for the clinical response evaluation15.
Representative photomicrographs of HER2 in immunohistochemical sections in breast cancer. (A) HER2-negative (× 100 magnification). (B) HER2-low expression (IHC scores of 1 +) (× 100 magnification). (C) HER2-low expression (IHC scores of 2 + with FISH non-amplified) (× 100 magnification). (D) HER2-enriched (× 100 magnification).
Treatment
The NACT criteria for breast cancer patients were as follows: patients with the advanced stage disease, such as patients with axillary lymph node metastasis or large mass or invasion of skin and chest wall. The patients who had a strong desire to conserve breast after operation but did not meet the indication of breast conserving surgery when diagnosed also met the criteria. NACT was performed in accordance with the local protocol and international guidelines of the current year. The treatments were predominantly anthracycline and taxane. The TEC (docetaxel 75 mg/m2, epirubicin 75 mg/m2, and cyclophosphamide 500 mg/m2) or EC (epirubicin 75 mg/m2, and cyclophosphamide 500 mg/m2) NACT regimens were administered every 3 weeks. After diagnosis, all patients started the first cycle of NACT in a week and received full cycle of NACT regimens (TEC 6 cycles; EC-T 8 cycles) we evaluated the clinical response.
After a series of standard treatments, such as chemotherapy, radiotherapy and surgery, all patients would take up the standard 5-year endocrine therapy (patients who conform the menopause criteria treated with selective non-steroidal aromatase inhibitors, and others treated with estrogen receptor antagonist).
Objective
For all patients enrolled, mastectomy or breast conserving surgery plus axillary lymphadenectomy was the basic surgical treatment after full cycle NACT. Two skilled pathologists blindly and independently diagnosed all resected breast and lymph node specimens. Then, pCR was defined as no residual invasive cancer in the breast or evidence of disease in the axillary lymph nodes (ypT0ypN0) after NACT. In our study, the patients without achieving pCR after NACT were screened for follow-up analysis. Next, disease-free survival (DFS, defined as the time from surgery to any relapse, secondary malignancy, or death from any cause) was the endpoint in this trial.
Statistical methods
Statistical analysis was performed by SPSS (Version 25.0). Categorical variables were compared using the chi-squared test or Fisher’s exact test. Then, follow-up was estimated by using the Kaplan–Meier method and the COX regression analysis was utilized to find the influencing factors of DFS. The two-sided log-rank test was used to compare results between groups. The intolerant abilities of the regression analysis results were assessed by calculating the area under the receiver operating characteristic (ROC) curve. P < 0.05 was defined as statistically significance.
Ethical approval
All procedures were performed in accordance with relevant guidelines. Ethical approvals for the study were obtained from the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (No. 2020-202).
Informed consent
Informed consents were waived due to the retrospective nature of this study. We declared that patient data was maintained with confidentiality and all the process complies with the requirements of the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
Results
Survival comparison of ER-positive breast cancer patients
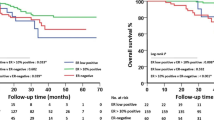
Our study included 618 patients with ER-positive breast cancer who received NACT. Among these patients, 81 were HER2 negative, 411 were HER2-low, and 126 were HER2-enriched (Fig. 3A). The pCR rate of HER2-enriched patients who did not receive targeted therapy was the lowest (10.3%), followed by HER2-low patients (11.9%) (Fig. 3B). The median follow-up periods for HER2-negative, HER2-low, and HER2-enriched patients were 81, 85, and 77 months, respectively. Next, we compared patients’ outcomes based on HER2 status (HER2-negative group vs. HER2-low group vs. HER2-enriched group). As shown in Fig. 3C, HER2-enriched patients had the worst 5-year disease free survival (DFS), while patients with HER2-negative and HER2-low statuses showed no significant difference in outcome (P = 0.440). Among the 411 HER2-low patients, 49 (11.9%) achieved pCR after NACT, whereas the remaining 382 (88.1%) had residual diseases. The outcome comparison between the two groups (pCR group vs. non-pCR group) also showed that patients who achieved pCR had a significant better 5-year DFS (P = 0.020) (Fig. 3D). Consequently, we enrolled the non-pCR patients with poor outcomes in the followed study (n = 362).
