Abstract
This study compares the effectiveness of Conbercept and Aflibercept in treating neovascular age-related macular degeneration (nAMD). Conducted at the First Affiliated Hospital of Chongqing Medical University's Ophthalmology Department (May 2020–May 2023), this prospective study enrolled 159 nAMD patients. Participants were randomly divided into two groups: one receiving 0.5 mg Conbercept and the other 2 mg Aflibercept intravitreal injections. Over 12 months, the study, employing a Treat-and-Extend (T&E) regimen, assessed Best-Corrected Visual Acuity (BCVA), Central Retinal Thickness (CRT) changes and injection frequency. Of the 159 patients, 137 (149 eyes) completed the study. No significant age difference was found between the groups (P = 0.331). After 12 months, BCVA improved similarly in both groups (Conbercept: 52.8 ± 18.9, Aflibercept: 52.0 ± 19.7 letters; P = 0.820). CRT reduction was also comparable (Conbercept: 246.3 ± 82.8 µm, Aflibercept: 275.9 ± 114.3 µm; P = 0.079). Injection frequencies averaged 6.9 ± 0.7 (Conbercept) and 6.7 ± 0.7 (Aflibercept; P = 0.255). Subtype analysis revealed Type 1 MNV had higher baseline BCVA and lower CRT, with more frequent injections compared to other types. Both Conbercept and Aflibercept are clinically similar in efficacy for nAMD, with the T&E regimen proving therapeutically effective and potentially reducing patient costs. Anti-VEGF treatment efficacy varies across nAMD subtypes, indicating a potential benefit in tailored treatments for specific subtypes.
Clinical trial registration number NCT05539235 (Protocol Registration and Results System).
Similar content being viewed by others
Introduction
Macular degeneration, the primary cause of severe, irreversible vision loss in those over 55, is commonly termed age-related macular degeneration (AMD)1. It manifests in two forms: dry AMD and the more rapidly vision-impairing wet AMD. In wet AMD, accelerated by vascular endothelial growth factor (VEGF) and placental growth factor (PLGF), choroidal neovascularization (CNV) occurs in the macula. This leads to retinal exudation, hemorrhage, and ultimately central vision loss2, hence its alternative name, neovascular AMD (nAMD).
In clinical practice, nAMD diagnosis integrates fundoscopic exams with imaging techniques like fluorescein fundus angiography (FFA) and indocyanine green angiography (ICGA), previously gold standards. Recent high-resolution tomography, including optical coherence tomography (OCT) and OCT angiography (OCTA), offers detailed, three-dimensional views of the retinal and choroidal vasculature3, revealing new vessel size, shape, and location.Based on the classification of the anatomical levels of neovascularization, the International Consensus on Naming for Age Related Macular Degeneration Research Group has formed a consensus on the naming of nAMD4. The consensus suggests using macular neovascularization (MNV) instead of CNV, and categorizing nAMD into three types based on the anatomical location of MNV: Type 1 (MNV between the retinal pigment epithelium and Bruch membrane, including polypoidal choroidal vasculopathy), Type 2 (MNV from the choroidal capillaries, growing under the retinal nerve fiber layer), and Type 3 (MNV from the retina, extending to the outer retina and potentially leading to pigment epithelial detachment and CNV). Besides these, mixed-type lesions exhibit features of multiple types."
Internationally, nAMD treatments include drug therapy, surgical options, and photodynamic therapy, with anti-VEGF drug therapy as the recommended first-line approach5. This category mainly involves receptor fusion proteins like Conbercept and Aflibercept.
Conbercept, developed in China, is a recombinant fusion protein with high affinity for all VEGF-A, VEGF-B, VEGF-C, PLGF subtypes, and a longer half-life6. It uniquely includes VEGFR-2's domain 4, enhancing its efficacy7,8, and has shown 50 times higher VEGF affinity than bevacizumab9. In the PHOENIX trial10, Conbercept improved nAMD visual outcomes with less frequent dosing.
