Abstract
The increasing prevalence of heart failure (HF) in ageing populations drives demand for echocardiography (echo). There is a worldwide shortage of trained sonographers and long waiting times for expert echo. We hypothesised that artificial intelligence (AI)-enhanced point-of-care echo can enable HF screening by novices. The primary endpoint was the accuracy of AI-enhanced novice pathway in detecting reduced LV ejection fraction (LVEF) < 50%. Symptomatic patients with suspected HF (N = 100, mean age 61 ± 15 years, 56% men) were prospectively recruited. Novices with no prior echo experience underwent 2-weeks’ training to acquire echo images with AI guidance using the EchoNous Kosmos handheld echo, with AI-automated reporting by Us2.ai (AI-enhanced novice pathway). All patients also had standard echo by trained sonographers interpreted by cardiologists (reference standard). LVEF < 50% by reference standard was present in 27 patients. AI-enhanced novice pathway yielded interpretable results in 96 patients and took a mean of 12 min 51 s per study. The area under the curve (AUC) of the AI novice pathway was 0.880 (95% CI 0.802, 0.958). The sensitivity, specificity, positive predictive and negative predictive values of the AI-enhanced novice pathway in detecting LVEF < 50% were 84.6%, 91.4%, 78.5% and 94.1% respectively. The median absolute deviation of the AI-novice pathway LVEF from the reference standard LVEF was 6.03%. AI-enhanced novice pathway holds potential to task shift echo beyond tertiary centres and improve the HF diagnostic workflow.
Similar content being viewed by others
Introduction
Due to rapidly ageing societies, the prevalence of heart failure (HF) is increasing worldwide1. Advances in HF therapeutics have significantly improved patient outcomes2. Delayed initiation of guideline-recommended therapies for HF can lead to unnecessary hospitalisations and deaths3,4. Therefore, early diagnosis and subsequent treatment are critical5,6,7,8,9,10.
Echocardiography, the primary method for evaluating the HF subtype, guiding HF treatment decisions and counselling, requires trained sonographers and cardiologists to acquire and interpret images11. Unfortunately, the lack of highly trained sonographers and cardiologists is a rate-limiting step in the HF diagnostic care pathway. This delays diagnosis and treatment access, deprives patients of the benefits of proven therapies and leads to worse patient outcomes12,13,14,15.
Established guidelines have demonstrated a difference in treatment strategies between HF with reduced ejection fraction (< 40%) and HF with preserved ejection fraction (≥ 50%)16, while studies and post hoc analyses suggested that patients with HF with mildly reduced ejection fraction (40–49%) benefit from therapies similar to their counterparts with reduced ejection fraction17,18,19,20. Hence, for purposes of initiation of treatment, knowledge of the presence of left ventricular systolic dysfunction, defined as left ventricular ejection fraction (LVEF < 50%) is vital. Moreover, the first HF presentation for most patients is usually to a primary care clinic, where without appropriate evaluation tools, diagnosis may be missed and treatment delayed14,21. With the increased uptake of point-of-care (POC) ultrasound devices in primary care clinics22,23, there is opportunity to improve HF care using artificial intelligence (AI).
Previous studies demonstrated that untrained novices could acquire echocardiographic images of sufficient quality after only limited training24,25. However, image interpretation was the rate-limiting step, requiring trained cardiologists or sonographers. Advances in deep learning have shown the potential of automated algorithms to interpret cardiac structural and functional information effectively26,27,28. Combined with POC echocardiographic devices, these algorithms could empower novices to perform echocardiographic examinations, thus overcoming barriers to access, allowing prompt initiation of treatment and reducing delays in the HF diagnostic pathway29,30.
Therefore, this study investigated the diagnostic accuracy of an end-to-end package by novice and AI for detecting left ventricular systolic dysfunction, without intervention from trained sonographers or cardiologists.
Methods
Study design, study population and sample size
In this cross-sectional study, all patients aged ≥ 21 years with a at least one HF symptom (pedal oedema, New York Heart Association (NYHA) II-III effort limitation, orthopnoea, breathlessness), were eligible for inclusion. Patients were recruited over a 6-month period.
