Abstract
This network meta-analysis (NMA) aimed to compare the efficacy of five non-pharmacological interventions, including exercise intervention (EI), nutritional intervention (NI), respiratory intervention (RI), psychological intervention (PSI), and integrated physical intervention (IPI), on functional status, quality of life, muscle strength, pulmonary function, and safety in patients with amyotrophic lateral sclerosis (ALS). We searched nine databases, PubMed, Cochrane, Embase, Scopus, Web of Science, CNKI, CBM, WFPD, and CSTJ, for randomized controlled trials of ALS patients. The primary outcome was the Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-R) score. Secondary outcomes were the McGill Quality of Life Questionnaire (McGill-QoL), Medical Research Council (MRC)-sum score, Forced Vital Capacity (FVC), and Fatigue Severity Scale (FSS) score. This NMA was conducted using random-effect models to calculate the standard mean difference (SMD) and 95% confidence interval (CI). All types of supplemental interventions had some benefit for patients with ALS. EI had a beneficial effect on the ALSFRS-R score (SMD: 1.01; 95% CI 0.50–1.51), FVC (SMD: 0.78; 95% CI 0.02–1.55), McGill-QoL (SMD: 0.71 95% CI 0.33–1.08), and MRC (SMD: 1.11; 95% CI 0.08–2.14). RI had a beneficial effect on the ALSFRS-R score (SMD: 0.83 95% CI 0.12–1.55). IPI had a beneficial effect on the ALSFRS-R score (SMD: 0.65 95% CI 0.06–1.24). NI had a beneficial effect on the McGill-QoL (SMD: 0.63 95% CI 0.02–1.23). The current study findings support a multimodal intervention strategy with an emphasis on EI for slowing disease progression in patients with ALS.
Similar content being viewed by others
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease that affects the front part of the spinal cord, motor nerve nuclei in the brainstem, the pyramidal tract, and motor neurons in the upper and lower limbs1. ALS results in muscle weakness, myasthenia gravis, myofascial fibrillation, medullary palsy, pyramidal signs, dyspnea, weight loss, and cardiac arrhythmia2,3. Respiratory failure, malnutrition, and aspiration pneumonia are the leading causes of death in ALS patients4.
ALS has remained an incurable disease5. After being diagnosed with ALS, patients should seek multidisciplinary care teams to control their symptoms, according to the most recent ENFS and EALSC working group guidelines6. Three ALS medications, Riluzole, Edaravone and Relyvrio, can be prescribed to slow disease progression and increase life expectancy7,8,9. However, Edaravone has been linked to serious side effects such as bruising, headaches, dermatitis, and gait disturbances10, while Riluzone is associated with increased liver enzymes, vomiting, weakness, and stomach upset11. Relyvrio has several adverse effects, including diarrhea, stomach pain, nausea, cold symptoms, upper respiratory tract infection, tiredness, drooling a lot, and dizziness12.
Along with medications, ALS patients can choose non-pharmacological interventions such as respiratory intervention, psychological, integrated physical intervention, nutritional intervention, and exercise intervention. All of these can help patients improve their functional ability, stabilize their mood, improve their quality of life, and slow the progression of ALS pathology13,14,15. To the best of our knowledge, however, no previous studies compared the efficacy of the five interventions on the clinical symptoms of ALS patients.
Although choosing a safe and effective intervention is an important factor to consider as part of a multidisciplinary care plan, the relative efficacy of the plans is unclear16,17. As a result, healthcare providers have struggled to find the optimal treatment plan for their patients. The purpose of this study was to compare the efficacy of supplemental interventions for ALS patients.
Materials and methods
Program and registration
This NMA was conducted under the guidelines on preferred reporting items for systematic reviews and meta-analysis (PRISMA). The protocol for this systematic review and network meta-analysis has been registered within PROSPERO (CRD42023459965).