Clinicopathological characteristics of patients
We collected pre- and post-NACT comprehensive clinicopathological data of ER-positive, HER2-low patients with residual diseases. As shown in Table 1, a total of 362 patients were identified (mean age 49.0 years (range 20–73 years)) and nearly half of the patients (43.1%) met the menopausal standard. In addition, the comparison of pre- and post-NACT data illustrated a significant reduction in disease stages, with a noticeable decrease in the number of patients with large tumors (25.7–6.4%) and half of the cases having local tumors with a diameter of less than 2 cm after NACT (49.1%). Furthermore, the rate of lymph node metastasis decreased after NACT (75.1–67.4%). In terms of routine pathological indicators of breast cancer, such as ER, PR, and Ki67 status, NACT was found to significantly reduce the proliferation index and 27 patients (7.5%) became ER-negative after chemotherapy.
The pre-NACT factors affecting the outcomes
To begin with, we conducted an analysis of the data collected prior to the NACT and its impact on patient outcomes. Through univariate analysis, the influence of stage_cN (P = 0.002), pre-ER status (P < 0.001), pre-Ki67 index (P = 0.011) on prognosis were studied in the multivariate analysis. We found that stage_cN (P = 0.002), pre-ER status (P = 0.002), pre-Ki67 index (P = 0.023) were independent factors affecting DFS in ER-positive, HER2-low patients with residual diseases (Table 2).
The post-NACT factors affecting the outcomes
Similarly, the analysis of the pathological data from surgical specimens and general information revealed that stage_pT (P = 0.015), stage_pN (P = 0.029), post-ER status (P = 0.010), post-Ki67 index (P < 0.001) were influencing factors for DFS, as determined through univariate and multivariate COX regression analysis (Table 3).
Discussion
At present, neoadjuvant chemotherapy is extensively applied to reduce the disease stage of advanced breast cancer and enable the possibility of breast-conserving treatment. Our study presents retrospective real-world clinicopathological data and prognostic outcomes for patients with ER-positive, HER2-low breast cancer treated with NACT. As reported in previous studies, the proportion of patients with HER2-low breast cancer was greater in the ER-positive group than in the ER-negative group10,16. Additionally, our previous study showed that patients with ER-positive, HER2-low breast cancer exhibit poor sensitivity to NACT. In this cohort, the rates of achieving pCR between HER2-low and HER2-negative patients were significantly different (HER2-low: 11.9%, HER2-negative: 21.0%, P < 0.001)12. These findings are in line with those in other reports, for instance, Baez-Navarro et al.17 reported that patients with HR-positive, HER2-low breast cancer had the worst pCR rate (3.9%). Poor responsiveness to chemotherapy and the relatively high proportion of overall cases result in numerous cases of ER-positive, HER2-low patients with residual disease.
Although the prognosis of HER2-enriched breast cancer has been greatly improved through the application of anti-HER2 targeted therapy, it is still the subtype of ER-positive breast cancer with the worst prognosis18,19. Interestingly, extremely similar DFS rates were observed in the HER2-negative and HER2-low cohorts, which aligns with the findings in previous studies9,10,20,21. Subsequently, we examined the prognosis of HER2-low patients based on NACT efficacy. Consistent with other findings, patients with ER-positive, HER2-low breast cancer who did not achieve pCR after NACT had a significantly greater recurrence risk than those who achieved pCR (P = 0.020)22,23. To further explore the independent factors affecting recurrence in ER-positive, HER2-low patients with residual disease, we conducted a detailed analysis.
Pathological evaluation of primary disease and metastatic lymph nodes is highly important for predicting prognosis. Similarly, the presence of residual tumors reflects the sensitivity of tumors to chemotherapy. In our study cohort, although the pCR rate was only 11.9%, approximately half of the other patients had residual tumors with a maximum diameter of less than 2 cm, which sufficiently showed the importance of NACT in breast conservation strategies. After conducting a comprehensive analysis, we found that DFS was related to post-NACT tumor size (P = 0.015) but not to pre-NACT tumor size (P = 0.837). This suggests that the sensitivity of the tumor to chemotherapy, as indicated by the reduction in tumor size, has a relatively greater impact on prognosis. Notably, lymph node status is closely related to the risk of recurrence. Our prognostic analyses of both pre- and post-NACT data revealed that lymph node status was significantly correlated with recurrence. Likewise, several studies have demonstrated that post-NACT lymph node status has a substantial impact on prognosis24,25,26. For instance, Lee et al. reported that the prognosis in patients without lymph metastasis after NACT was not significantly different regardless of the lymph node status before NACT (P = 0.152)24. Overall, diseases with more advanced stages after NACT challenged the ability of the operator and were more inclined to recurrence.