Eligible patients presenting to the cardiac imaging laboratory at the National Heart Centre Singapore for physician-ordered clinical echocardiogram for investigation or follow up for HF were approached for recruitment. Recruited patients underwent standard cart-based sonographer-performed, cardiologist-reported echocardiogram as per clinical practice, and novice-performed AI-supported POC echocardiogram.
Pregnant women were excluded. All patients provided written informed consent before participation. Ethics approvals were obtained from the local institutional review committee (Singhealth Centralised Institutional Review Board) for this study, and this study conformed to the ethical guidelines in the Declaration of Helsinki.
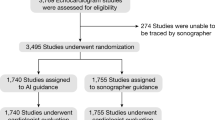
The sample size was calculated based on previously published standard deviations31 and the assumption that the study population’s mean left ventricular ejection fraction (LVEF) was 50%, with an equivalence region of 5%32. The sample size required for non-inferiority with 90% statistical power was at least 79 patients. We considered potential difficulties in image acquisition, hence 100 patients were recruited for this study.
Novice selection and training
A clinical study coordinator (she/her) without prior sonography experience was invited to participate. She was trained to identify cardiac structures and key landmarks on ultrasound using the 2015 American Society of Echocardiography Chamber Quantification33. She was then given the POC ultrasound device (EchoNous Kosmos) and was attached to the cardiac imaging laboratory under the supervision of sonographers for 2 weeks. In the first week, she observed the sonographers in image acquisition. She was allowed hands-on practice with guidance in acquiring seven standard views (apical 4,3 and 2 chambers and parasternal long, short axis at the mitral, mid and apical levels) for LVEF assessment. In the second week, after independently acquiring the seven standard images using the POC ultrasound device in 10 patients, she started recruitment.
POC device and software
This study used the Torso-One cardiac probe and the Kosmos Bridge tablet with image processing capabilities. The AI TRIO, the background AI image acquisition program, provides real-time image guidance on fanning, rotating, and repositioning the probe to achieve better image quality. It contains anatomic labelling of the structures in the image and grades the image in real time, so users know via a green light indicator when they have acquired an adequate picture.
Us2.ai, an AI-driven vendor-independent cardiac ultrasound image processing platform, was used for image interpretation. This software’s external and United States Food and Drug Administration (FDA) validation results were published previously31. Briefly, Us2.ai is a deep learning-based workflow which automates the entire process by rapidly analysing the digital imaging and communications in medicine (DICOM) files of a patient’s echocardiographic exam without the need for human intervention, from the classification of views, identifying cardiac phases to giving readouts of relevant cardiac measurements such as the LVEF.
During the study, the novice attempted to capture seven standard views (apical 4,3 and 2 chambers and parasternal long, short axis at the mitral, mid and apical levels) for LVEF interpretation. The LVEF readout by the AI followed the priority dependent on image quality, biplane followed by monoplane.
Study outcomes
The primary outcome of this study was the accuracy of AI-enhanced novice-performed POC echocardiogram and AI-interpreted LVEF (AI-enhanced novice pathway) to detect a reduced LVEF < 50%. The cardiologist-reported LVEF based on the sonographer-acquired traditional cart-based clinical echocardiogram was the reference standard.
Secondary outcomes were the yield and the learning curve of a novice performing POC cardiac ultrasound. The yield was defined as the proportion of POC ultrasound exams with an AI-measurable LVEF on any of the views using the number of cart-based cardiologist reported LVEFs as the denominator. The learning curve was assessed by the time taken for image capture for each study over the study duration. The time taken for image capture was defined as the difference between the first and last image recorded for each study.
Statistical analysis
Continuous variables with a normal distribution were presented as means ± one standard deviation. Variables with a non-normal distribution were expressed as median and interquartile range. Binary variables were expressed as a number count and proportion. The accuracy by area under the curve (AUC), sensitivity, specificity, positive predictive and negative predictive values were tabulated. The agreement between AI-enhanced novice pathway and reference standard LVEF was assessed by percent agreement and Cohen’s Kappa. We also calculated the mean absolute error (MAE), root mean square error, median absolute and relative (percentage) deviation, and the Pearson correlation coefficient, r, for AI- enhanced novice pathway versus reference standard measurements.