Inclusion and exclusion criteria
Inclusion criteria were: (1) 18 years or older with definite or probable ALS as determined by the EI Escorial criteria or its revised version18,19; (2) respiratory treatment (RI) that included respiratory muscle training and inspiratory muscle training; psychological treatment (PSI) that included meditation training and non-meditative mindfulness intervention; integrated physical intervention (IPI) that included repetitive transcranial magnetic stimulation; or massage, acupuncture, and pharyngeal electrical stimulation; nutritional treatment (NI) that included supplementation of branched-chain amino acids, L-threonine, vitamin E, creatine, CoQ10, milk whey proteins, acetyl‐L‐carnitine, hypercaloric enteral nutrition, and high-calorie foods; exercise treatment (EI) that included muscle exercise, resistance exercise, a monitored exercise program, aerobic exercise, endurance exercise, and a combination of aerobic exercise and strength program; control (CON) that included receiving standard care, usual care, no treatment, sham expiratory training, sham inspiratory training, sham repetitive transcranial magnetic stimulation, continuous care, placebo, or usual stretching; (3) outcomes included any of the following measurements: Amyotrophic Lateral Sclerosis Functional Rating Scale Revised (ALSFRS-R) score, McGill quality of life questionnaire (McGill-QoL), and 36-item Short Form Survey (SF-36). Secondary outcomes included Forced Vital Capacity (FVC), Maximal Inspiratory Pressure (MIP), Medical Research Council (MRC)-sum score, Manual Muscle Testing (MMT), Maximum Voluntary Isometric Contraction (MVIC), Lower-Extremity Muscle Strength, Fatigue Severity Scale (FSS) score, and Visual Analogy Scale for Fatigue (VAS-F) score; and (4) the study design of a randomized controlled trial. Exclusion criteria were conference abstracts, reviews, syntheses, letters, guidelines, non-randomized controlled trials, studies with incomplete data, and non-English publications.
Search strategies
Two independent researchers searched nine databases: PubMed, Cochrane, Embase, Scopus, Web of Science, CNKI, CBM, WFPD, and CSTJ, from inception to June 2023. We conducted the online search for the articles published between the periods using the following logic: (1) "amyotrophic lateral sclerosis" or "ALS"; (2) "respiratory treatment," "psychology treatment," "respiratory treatment," "psychology treatment," "physiotherapy," "nutritional treatment," and "exercise treatment"; and (3) "randomized controlled trial" or "RCT" or "randomized" or "placebo.” The search strategy was developed first for the PubMed database and then adapted for the other sources. Experts in ALS and intervention then reviewed these sources to make sure they were complete and accurate. The detailed search strategy is presented in Supplementary Appendix 1.
Study selection
The Endnote 21 software was used to select eligible articles, exclude duplicates, and filter out articles that did not meet the inclusion criteria. Two researchers independently read the titles and abstracts, initially selected potential studies, screened the full texts, and chose the studies that ultimately met the requirements. A third investigator resolved any discrepancies, if necessary. Finally, eligible studies were documented and recorded using a PRISMA flow chart.
Data extraction
Two independent investigators extracted the data. A third investigator was called in to help resolve data discrepancies and provide a consensus for decision-making. The data were then recorded by author and year, number of participants (male and female ratio), age, treatments, and outcome index.
Risk-of-bias assessment
The Cochrane risk-of-bias assessment tool (Review Manager 5.4) was used to evaluate selection bias, implementation bias, measurement bias, follow-up bias, reporting bias, and other potential biases. The quality of studies was graded by two evaluators and classified as “low, high, or unclear risk of bias.” If a disagreement arose, a third evaluator stepped in to resolve the issue. The risk of bias is presented in Supplementary Appendix 2.
Statistical analyses
NMA was performed using STATA 14.0 MP software to incorporate an indirect comparison of non-pharmacological interventions for ALS. Because the data in this NMA are all continuous variables with different scales for each outcome indicator, the standard mean difference (SMD) and 95% confidence interval (CI) were calculated. A p-value ≤ 0.05 was considered statistically significant for between-group differences. Consistency and inconsistency tests between studies were used to improve study stability. A funnel plot was used to detect publication bias. The effectiveness of the interventions was ranked using the surface under the cumulative ranking curve (SUCRA) and mean rank, which considers all possible rankings and uncertainties in intervention effects; a greater SUCRA value indicated a more effective intervention.
Results
Literature search and study inclusion
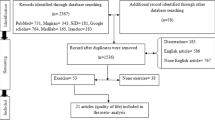
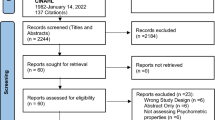
As shown in Fig. 1, the initial research on the nine databases yielded 3,968 studies. After 835 duplicates were removed, the first round of screening resulted in the selection of 537 studies based on the titles and abstracts. After reading the full articles, 62 studies were chosen. Thirty-seven of these were subsequently excluded due to an incompatible outcome index (n = 16) or not being an RCT (n = 21). The remaining 25 eligible studies were included in the NMA.