In addition, the results of pathological analysis after NACT treatment revealed a noticeable reduction in malignancy, including decreases in the Ki67 index and tumor grade. The Ki67 index serves as an indicator of malignant proliferation, which is closely related to local recurrence and distant metastasis of breast cancer27. In our retrospective analysis of ER-positive patients with tissue available for Ki67 analysis, we found that patients with higher pre-NACT Ki67 levels were more likely to achieve pCR than those with lower pre-NACT Ki67 levels14. Furthermore, several studies have reported that a decreased Ki67 index after NACT is associated with favorable clinical outcomes28,29,30,31. Consistent with this, we found that both pre- and post-NACT Ki67 expression levels were associated with DFS, particularly highlighting the increased risk of relapse for patients with residual tumors with higher proliferation activity (P < 0.001). However, some studies have indicated that the post-NACT Ki67 level of residual tumors has independent prognostic significance, whereas the pre-NACT Ki67 level does not have the same significance22,31,32,33. In addition, there was no significant correlation between histological grade and DFS in our study, which may indicate that cancer cells scattered throughout the human body are the primary cause of recurrence34,35. Then, we observed variations in the expression levels of ER and PR after NACT. Heterogeneity is likely the reason for these differences, leading to distinct biological characteristics within the tumor36. Previous studies reported that NACT leads to a transformation between HR positivity and HR negativity, and 26 of the ER-positive patients experienced negative conversion after NACT in our study. Consistent with most findings, a higher expression level of ER was associated with a better prognosis37,38. Moreover, multivariate analysis suggested that even in patients in whom the ER expression level is positive after NACT, if it remains below 10%, the outcomes are similar to those of ER-negative patients.
Despite the extensive efforts made to enhance the rigor and dependability of this research, there are still several limitations. For instance, the number of included patients was restricted due to missing patient historical data or loss to follow-up, and our sample was insufficient for establishing a clinical prediction model and validating it. Furthermore, the characteristics of retrospective studies hinder the improvement of many clinically and pathologically significant features, such as the P53 mutation status. Our collection of the pre- and post-NACT prognostic factors cannot guarantee subjective consistency between the two sets. Additionally, this study involved only Chinese individuals, limiting the generalizability of the results to populations with diverse racial backgrounds. The single-center and retrospective design of the study may have resulted in both selection and information biases. Moreover, with the development of chemotherapy drugs, NACT may have greater efficacy than before, so our research can provide limited guidance for NACT in patients with low HER-2 expression in current clinical practice. It is worth mentioning that the pCR rates for both groups were higher than in our previous study conducted between 2012 and 2016. Consequently, with the development of chemotherapy medications, our investigation may provide limited guidance for the use of NACT in patients with ER-positive, HER2-low breast cancer in present-day clinical practice12.
Conclusion
Previously, we found that ER-positive, HER2-low patients have low sensitivity to NACT; thus, we further analyzed the outcomes in these patients. We collected comprehensive patient data as much as possible to identify the factors affecting recurrence. We found that advanced disease stage, a greater Ki67 index and lower ER expression were associated with a superior five-year recurrence rate in ER-positive, HER2-low patients with residual disease after NACT. Patients with a high risk of recurrence will be candidates for new ADC drugs in the future.
Data availability
The datasets generated and/or analysed during the current study are available in the figshare repository, [https://doi.org/10.6084/m9.figshare.22294111].
References
Cancer Genome Atlas, N. Comprehensive molecular portraits of human breast tumours. Nature 490(7418), 61–70. https://doi.org/10.1038/nature11412 (2012).
Cronin, K. A. et al. Population-based estimate of the prevalence of HER-2 positive breast cancer tumors for early stage patients in the US. Cancer Invest. 28(9), 963–968. https://doi.org/10.3109/07357907.2010.496759 (2010).
Wang, S. L. et al. Triple-negative or HER2-positive status predicts higher rates of locoregional recurrence in node-positive breast cancer patients after mastectomy. Int. J. Radiat. Oncol. Biol. Phys. 80(4), 1095–1101. https://doi.org/10.1016/j.ijrobp.2010.03.038 (2011).
Slamon, D. J. et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785), 177–182. https://doi.org/10.1126/science.3798106 (1987).
Swain, S. M. et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 372(8), 724–734. https://doi.org/10.1056/NEJMoa1413513 (2015).
Geyer, C. E. et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 355(26), 2733–2743. https://doi.org/10.1056/NEJMoa064320 (2006).