The learning curve of the novice was calculated by tabulating the rolling cumulative average of scan durations by chronological patient order and fitting a Lowess curve of time taken to complete a scan against the chronological patient number recruited across time. The learning rates were tabulated for the first 20, next 20 to 40, and 40 to 60 patients, using Wright’s model34 where Y = aXb; Y = the cumulative average time per study, X = the cumulative number of studies performed, a = time required for the first study and b = slope of the function when plotted on log–log paper, which is also the log of the learning rate/log of 2.
All analyses were performed using Stata statistical software (version 14.0, Stata Corporation, College Station, Texa ds, USA). All statistical analyses were conducted at the significance level of 0.05 and all tests were two-tailed whenever appropriate.
Ethical approval
Ethics approvals were obtained from the local institutional review committee (Singhealth Central Institutional Review Board) for this study, and this study conformed to the ethical guidelines in the Declaration of Helsinki.
Results
Demographics
Out of 100 participants, 56 (56%) were men, and the average age of the cohort was 61.2 ± 15.0 years. Forty (40%) patients had a history of HF, while others had symptoms suggestive of HF, including peripheral edema (54%), orthopnea (9%), and breathlessness (48%). Ninety-five patients were in sinus rhythm, four were in atrial fibrillation, and one had a paced rhythm (Table 1). Ninety-six studies in the AI-enhanced novice pathway had sufficient image quality for AI interpretation to produce an LVEF readout; hence the yield was 96%.
Performance of AI-enhanced novice pathway in predicting LVEF < 50%
The sensitivity, specificity, positive predictive and negative predictive values of the AI-enhanced novice pathway in detecting LVEF < 50% were 84.6%, 91.4%, 78.5% and 94.1% respectively. The AUC of the AI-enhanced novice pathway in detecting LVEF < 50% was 0.880 (95% CI 0.802, 0.958). The agreement between the reference standard and AI-enhanced novice pathway in the detection of LVEF < 50% was percent agreement 0.895, Cohen Kappa 0.742 ± 0.101.
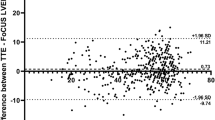
The correlation between the reference standard LVEF and the AI-novice LVEF was 0.713. When comparing actual LVEF values by the AI-enhanced novice pathway and reference standard, the median absolute deviation was 6.03%, with a median relative percentage deviation of 10.9%. The root mean square error of the AI-enhanced novice pathway against the reference standard was 8.31, with a mean absolute error (MAE) of 7.74. The absolute difference in LVEF between reference standard and AI-enhanced novice pathway, and subjects incorrectly classified at the individual patient level is shown in Fig. 1.
Learning curve of novice
The mean time required for the layperson to perform an AI-guided handheld echocardiogram was 12 min and 51 s. The learning rate for the first 20 patients was 60.2%, 30.4% for patients 20 to 40, and plateaued at 3.5% for patients 40 to 60 (Fig. 2).
There was no significant difference in accuracy by AUC in predicting LVEF < 50% between the first 40 and next 60 patients (p = 0.652). There was also no difference in the LVEF mean absolute deviation between both pathways in the first 40 and next 60 patients (p = 0.468).
Discussion
This study demonstrated that an AI-enhanced novice with no prior sonography background could successfully perform LVEF assessments for patients with suspected or current HF. The novice obtained sufficient AI-interpretable images in 96% of studies performed with a high AUC of more than 0.80. This is important as we view HF across a spectrum of ejection fraction, with individualised therapies for each ejection fraction category35. Apart from diagnosing HF, the ejection fraction is an important marker for therapy initiation. Our study timely demonstrates that a new workflow can significantly increase accessibility to LVEF measurements.