General characteristics
Table 1 summarizes the 25 RCTs that included 1226 patients eligible for data analysis. The studies included four respiratory interventions, two psychological interventions, six physiotherapy interventions, six nutritional interventions, and seven exercise interventions conducted between 1996 and 2022 and were published in English.
Quality assessment
The randomization methods used in the 25 RCTs were random sequence generation (n = 14), random number table method (n = 3), computer-generated randomization lists (n = 3), permuted block randomization (n = 4), and duplo-cego randomization (n = 1). Except for EI and PSI, allocation concealment was used to prevent selection bias in all remaining RCTs. For participant and personnel blinding, 11 studies used double blinding, two studies used single blinding, and the remaining studies did not specify. For detection bias, eight studies were assessor-blinded, while the remaining studies did not report. One study had a selective bias. Our study was free of attrition and other biases. Overall, the quality assessment of the included studies was high.
Small sample effect
Publication bias is a critical factor in determining the validity and generalization of conclusions in systemic reviews and meta-analyses. In this meta-analysis, a funnel plot was used to detect publication bias, with effect size on the horizontal coordinate and standard error on the vertical coordinate. As the sample size increases and the standard error decreases, trials are likely to converge around the true underlying effect size. The funnel plots of the trials used in the current study indicate a lower likelihood of publication bias in the ALSFRS-R score (Fig. 2a), FVC (Fig. 2b), McGill-QoL (Fig. 2c), MRC (Fig. 2d), and FSS score (Fig. 2e).
Consistency and inconsistency analysis
An inconsistency analysis of our network meta-analysis indicates a stable model with no significant evidence of discrepancy in indirect comparisons. The restricted maximum likelihood method was used in conjunction with a variance–covariance matrix proportional to 0.5I (3) + 0.5 J (3,3,1). Furthermore, the specific test for inconsistency within the model yielded a chi-square value and probability value, which are shown in Supplementary Appendix 3, supporting our conclusion that the observed data do not deviate significantly from what would be expected under a consistent model. This analysis provides strong evidence to support the consistency assumption in our network meta-analysis.
Eligible comparison network plots
Figure 3a depicts the network diagram of eligible comparisons for the primary outcome of the ALSFRS-R scores. Figure 3b–e show the network diagrams of eligible comparisons for the secondary outcomes of FVC, McGill-QoL, MRC, and FSS scores. Five nodes represented five different interventions, and controls served as a bridge for indirect comparison and resulted in five pairs of comparisons.
Network of eligible comparisons for all interventions included in the analysis. ALSFRS-R Amyotrophic lateral sclerosis Functional Rating Scale-Revised, FVC Forced Vital Capacity, McGill-QoL McGill Quality of Life Questionnaire, MRC Medical Research Council, FSS Fatigue Severity Scale. The size of the nodes is proportional to the number of participants assigned to the intervention and the thickness of the lines is proportional to the number of randomized trials that studied the respective direct comparison.
Primary outcomes
Table 2 depicts the SUCRA rankings of the five interventions on ALSFRS-R score. EI had the best SUCRA (84.5%) on functional capacity, followed by RI (70.9%), IPI (56.8%), NI (43%), and PSI (37.3%).
Figure 4a depicts a forest plot of the ALSFRS-R score. Compared to Con, the effect on functional capacity was greater in EI (SMD = 1.01, 95% CI 0.50 ~ 1.51), RI (SMD = 0.83, 95% CI 0.12 ~ 1.55), IPI (SMD = 0.65, 95% CI 0.06 ~ 1.24), NI (SMD = 0.47, 95 CI − 0.11 ~ 1.04), and PSI (SMD = 0.33, 95 CI − 0.90 ~ 1.55). Taken together, the primary outcomes of the NMA indicate that EI is the most effective strategy for slowing the functional capacity decline due to ALS.