Aflibercept, a fully humanized fusion protein combining VEGFR-1 and -2 domains with IgG1 Fc fragment, binds to all VEGF-A, VEGF-B, PLGF subtypes11, offering superior affinity and half-life over monoclonal antibodies.Its bi-monthly administration matches the effectiveness of monthly ranibizumab in maintaining visual acuity in new nAMD cases12. Despite studies comparing monoclonal antibodies and receptor fusion proteins13,14,15,16, there's limited research directly comparing receptor fusion proteins like Conbercept and Aflibercept in nAMD. This study addresses this gap, evaluating their efficacy differences using a Treatment and Extend (T&E) regimen in nAMD."
Methods
Study design
This 12-month, randomized, phase IV trial at The First Affiliated Hospital of Chongqing Medical University compared the efficacy of 0.5mg Conbercept and 2mg Aflibercept in treating nAMD. Participants were equally randomized to each treatment group. Written informed consent was secured before examinations. The study adhered to the Declaration of Helsinki and received approval from the Institutional Review Board of Chongqing Medical University, the First Affiliated Hospital (Approval No. 20199401).
Sample size determination
This study is a randomized controlled trial comparing the efficacy of Conbercept group versus Aflibercept group. The primary outcome measure is the change in Best Corrected Visual Acuity (BCVA) among the subjects. Based on the preliminary experiment results at the sixth month, the mean change in BCVA for the Conbercept group was 5.6 ± 5.85 letters, and for the Aflibercept group, it was 1.2 ± 8.16 letters. Setting a two-sided α at 0.05 and a power of 90%, the sample size was calculated using the following formula:
As a result, n = 55 cases per group were determined. Considering a 1:1 randomization for treatment and control groups, each group requires 55 subjects. Accounting for a 15% dropout and refusal rate, each group should ultimately include at least 65 participants, totaling a minimum of 130 subjects for the study.
Examination and enrolment
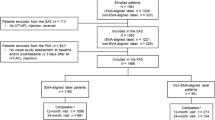
In this prospective study at the First Affiliated Hospital of Chongqing Medical University (May 2020 to May 2023), patients were randomly assigned to either the Conbercept or Aflibercept group. Inclusion criteria were age ≥ 50 years and confirmed nAMD via mydriatic slit lamp exam, OCT, OCTA, FFA, and ICGA, with no prior nAMD treatments. Only patients able to follow up for at least 12 months were included. Exclusion criteria encompassed coexisting fundus diseases (like retinal detachment, pathological myopia, proliferative diabetic retinopathy, glaucoma, retinal vascular diseases, macular membrane, macular hole), past internal eye surgery or fundus laser treatment, severe ocular trauma history, significant refractive medium clouding hindering fundus observation, and inability to maintain regular treatment follow-up or follow-up less than 12 months.
BCVA BCVA was recorded for all eligible patients pre-treatment, and at 1, 3, 6 months, and 1 year post-treatment, using the ETDRS chart for final assessment.
OCT and OCTA Examinations utilized the CIRRUS HD-OCT 5000 with pupil dilation to approximately 7mm.
FFA and ICGA Examinations used the Heidelberg Spectralis HRA angiography system from Germany.
Treatment
After routine preoperative exams and assessments, informed consent was obtained from patients and their families. Both groups underwent standardized intravitreal drug injections. According to different groups, the patients were given intravitreal injection of Conbercept (0.5mg) or Aflibercept ( 2 mg).
The patients in both groups adopted the T&E protocol17, namely, the initial treatment stage was three load injections, Conbercept (0. 5mg) or Aflibercept (2mg) was injected once every four weeks for three times, and the treatment interval was extended to eight weeks after that. Following the fourth injection, the subsequent treatment interval will be determined based on the patient's BCVA, CRT, OCT, and fundus photography. The inter-treatment interval will not be shorter than 8 weeks and shall not exceed 16 weeks. Treatment interval extension: an extension of the treatment interval is warranted when the following conditions are concurrently satisfied: (1) Vision stability with a < 5-letter decrease in sequential BCVA assessments; (2) Retinal thickness stability, with CRT increment of < 50μm; (3) Absence of persistent intraretinal fluid (IRF) and subretinal fluid (SRF), or stable persistent retinal fluid over three consecutive visits, attributed to long-standing anatomical changes or fibrosis without active CNV; (4) No new neovascularization; and (5) No new macular hemorrhage. Treatment interval retention: the established interval may be upheld when all specified parameters are met: (1) Visual acuity decline of < 5 letters; (2) OCT imaging reveals no new retinal fluid accumulation or a decrease in fluid volume relative to the last visit; (3) Absence of new neovascularization; and (4) No new macular hemorrhage. Treatment interval reduction: treatment intervals should be decreased upon the occurrence of any of the following conditions: (1) A reduction in visual acuity of ≥ 5 letters (or 1 line); (2) Recurrent or persistent retinal fluid associated with a decrease in vision; (3) Development of new neovascularization; (4) Onset of new macular hemorrhage; (5) Continued activity of the lesion following an attempt to extend the treatment interval.