The AI-enhanced novice pathway offers a more sensitive and objective alternative for physicians to confidently initiate appropriate guideline directed medical therapy based on LVEF, especially in primary care clinics, before confirmatory echocardiograms are performed. In contrast NT-proBNP, a common initial diagnostic test for patients presenting with HF symptoms, is confounded by common comorbidities such as atrial fibrillation, renal dysfunction and obesity36, and is unable to give the LVEF readout for therapy initiation. Given its high negative predictive value, the AI-enhanced novice pathway is also a valuable screening tool for at-risk Stage B heart failure patients with comorbid conditions, but do not exhibit symptoms of heart failure yet.
Prior studies, which largely compared differences in measurements between manual readers and automated AI based on the same sonographer-acquired images, reported an MAE range in LVEF to be 6–10%37 and median percentage deviation of automated AI LVEF was approximately 6%26. Although the novice acquired images in this study, the MAE was favourably comparable at 6.03% with a median percentage deviation of 10.9%.
We also looked at the performance of the novice in the real-world situation where patients of different habitus and acoustic windows were recruited, instead of a small controlled patient cohort in the previous study by Schneider et al.25. We achieved comparable LVEF agreement of 0.713 between AI and human expert (versus correlation coefficient r 0.79–0.92) and a similar incidence (96% versus 91%) of diagnostic quality images. Further information on the learning curve of the novice also provides background to determine adequacy of training before novices reach their optimal scan time for image acquisition.
Additionally with the help of AI, a relatively short time was taken to perform the scans (approximately 12 min); hence novices using POC ultrasound devices to acquire images for LVEF assessment in suspected HF patients is a feasible, implementable service in busy primary care settings. Use of AI in echocardiogram reduces scanning time and allows almost instantaneous reporting of results. The LVEF is both diagnostic and prognostic in assessing a wide variety of cardiac conditions, such as coronary artery disease, arrhythmias, syncope, suspected HF etc38. This AI-enhanced novice pathway holds potential for new workflows to improve resource allocation and increase diagnostic throughput without sacrificing accuracy. This is invaluable in addressing real-world shortage of echocardiogram services39 by increasing accessibility to LVEF assessments in conditions where only LVEF is required for screening and therapy initiation (e.g. non-valvular atrial fibrillation, palpitations, monitoring during chemotherapy) and in turn improving wait times for conditions where detailed sonographer echocardiogram assessments are required (e.g. valvular heart disease, infective endocarditis). Integrating both the AI-enhanced novice pathway and traditional sonographer-cart-based workflows will enable cardiac imaging laboratories to meet the increasing demands for cardiac imaging better and allow physicians to provide better, timely care for cardiac patients.
Limitations
This study has multiple limitations. First, our sample of a small Asian population could limit generalisation as real-world patients with HF. Next, our data consists of patients with sinus rhythm (94%) – it would be helpful to assess the system under stress with arrhythmias. Finally, it is unclear if suboptimal image quality in 4% of our population is attributable to end-user or AI, as only one AI-novice pair was included in this study. Additionally, as a significant proportion of reference LVEF was reported by visual estimation, we did not manage to compare the LV volumes reported by both pathways.
Conclusion
In conclusion, our study affirms AI's role in empowering a novice to perform POC ultrasound to obtain accurate diagnostic information to diagnose HF. This augments the supply of echocardiogram services by minimising the sonographic expertise required in the traditional cart-based setting. Future studies involving a larger population and integrating this AI-enhanced novice pathway are underway.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AUC:
-
Area under the curve
- DICOM:
-
Digital imaging and communications in medicine
- FDA:
-
United States food and drug administration
- HF:
-
Heat failure
- LVEF:
-
Left ventricular ejection fraction
- MAE:
-
Mean absolute error
- NYHA:
-
New York Heart Association
- NT-proBNP:
-
N-terminal pro–B-type natriuretic peptide
- POC:
-
Point-of-care
References
van Riet, E. E. S. et al. Epidemiology of heart failure: The prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur. J. Heart Fail. 18, 242–252 (2016).
Tromp, J. et al. A systematic review and network meta-analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 10, 73–84 (2022).
Shen, L. et al. Accelerated and personalized therapy for heart failure with reduced ejection fraction. Eur. Heart J. 43, 2573–2587 (2022).
Tromp, J. & Voors, A. A. Heart failure medication: Moving from evidence generation to implementation. Eur. Heart J. 43, 2588–2590 (2022).