Secondary outcomes
Table 2 depicts the SUCRA rankings of the five interventions on the secondary outcomes. On FVC, EI had the highest SUCRA (81.7%), followed by IPI (65.9%), RI (64.6%), and NI (11.4%). On the McGill-QoL scale, EI had the highest SUCRA (85.8%), followed by NI (76.9%), PSI (40.7%), and IPI (33.3%). On MRC, EI had the highest SUCRA (89.9%), followed by IPI (60.5%) and NI (26.4%). On the FSS score, IPI had the highest SUCRA (61.4%), followed by CON (56.5%), NI (55.9%), and EI (26.3%).
Figure 4 represents the forest plots of the five different interventions on the secondary outcomes. Compared to CON, the effect on FVC was greater in EI (SMD = 0.78, 95% CI 0.02 ~ 1.55), IPI (SMD = 0.57, 95% CI − 0.70 ~ 1.84), RI (SMD = 0.48, 95% CI − 0.22 ~ 1.18), and NI (SMD = − 0.19, 95% CI − 0.69 ~ 0.31). Furthermore, EI had a greater effect on FVC (SMD = − 0.97, 95% CI − 1.89 ~ − 0.06) than NI. Compared to Con, the effect on McGill-QoL was greater in EI (SMD = 0.71, 95% CI 0.33 ~ 1.08) and NI (SMD = 0.63, 95% CI 0.02 ~ 1.23), while the effect on MRC was greater in EI (SMD = 1.11, 95 CI 0.08 ~ 2.14) and IPI (SMD = 0.53, 95 CI − 0.54 ~ 1.60). Compared to CON, none of the five interventions had a significant effect on the FSS score. Taken together, the outcomes of the study suggest that EI is the most effective intervention strategy for the secondary outcomes of ALS patients.
Discussion
This study examined the efficacy of five interventions on functional status, pulmonary function, muscle strength, quality of life, and the severity of fatigue by performing a systematic review and network meta-analysis of 25 RCTs involving 1,226 ALS patients. To the best of our knowledge, this is the first study to show that, despite intervention-specific benefits, EI outperformed RI, IPI, NI, and PSI on all metrics except severity of fatigue.
In agreement with the current study findings, the beneficial effect of EI on the functional status of ALS patients has been reported in many previous studies. For example, unsupervised structured home exercise or progressive muscle training combined with aerobic exercise resulted in improved functional status in terms of the ALSFRS-R score, Functional Independence Measure (FIM), and Barthel scores, as well as improved quality of life in ALS patients20,21,22. The beneficial effects of EI are also reported in animal studies. For instance, swimming training modifies muscle metabolism by increasing muscle bioenergetics and decreasing oxidative stress. This training also slows grip strength loss and increases lifespan in the G93A-SOD1 mouse, which expresses a G93A mutant form of human copper-zinc superoxide dismutase 1 (SOD1) and can be used for ALS research23.
There are some explanations for the suppressive effects of EI on disease progression in ALS patients. First, exercise training activates satellite cells that reside between the basal lamina and sarcolemma of adult skeletal myofibers. These cells aid in muscle repair and regeneration in ALS patients. Second, exercise training increases the production of neuroprotective hormones24,25. Resistance training and aerobic exercise, for example, have been shown to increase growth hormone secretion26,27,28,29,30, which promotes growth hormone (GH) binding to intracellular receptors on the cell membrane and stimulates target cell proliferation31.
Because respiratory failure is unavoidable and a leading cause of death in ALS patients, RI has become a focal point for the disease. For example, ALS rodent models lose a lot of intercostal motor neurons and phrenic neurons. This makes the respiratory system much harder to use by placing more metabolic stress on the remaining neurons32,33. This type of unfavorable compensatory mechanism hastens motor neuron death34. In contrast, RI has been shown to improve brain-derived neurotrophic factor (BDNF)-dependent synthesis of phrenic neuronal output rates, which improves ALS patients' quality of life, ability to perform actions, and ability to breathe35,36. One systematic review demonstrated that RI improves respiratory muscle function with an effect size of 1.48 and a 12-month mean survival extension37.
IPI has also demonstrated unique therapeutic benefits on the ALSFRS-R scale score. Scalp acupuncture, which is widely used in research and clinical settings for neurodegenerative diseases, has shown promise as a treatment for ALS. For example, eight-week acupuncture therapy helped a 55-year-old female ALS patient by improving dorsiflexion strength and easing cramps, fasciculations, and lower limb pain38. Four-week acupuncture injection point therapy improved speech clarity, motor function, and muscle strength in two ALS patients39. Additionally, massage has been shown to help relieve clinical symptoms of ALS, such as muscle stiffness and cramping, by activating the parasympathetic nervous system through force on the skin surface and exerting pressure on the muscles to increase blood flow40.