Follow-up
Outpatient follow-ups recorded BCVA, CRT, intraocular pressure, and total anti-VEGF treatments at 12 months, at baseline, and at 1, 3, 6, and 12 months of treatment. Fundus lesions were monitored before and after treatment. MNV classification was based on baseline OCTA, with neovascular regression assessed at each follow-up. The study also observed ocular and systemic complications related to drug injections, including subconjunctival hemorrhage, endophthalmitis, vitreous hemorrhage, and retinal detachment.
Masking
Researcher A logged into the central randomization system to obtain a random number, and finally formed a random allocation table. They were randomly divided into the Conbercept group and the Aflibercept group in a 1:1 ratio without blinding. Doctor B consistently measured and recorded all patients' BCVA. Additionally, Doctor C from the Photographic Reading Centre evaluated and classified all OCT, OCTA, FFA, and ICGA images. Both Doctors B and C, as well as the patients, remained unaware of the group allocations.
Statistical analyses
Data from both groups were analyzed using SPSS22.0. The independent sample t-test was applied to measurement data, presented as mean ± standard deviation. Enumeration data were evaluated using the Chi-square test and expressed as proportions. A P-value < 0.05 was considered statistically significant.
Ethical approval and consent to participate
The Ethics Committee of Clinical Research of The First Affiliated Hospital of Chongqing Medical University approved and reviewed the studies involving human participants(Grant number, 20190401). The patients/participants provided their written informed consent to participate in this study.
Consent for publication
The patient’s written and informed consent was obtained.
Results
The study enrolled 159 patients (May 2020 to May 2023), including 79 patients in the Conbercept group and 80 patients in the Aflibercept group, with 137 (149 eyes) completing the 12-month follow-up, 82 eyes received Conbercept and 67 Aflibercept. 9 participants in the Conbercept group and 13 in the Aflibercept group voluntarily withdrew from the study, citing personal reasons. Baseline characteristics (age, gender, BCVA, CRT, OCTA MNV classification) showed no significant differences between groups, detailed in Table 1.
BCVA
At 12 months, no significant differences in BCVA or mean changes were observed between groups, detailed data are shown in Fig. 1a and Table 2. 50.0% of Conbercept and 64.2% of Aflibercept patients showed > 5 letter BCVA improvement (Fig. 2). Type 1 MNV had a higher BCVA baseline (57.1 ± 16.0 letters) than other types (P < 0.05). At 12 months, no significant differences in mean changes were observed between four types, detailed data are shown in Table 3. After grouping all patients according to MNV subtype, an analysis was conducted on baseline BCVA and changes in BCVA (Fig. 1c, Fig. 1d, Table 3).
(a) Mean BCVA (letter Score) at baseline and at each follow-up time until month 12 in the two groups; (b) Mean CRT (μm) at baseline and at each follow-up time until month 12 in the two group; (c) BCVA baseline (letter Score) of four types of MNV; (d) BCVA variation from month-12 to baseline (letter Score) of four types of MNV; (e) CRT baseline of four types of MNV; f:CRT variation from month-12 to baseline (letter Score) of four types of MNV. “**” means P < 0.01, “***” means P < 0.001.
At month-12, the proportion of patients with stable or improved vision in Conbercept group is higher than Aflibercept group, while the proportion of patients with reduced vision is lower than that Aflibercept group (Fig. 2).
CRT
During the 12-month treatment period, both groups of patients exhibited a continuous decrease in CRT compared to baseline levels (Fig. 1b). At the 1st and 6th months, the Conbercept group showed a significantly greater reduction in CRT compared to the Aflibercept group, as indicated in Table 3. After grouping all patients according to MNV subtype, an analysis was conducted on CRT baseline and changes in CRT (Fig. 1e,f, Table 3). At month-12 the CRT baseline for Type 1 MNV was 325.6 ± 128.1 µm, significantly lower than that of other types of MNV (P < 0.05)(Table 3).