Tsao, C. W. et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 6, 678–685 (2018).
Gerber, Y. et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern. Med. 175, 996–1004 (2015).
Tromp, J. et al. Post-discharge prognosis of patients admitted to hospital for heart failure by world region, and national level of income and income disparity (REPORT-HF): A cohort study. Lancet Glob. Health 8, e411–e422 (2020).
Buddeke, J. et al. Mortality after hospital admission for heart failure: Improvement over time, equally strong in women as in men. BMC Public Health 20, 1–10 (2020).
Taylor, C. J. et al. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000–2017: Population based cohort study. BMJ https://doi.org/10.1136/bmj.l223 (2019).
Bytyçi, I. & Bajraktari, G. Mortality in heart failure patients. Anadolu Kardiyoloji Dergisi 15, 63–68. https://doi.org/10.5152/akd.2014.5731 (2015).
Feigenbaum, H. Re: “American Society of echocardiography recommendations for quality echocardiography laboratory operations”. J. Am. Soc. Echocardiogr. 24, 930 (2011).
Waring, L., Miller, P. K., Sloane, C. & Bolton, G. Charting the practical dimensions of understaffing from a managerial perspective: The everyday shape of the UK’s sonographer shortage. Ultrasound 26, 206–213. https://doi.org/10.1177/1742271X18772606 (2018).
NHS Diagnostic Waiting Times and Activity Data NHS England and NHS Improvement 2 NHS Diagnostic Waiting Times and Activity Data January 2022 Monthly Report (2022).
Kwok, C. S. et al. Missed opportunities in the diagnosis of heart failure: Evaluation of pathways to determine sources of delay to specialist evaluation. Curr. Heart Fail. Rep. 19, 247–253 (2022).
van Riet, E. E. S. et al. Prevalence of unrecognized heart failure in older persons with shortness of breath on exertion. Eur. J. Heart Fail. 16, 772–777 (2014).
Heidenreich, P. A. et al. 2022 AHA/ACC/hfsa guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 79, e263–e421 (2022).
Cleland, J. G. F. et al. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: An individual patient-level analysis of double-blind randomized trials. Eur. Heart J. 39, 26 (2018).
Reiner, Z., Capatano, A., De Backer, G. & Graham, I. ESC/EAS guidelines for the management of dyslipidemias. Eur. Heart J. https://doi.org/10.1093/eurheartj/ehr158 (2011).
Lund, L. H. et al. Heart failure with mid-range ejection fraction in CHARM: Characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur. J. Heart Fail. 20, 1230–1239 (2018).
Solomon, S. D. et al. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur. Heart J. 37, 455–462 (2016).
Kahn, M. et al. Primary care heart failure service identifies a missed cohort of heart failure patients with reduced ejection fraction. Eur. Heart J. 43, 405–412 (2022).
Sorensen, B. & Hunskaar, S. Point-of-care ultrasound in primary care: A systematic review of generalist performed point-of-care ultrasound in unselected populations. Ultrasound J. 11, 1–29 (2019).
Fraleigh, C. D. M. & Duff, E. Point-of-care ultrasound: An emerging clinical tool to enhance physical assessment. Nurse Pract. 47, 14 (2022).
Francis, J. R. et al. single-view echocardiography by nonexpert practitioners to detect rheumatic heart disease: A prospective study of diagnostic accuracy. Circ. Cardiovasc. Imaging 14, e011790–e011790 (2021).
Schneider, M. et al. A machine learning algorithm supports ultrasound-naïve novices in the acquisition of diagnostic echocardiography loops and provides accurate estimation of LVEF. Int. J. Cardiovasc. Imaging 37, 577–586 (2021).
Zhang, J. et al. Fully automated echocardiogram interpretation in clinical practice: Feasibility and diagnostic accuracy. Circulation 138, 1623–1635 (2018).
Tromp, J. et al. Automated interpretation of systolic and diastolic function on the echocardiogram: A multicohort study. Lancet Digit. Health 4, e46–e54 (2022).