Okamoto, H. et al. Pharmacokinetics of trastuzumab deruxtecan (T-DXd), a novel anti-HER2 antibody-drug conjugate, in HER2-positive tumour-bearing mice. Xenobiotica 50(10), 1242–1250. https://doi.org/10.1080/00498254.2020.1755909 (2020).
Modi, S. et al. Antitumor activity and safety of Trastuzumab Deruxtecan in patients with HER2-low–expressing advanced breast cancer: Results from a phase Ib study. J. Clin. Oncol. 38(17), 1887–1896. https://doi.org/10.1200/JCO.19.02318 (2020).
Horisawa, N. et al. The frequency of low HER2 expression in breast cancer and a comparison of prognosis between patients with HER2-low and HER2-negative breast cancer by HR status. Breast Cancer 29(2), 234–241. https://doi.org/10.1007/s12282-021-01303-3 (2022).
de Nonneville, A. et al. Pathological complete response rate and disease-free survival after neoadjuvant chemotherapy in patients with HER2-low and HER2-0 breast cancers. Eur. J. Cancer 176, 181–188. https://doi.org/10.1016/j.ejca.2022.09.017 (2022).
Denkert, C. et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 22(8), 1151–1161. https://doi.org/10.1016/S1470-2045(21)00301-6 (2021).
Tang, L. et al. Efficacy evaluation of neoadjuvant chemotherapy in patients with HER2-low expression breast cancer: A real-world retrospective study. Front Oncol. 12, 999716. https://doi.org/10.3389/fonc.2022.999716 (2022).
van der Hage, J. A. et al. Preoperative chemotherapy in primary operable breast cancer: Results from the European organization for research and treatment of cancer trial 10902. J. Clin. Oncol. 19(22), 4224–4237. https://doi.org/10.1200/JCO.2001.19.22.4224 (2001).
Tang, L., Shu, X. & Tu, G. Exploring the influencing factors of the pathologic complete response in estrogen receptor-positive, HER2-negative breast cancer after neoadjuvant chemotherapy: A retrospective study. World J. Surg. Oncol. 20(1), 27. https://doi.org/10.1186/s12957-022-02492-7 (2022).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 45(2), 228–247. https://doi.org/10.1016/j.ejca.2008.10.026 (2009).
de Moura, L. L. et al. HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer. Breast Cancer Res. Treat. 190(1), 155–163. https://doi.org/10.1007/s10549-021-06365-7 (2021).
Baez-Navarro, X. et al. HER2-low breast cancer and response to neoadjuvant chemotherapy: A population-based cohort study. Pathology 56(3), 334–342. https://doi.org/10.1016/j.pathol.2023.10.022 (2024).
Gianni, L. et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 13(1), 25–32. https://doi.org/10.1016/S1470-2045(11)70336-9 (2012).
Schneeweiss, A. et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 24(9), 2278–2284. https://doi.org/10.1093/annonc/mdt182 (2013).
Tarantino, P. et al. Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur. J. Cancer 163, 35–43. https://doi.org/10.1016/j.ejca.2021.12.022 (2022).
Schettini, F. et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 7(1), 1. https://doi.org/10.1038/s41523-020-00208-2 (2021).
van der Hage, J. A. et al. Preoperative chemotherapy in primary operable breast cancer: Results from the European organization for research and treatment of cancer trial 10902. J. Clin. Oncol. 19(22), 4224–4237. https://doi.org/10.1200/JCO.2001.19.22.4224 (2001).
Pan, Z. et al. Concurrent radiotherapy and intrathecal methotrexate for treating leptomeningeal metastasis from solid tumors with adverse prognostic factors: A prospective and single-arm study. Int. J. Cancer 139(8), 1864–1872. https://doi.org/10.1002/ijc.30214 (2016).
Lee, S. B. et al. Prognosis according to clinical and pathologic lymph node status in breast cancer patients who underwent sentinel lymph node biopsy alone after neoadjuvant therapy. PLoS One 16(5), e0251597. https://doi.org/10.1371/journal.pone.0251597 (2021).
Gerber, B. et al. Pathological response in the breast and axillary lymph nodes after neoadjuvant systemic treatment in patients with initially node-positive breast cancer correlates with disease free survival: An exploratory analysis of the GeparOcto 23 trial. Cancers (Basel) 14(3), 521. https://doi.org/10.3390/cancers14030521 (2022).