Injection times
At the 12-month follow-up, the average number of intravitreal injections in the Conbercept group and the Aflibercept group, respectively, with no statistically significant difference between the two groups (p = 0.255). Detailed data of the intervals for the last injection are shown in Fig. 3b.
Over the course of 52 weeks (12 months), the distribution of injection frequencies for 6, 7, and 8 injections in the Conbercept group was 30 people (36.59%), 39 people (47.56%), and 13 people (15.85%), respectively, while in the Aflibercept group, it was 32 people (47.76%), 25 people (37.31%), and 10 people (14.93%). There was no statistically significant difference between the groups in individuals who received 6 injections (P = 0.36).
Injection frequency across various subtypes was systematically analyzed. The mean injection count for Type 1 was 7.2 ± 0.6, significantly surpassing Type 2's 6.4 ± 0.5 and the mixed type's 6.6 ± 0.7 (P < 0.05). Notably, Type 3's mean injection count stood at 7.2 ± 0.8, exceeding that of Type 2 (P < 0.05), as detailed in Fig. 3a and Table 3.
Postoperative complications
In this study, a total of 5 cases of subconjunctival hemorrhage were observed (4 cases in the Conbercept group and 1 case in the Aflibercept group). These cases did not receive any specific treatment, and the symptoms resolved spontaneously after observation. There were no postoperative complications such as elevated intraocular pressure, vitreous hemorrhage, intraocular inflammation, or retinal detachment observed in either group of patients.
Discussion
This study, the first prospective analysis over a year comparing Conbercept and Aflibercept in a real-world context, reveals their similar efficacy and safety for nAMD. With no significant differences in injection frequency or safety, both are viable nAMD treatment options.
Conbercept, a 143 kDa fusion protein, combines VEGFR1Ig2, VEGFR2Ig3, and VEGFR2Ig4 of human VEGF receptors with IgG1 Fc10, binding all VEGFA, VEGF-B, VEGF-C, and PlGF subtypes18,19. Aflibercept, at 115 kDa, fuses VEGFR1Ig2 and VEGFR2Ig3 with IgG1 Fc20,21, targeting VEGF-A, VEGF-B, and PlGF 22.In rabbit vitreous, Conbercept has a 4.2-day half-life, longer than Aflibercept's 3.63 days23,24. Conbercept's unique inclusion of VEGFR2's fourth domain enhances VEGF affinity and lowers extracellular matrix adhesion, potentially boosting its anti-angiogenic effectiveness9. Despite these differences, comparative studies in nAMD show no clear superiority of either drug in improving visual acuity, anatomical outcomes, or treatment frequency.In studies utilizing the 3 + T&E regimens (injection intervals extended or shortened by 4 weeks), the COCOA study, a pioneering multicentric prospective Chinese trial, compared Conbercept 3 + T&E (treatment intervals adjusted by 4 weeks) against a 3 + PRN regimen in 501 patients. By week 48, more than half of the participants had received 6 or fewer injections. In the 3 + T&E cohort, visual acuity improved by an average of 8.6 letters, with about 62% of patients having ≥ 12-week intervals and 42% extending to 16 weeks20.The ALTAIR Study, administering 2 mg Aflibercept intravitreally, observed an average improvement of 8.4 letters in visual acuity at 52 weeks and an average of 6.9 injections over 12 months, accompanied by a mean CRT reduction of 126.1 μm. Approximately 49.6% of the patients in this study extended the final injection interval to ≥ 12 weeks, and 40.7% to 16 weeks 25. Moreover, in both AURORA and PHOENIX studies assessing Conbercept and the VIEW1 and VIEW2 studies evaluating Aflibercept, the comparative assessment in terms of BCVA improvement, CRT reduction, and injection frequency did not demonstrate clear dominance of either treatment10,26,27.
Drug clearance from the vitreous hinges on molecular size. Due to their larger molecular weight, these drugs minimally penetrate the blood-ocular barrier, ensuring high selectivity and few systemic adverse reactions, confirming their safety. This study finds no significant difference between the two drugs in efficacy, injection frequency, or adverse reactions, consistent with previous research. While Conbercept's additional structural domain might suggest greater efficacy, this doesn't necessarily translate to clinical significance.As AMD is influenced by various factors, patients' responses to anti-VEGF treatments can vary, with some experiencing reduced effectiveness over time.Notably, despite these treatments, AMD-related blindness incidences continue to increase annually28.