Tromp, J. et al. A formal validation of a deep learning-based automated workflow for the interpretation of the echocardiogram. Nat. Commun. 13, 1–9 (2022).
Smallwood, N. & Dachsel, M. Point-of-care ultrasound (POCUS): Unnecessary gadgetry or evidence-based medicine?. Clin. Med. (Lond.) 18, 219–224 (2018).
Hashim, A. et al. The utility of point of care ultrasonography (POCUS). Ann. Med. Surg. (Lond.) 71, 102982 (2021).
Tromp, J. et al. A formal validation of a deep learning-based automated workflow for the interpretation of the echocardiogram. Nat. Commun. https://doi.org/10.1038/s41467-022-34245-1 (2022).
Kusunose, K. et al. Reduced variability of visual left ventricular ejection fraction assessment with reference images: The Japanese Association of Young Echocardiography Fellows multicenter study. J. Cardiol. 72, 74–80 (2018).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 28, 1-39.e14 (2015).
Smunt, T. L. Learning curve analysis. In Encyclopedia of Production and Manufacturing Management (ed. Swamidass, P. M.) 353–360 (Springer US, 2000). https://doi.org/10.1007/1-4020-0612-8_504.
Docherty, K. F. et al. The four pillars of HFrEF therapy: Is it time to treat heart failure regardless of ejection fraction?. Eur. Heart J. Suppl. 24, L10–L19 (2022).
Januzzi, J. L. & Myhre, P. L. The challenges of NT-proBNP testing in HFpEF: Shooting arrows in the wind∗. Heart Fail. 8, 382–385 (2020).
Thavendiranathan, P. et al. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: Application to patients undergoing cancer chemotherapy. J. Am. Coll. Cardiol. 61, 77–84 (2013).
Doherty, J. U. et al. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2019 appropriate use criteria for multimodality imaging in the assessment of cardiac structure and function in nonvalvular heart disease: A report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions. Soc. J. Am. Coll. Cardiol. 73, 488–516 (2019).
American Society of Echocardiography News. J. Am. Soc. Echocardiogr. President's Message Where are the sonographers? 15, 25A. https://doi.org/10.1016/S0894-7317(02)70041-2 (2002).
Acknowledgements
The PANES-HF is supported by a research grant from AstraZeneca. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
WTH had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors critically reviewed and contributed to the intellectual content of the manuscript. WTH, JT, CC and CSPL were involved with the conception of the study. Initial data preparation was done by WTH. WTH and TK performed the statistical analyses, supported by JT, FH, YH and CC and drafted the manuscript. CSPL, SHE, ASYL, CTN and LLYT provided the clinical expertise. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
JT is supported by the National University of Singapore Start-up grant, the tier 1 grant from the ministry of education and the CS-IRG New Investigator Grant from the National Medical Research Council; has received consulting or speaker fees from Daiichi-Sankyo, Boehringer Ingelheim, Roche diagnostics and Us2.ai, co-owns patent US-10702247-B2. CC reports philanthropic research grants from Lee Foundation Singapore and consulting/speaker fees from Boehringer Ingelheim, Sanofi Aventis and Us2.ai. YMH and FH are employees of Us2.ai. CSPL is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from NovoNordisk and Roche Diagnostics; has served as consultant or on the Advisory Board/ Steering Committee/ Executive Committee for Alleviant Medical, Allysta Pharma, Amgen, AnaCardio AB, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Darma Inc., EchoNous Inc, Eli Lilly, Impulse Dynamics, Intellia Therapeutics, Ionis Pharmaceutical, Janssen Research & Development LLC, Medscape/WebMD Global LLC, Merck, Novartis, Novo Nordisk, Prosciento Inc, Radcliffe Group Ltd., Recardio Inc, ReCor Medical, Roche Diagnostics, Sanofi, Siemens Healthcare Diagnostics and Us2.ai; and serves as co-founder & non-executive director of Us2.ai. All other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, W., Koh, T., Tromp, J. et al. Point-of-care AI-enhanced novice echocardiography for screening heart failure (PANES-HF). Sci Rep 14, 13503 (2024). https://doi.org/10.1038/s41598-024-62467-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62467-4
- Springer Nature Limited