Wu, S. G. et al. Using the lymph node ratio to evaluate the prognosis of stage II/III breast cancer patients who received neoadjuvant chemotherapy and mastectomy. Cancer Res. Treat. 47(4), 757–764. https://doi.org/10.4143/crt.2014.039 (2015).
Polley, M.-Y.C. et al. An international Ki67 reproducibility study. J. Natl. Cancer Inst. 105(24), 1897–1906. https://doi.org/10.1093/jnci/djt306 (2013).
Tokuda, E. et al. Differences in Ki67 expressions between pre-and post-neoadjuvant chemotherapy specimens might predict early recurrence of breast cancer. Hum. Pathol. 63, 40–45. https://doi.org/10.1016/j.humpath.2017.02.005 (2017).
Tanei, T. et al. Prognostic significance of Ki67 index after neoadjuvant chemotherapy in breast cancer. Eur. J. Surg. Oncol. 37(2), 155–161. https://doi.org/10.1016/j.ejso.2010.10.009 (2011).
Ellis, M. J. et al. Aromatase expression and outcomes in the P024 neoadjuvant endocrine therapy trial. Breast Cancer Res. Treat. 116(2), 371–378. https://doi.org/10.1007/s10549-008-0161-8 (2009).
Garufi, G. et al. Development of a nomogram for predicting pathological complete response in luminal breast cancer patients following neoadjuvant chemotherapy. Ther. Adv. Med. Oncol. 15, 17588359221138656. https://doi.org/10.1177/17588359221138657 (2023).
Chen, C. et al. Decrease in the Ki67 index during neoadjuvant chemotherapy predicts favorable relapse-free survival in patients with locally advanced breast cancer. Cancer Biol. Med. 16(3), 575–586. https://doi.org/10.20892/j.issn.2095-3941.2018.0423 (2019).
Matsubara, N. et al. Survival outcome and reduction rate of Ki-67 between pre-and postneoadjuvant chemotherapy in breast cancer patients with non-Pcr. Breast Cancer Res. Treat. 147(1), 95–102. https://doi.org/10.1007/s10549-014-3084-6 (2014).
Luskin, M. R. et al. Targeting minimal residual disease: A path to cure?. Nat. Rev. Cancer. 18(4), 255–263. https://doi.org/10.1038/nrc.2017.125 (2018).
Bystricky, B. & Mego, M. Circulating tumor cells in breast cancer patients. Neoplasma 63(1), 18–29. https://doi.org/10.4149/neo_2016_003 (2016).
Roulot, A. et al. Tumoral heterogeneity of breast cancer Hétérogénéité tumorale des cancers du sein. Ann. Biol. Clin. (Paris) 74(6), 653–660. https://doi.org/10.1684/abc.2016.1192 (2016).
Miglietta, L. et al. A prognostic model based on combining estrogen receptor expression and Ki-67 value after neoadjuvant chemotherapy predicts clinical outcome in locally advanced breast cancer: Extension and analysis of a previously reported cohort of patients. Eur. J. Surg. Oncol. 39(10), 1046–1052. https://doi.org/10.1016/j.ejso.2013.06.024 (2013).
Kasami, M. et al. Comparison of estrogen receptor, progesterone receptor and Her-2 status in breast cancer pre- and post-neoadjuvant chemotherapy. Breast 17(5), 523–527. https://doi.org/10.1016/j.breast.2008.04.002 (2008).
Acknowledgements
The authors wish to thank all the study participants, research staff and students who participated in this work.
Funding
The key research and development project of Chongqing’s technology innovation and application development special big health field to Shengchun Liu (Project approval number: CSTC2021jscx-gksb-N0027). The First Clinical College Clinical medicine first-class discipline construction project to department of Breast and Thyroid Surgery (CYYY-BSYJSCXXW-202322).
Author information
Authors and Affiliations
Contributions
Conceptualization, L.T. and S.L.; Data curation, X.S.; Formal analysis, X.S.; Investigation, L.T.; Methodology, L.T., H.Y. and S.L.; Resources, L.T., Y.J. and H.Y.; Software, L.T.; Supervision, H.Y. and S.L.; Writing—original draft, L.T., X.S., L.J.; Writing—review & editing, L.J., L.T. and S.L.; Revision, L.T. and L.J.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, L., Jiang, L., Shu, X. et al. Prognosis and influencing factors of ER-positive, HER2-low breast cancer patients with residual disease after neoadjuvant chemotherapy: a retrospective study. Sci Rep 14, 11761 (2024). https://doi.org/10.1038/s41598-024-62592-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62592-0
- Springer Nature Limited