A U.S. study of 98,821 AMD eyes undergoing intravitreal anti-VEGF therapy showed a general visual acuity decline over four years29. This decrease is partly due to these treatments' inability to address factors like age, genetics, and environmental and cardiovascular influences. AMD's Geographic Atrophy (GA) grows about 0.33 mm/year, with a 38% incidence rate five years after starting anti-VEGF therapy30. Normally, ocular VEGF is essential for RPE survival and choroidal endothelial cell function31, so anti-VEGF treatments might increase GA risk32, possibly diminishing or even worsening visual acuity over time. This could explain the converging efficacy in long-term therapy. However, studies suggest no direct link between anti-VEGF dosing or injection frequency and macular atrophy rates in nAMD33.
After a year, visual acuity gains in both groups were 4.18 ± 8.80 and 5.25 ± 11.23 letters, lower than previous CATT study results.
Contributing factors include lower baseline visual acuity (Conbercept: 48.59 ± 18.33, Aflibercept: 46.79 ± 14.70 letters) compared to the 2012–2015 average of 53.1 letters29. This suggests more advanced disease at treatment onset, typically offering limited visual improvement.Also, about 46% of patients had Type 2 MNV, which, despite initial responsiveness to treatment, often leads to fibrotic scarring, potentially impairing long-term vision34. The high proportion of Type 2 cases may limit overall BCVA improvement. Furthermore, the T&E regimen in this study, with 4-week extension and shortening intervals (minimum 8 weeks, maximum 16 weeks), lacked adaptability for patients needing more frequent treatments, potentially leading to lesser visual improvement and not fully addressing specific needs. The fixed interval scheme could explain why more patients extended their last treatment interval to ≥ 12 weeks compared to prior studies.
In a subtype-based analysis, Type 1 neovascularization patients, with higher initial BCVA, needed more frequent injections but didn't show significant visual improvement over other types. Typically, Type 1 patients have higher baseline BCVA and better vision improvement, requiring more treatments35. This MNV, located beneath the RPE, preserves visual acuity by not damaging the RPE and maintaining its barrier and transport functions, reflected in this study's lower average CRT for Type 1. However, the deeper neovascularization location reduces the concentration of anti-VEGF medication reaching it to just 11.9% of that in the vitreous cavity23. Consequently, maintaining anti-VEGF treatment efficacy over time is challenging, and patients with this type often experience recurrent episodes, needing more injections for effective management.
In this study, Type 2 neovascularization incidence (51.7%) was higher than in past CATT studies (19.2–23.7%)36. This could reflect regional differences due to the study's single-center nature. Type 2 patients had lower baseline BCVA but needed fewer treatments, and their visual improvement was not significantly inferior to other subtypes.
Type 2 MNV, penetrating and located above the RPE, often presents with poorer vision.
Yet, its accessibility to anti-VEGF medications leads to better treatment response and fewer injections37, however, there are studies indicating that Type 2 neovascularization may require a higher frequency of treatment interventions35. This increased treatment frequency in Type 2 patients could be attributed to their higher susceptibility to fibrotic scarring38, it can impede improvements in visual acuity and the efficacy of treatment, potentially leading to more frequent injections in clinical practice. The prevalence of Type 2 in our study may have influenced average baseline BCVA, BCVA improvement, CRT reduction, and injection numbers. Type 3 patients, typically with poorer baseline vision, required more frequent injections, and experienced BCVA decline by 12 months. This subtype is characterized by significant RPE damage, with 6 patients (50%) already exhibiting atrophic changes in the macular RPE at the onset of treatment. By the 12th month, 2 more patients developed macular RPE atrophy. Previous studies have also indicated a higher propensity for Geographic Atrophy (GA) in patients with Type 3 neovascularization30. Mixed subtype patients had the poorest baseline BCVA and needed fewer injections, though the small sample size limits generalization.Considering these subtype differences, tailored treatments are essential, especially for unstable types like 1 and 3, where shorter interval dosing might be more effective. Future therapies should thus be subtype-specific for nAMD.
Limitations
This single-center study may limit the generalizability of population characteristics. Its small sample size, especially for Type 3 and mixed subtypes, could bias data analysis. The one-year follow-up might be insufficient for comparing long-term drug efficacy. Fixed treatment intervals, not tailored to patient conditions, may have affected anti-VEGF therapy effectiveness. Additionally, many patients had poor baseline visual acuity, suggesting advanced disease stages, which could influence OCT and OCTA subtype-based observations and outcomes.
Conclusion
Clinically, the efficacy of the two drugs may be similar. The T&E regimen, effective therapeutically, could also lessen patient economic burden. Responsiveness to anti-VEGF treatments differs among nAMD subtypes, suggesting future treatments should be customized by subtype for optimal patient benefit.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
References
Deng, Y. et al. Age-related macular degeneration: Epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes Dis. 9(1), 62–79 (2022).
Lipecz, A. et al. Microvascular contributions to age-related macular degeneration (AMD): From mechanisms of choriocapillaris aging to novel interventions. Geroscience 41(6), 813–845 (2019).
de Carlo, T. E. et al. A review of optical coherence tomography angiography (OCTA). Int. J. Retina Vitreous 1, 5 (2015).
Spaide, R. F. et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: Consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology 127(5), 616–636 (2020).
Flaxel, C. J. et al. Age-related macular degeneration preferred practice pattern®. Ophthalmology 127(1), 1 (2020).
Jia, H. et al. A randomized, controlled trial of treat-and-extend vs. pro re nata regimen for neovascular age-related macular degeneration. Front. Med. 9, 852519 (2022).
Stewart, M. W. & Rosenfeld, P. J. Predicted biological activity of intravitreal VEGF Trap. Br. J. Ophthalmol. 92(5), 667–668 (2008).
Shinkai, A. et al. Mapping of the sites involved in ligand association and dissociation at the extracellular domain of the kinase insert domain-containing receptor for vascular endothelial growth factor. J. Biol. Chem. 273(47), 31283–31288 (1998).
Zhang, M. et al. Recombinant anti-vascular endothelial growth factor fusion protein efficiently suppresses choridal neovasularization in monkeys [J]. Mol. Vis. 14, 37–49 (2008).
Liu, K. et al. Conbercept for treatment of neovascular age-related macular degeneration: Results of the randomized phase 3 Phoenix Study. Am. J. Ophthal. 197, 156–167 (2019).
Sarwar, S. et al. Fusion proteins: Aflibercept (VEGF Trap-Eye). Dev. Ophthalmol. 55, 282–294 (2016).
Schmidt-Erfurth, U. et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: Ninety-six-week results of the VIEW studies. Ophthalmology 121(1), 193–201 (2014).
Cui, J. et al. Comparison of effectiveness and safety between conbercept and ranibizumab for treatment of neovascular age-related macular degeneration. A retrospective case-controlled non-inferiority multiple center study. Eye (Lond., Engl.) 32(2), 391–399 (2018).
Wang, X. et al. Comparison of efficacy and safety between conbercept and ranibizumab in neovascular age-related macular degeneration: A meta-analysis of randomized controlled trials. Ophthalm. Res. 65(2), 140–151 (2022).
Ugurtay, O., Kockar, A. & Sengul, E. A. Real life data of treat and extend intravitreal ranibizumab and aflibercept therapy in wet age-related macular degeneration patients: 3-Year results. Kor. J. Ophthalmol.: KJO 35(4), 280–286 (2021).
Jin, K. W. et al. Long-term outcomes of ranibizumab vs aflibercept for neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Sci. Rep. 11(1), 14623 (2021).
Group T-A-E R F M O N A-R M D C E C. Chinese experts consensus of treat-and-extend regimen for management of neovascular age-related macular degeneration by intravitreal injection of aflibercept. Chin. J. Exp. Ophthalmol. 39(7), 577–84.
Kniggendorf, V., Dreyfuss, J. L. & Regatieri, C. V. Age-related macular degeneration: A review of current therapies and new treatments. Arquivos Brasileiros de Oftalmologia 83(6), 552–561 (2020).
Zhang, J. et al. Conbercept for patients with age-related macular degeneration: A systematic review. BMC Ophthalmol. 18(1), 142 (2018).
Zhao, M. W. 3+T&E regimen VS 3+PRN regimen efficiency of Conbercept for nAMD: 1-year result from COCOA, A prospective, multicenter and randomized controlled trial. The 23rd Euretina Congress (Amsterdam, 2023).https://doi.org/10.1016/S0140-6736(18)31550-2
Fogli, S. et al. Clinical pharmacology of intravitreal anti-VEGF drugs. Eye (Lond., Engl.) 32(6), 1010–1020 (2018).
García-Quintanilla, L. et al. Pharmacokinetics of intravitreal anti-VEGF drugs in age-related macular degeneration. Pharmaceutics 11(8), 1 (2019).
Park, S. J. et al. Intraocular pharmacokinetics of intravitreal vascular endothelial growth factor-Trap in a rabbit model. Eye (Lond. Engl.) 29(4), 561–568 (2015).
Li, H. et al. Pharmacokinetics of a long-lasting anti-VEGF fusion protein in rabbit. Exp. Eye Res. 97(1), 154–159 (2012).
Ohji, M. et al. Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-Week findings from ALTAIR : A randomized controlled trial. Adv. Therapy 37(3), 1173–1187 (2020).
Heier, J. S. et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 119(12), 2537–2548 (2012).
Li, X. et al. Safety and efficacy of conbercept in neovascular age-related macular degeneration: Results from a 12-month randomized phase 2 study: AURORA study. Ophthalmology 121(9), 1740–1747 (2014).
Johnston, R. L. et al. UK age-related macular degeneration electronic medical record system (AMD EMR) users group report IV: Incidence of blindness and sight impairment in ranibizumab-treated patients. Ophthalmology 123(11), 2386–2392 (2016).
Khanani, A. M. et al. SIERRA-AMD: A retrospective, real-world evidence study of patients with neovascular age-related macular degeneration in the United States. Ophthalmol. Retina 4(2), 122–133 (2020).
Grunwald, J. E. et al. Incidence and growth of geographic atrophy during 5 years of comparison of age-related macular degeneration treatments trials. Ophthalmology 124(1), 1 (2017).
Saint-Geniez, M. et al. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc. Natl. Acad. Sci. USA 106(44), 18751–18756 (2009).
Gemenetzi, M., Lotery, A. J. & Patel, P. J. Risk of geographic atrophy in age-related macular degeneration patients treated with intravitreal anti-VEGF agents. Eye (Lond., Engl.) 31(1), 1–9 (2017).
Spooner, K. L. et al. Macular atrophy incidence and progression in eyes with neovascular age-related macular degeneration treated with vascular endothelial growth factor inhibitors using a treat-and-extend or a Pro Re Nata Regimen: Four-year results of the MANEX Study. Ophthalmology 127(12), 1663–1673 (2020).
Mathis, T. et al. Characterisation of macular neovascularisation subtypes in age-related macular degeneration to optimise treatment outcomes. Eye (Lond., Engl.) 37(9), 1758–1765 (2023).
Izquierdo-Serra, J., Martin-Pinardel, R., Moll-Udina, A., et al. Macular neovascularization type influence on anti-VEGF intravitreal therapy outcomes in age-related macular degeneration. Ophthalmol. Ret. (2023).
Martin, D. F. et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. New Engl. J. Med. 364(20), 1897–1908 (2011).
Mrejen, S. et al. Long-term visual outcomes for a treat and extend anti-vascular endothelial growth factor regimen in eyes with neovascular age-related macular degeneration. J. Clin. Med. 4(7), 1380–1402 (2015).
Daniel, E. et al. Development and course of scars in the comparison of age-related macular degeneration treatments trials. Ophthalmology 125(7), 1037–1046 (2018).
Acknowledgements
The authors thank their collaborators for their help in surgical procedures, data collection, data analysis, and article writing.
Funding
The National Natural Science Foundation of China financially supported this study (Grant number, 81670881).
Author information
Authors and Affiliations
Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, H., Ju, H., Lu, J. et al. Comparative study on the efficacy of Conbercept and Aflibercept in the treatment of neovascular age-related macular degeneration. Sci Rep 14, 11997 (2024). https://doi.org/10.1038/s41598-024-62536-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62536-8
- Springer Nature Limited