Because oxidative stress is an important hypothesis for ALS pathogenesis, antioxidant research has become popular41. Vitamins, particularly vitamin B12 and vitamin E, play a protective role in the development of ALS42. Creatine monohydrate administration has a positive trend in the survival of ALS patients with no adverse events43. Malnutrition and weight loss are common in ALS patients44,45. High-calorie and ultra-high-calorie supplements can play an important role in increasing the body weight of ALS patients46.
PSI may help ALS patients with depression and improve their quality of life. For example, hypnosis and mindfulness-based psychological interventions improved patients' quality of life and relieved their pain, depression, and anxiety while also slowing disease progression47,48. Physiologically, PSI has been shown to increase telomere length and activity in leukocytes, promote telomeric DNA synthesis, and counteract the telomere depletion seen in the pathology of ALS49,50,51, while suppressing oxidative stress through increased levels of ROS-degrading enzymes52.
Fatigue is a common symptom seen at all stages of ALS pathology and is associated with worse functioning, poor quality of life, increased pain intensity and disease severity, and muscle weakness. Female ALS patients are more often affected by fatigue53. None of the non-pharmacological interventions included in the current study have shown a clear improvement in the FSS score compared to CON.
Even though it outperforms other interventions, selecting or recommending EI with the highest SUCRA ranking should not be a routine procedure for patients with ASL due to the following reasons. First, the efficacy of EI may vary depending on the stage of ALS54,55, potentially leading to bias in interpreting the intervention's results. That is, patients in the early stages of ALS are more likely to maintain the physical ability required for participation in such interventions, excluding those in later stages, for whom exercise may be impractical or, at the very least, significantly more difficult. Second, the potential bias in exercise modalities and durations adds another layer of complexity16,56, implying that the therapeutic benefits observed in this study may not be uniformly attributable to exercise itself, but rather to its specific characteristics, such as intensity, frequency, and patients' physical condition at the start of the intervention. This nuance highlights the possibility of varying response rates across the ALS population, complicating the task of generalizing our findings to the larger ALS community. To address these concerns, future research should take a more stratified methodological approach, ensuring that participants are not only representative of the broader ALS spectrum, but also categorized according to disease stage, physical capacity, and intervention specifics. Such stratification would enable a more granular analysis of the data, facilitating a nuanced understanding of the relationship between exercise interventions and ALS progression. Taken together, there may be insufficient evidence to draw a firm conclusion about the superior efficacy of EI for ALS patients, raising the risk of misinterpreting the efficacy hierarchy of the interventions in slowing the disease progression.
This meta-analysis included RCTs with varying degrees of blinding, ranging from double-blind to studies with unspecified blinding procedures57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81. We included studies regardless of blinding statutes due to a scarcity of ALS intervention research and methodological considerations. As a result, heterogeneity in blinding may introduce bias, compromising the internal validity of the results. Blinding, particularly double blinding, is the gold standard for RCTs, but its practical application in ALS trials presents significant challenges. They include the difficulty of masking certain interventions, ethical concerns about placebo control for life-altering conditions, and the possibility of unblinding due to the intervention's obvious effects57,59,61. Transparency in reporting the blinding process, including successes and failures, will allow for a more nuanced interpretation of trial results. It is critical to understand that developing blinding methods in ALS trials is a methodological and ethical requirement for ensuring the credibility of research findings and maintaining patient care integrity82,83.
Finally, the ALSFRS-R is a validated tool for tracking the progression of disability in the bulbar, motor, and respiratory domains in ALS84. Thus, individual subscale scores of the ALRFRS-R rather than total scores may provide more useful information about the progression of disability in ALS85,86, as the effects of the interventions included in the current study may be domain-specific. However, due to limited access to original datasets, our study relied on a total score rather than individual subscale scores. As a result, the therapeutic effects of the interventions on ALSFRS-R scores observed in the current study should be interpreted with caution and more specific in terms of bulbar, motor, and respiratory domains. This represents a limitation in our analysis framework and necessitates a thorough discussion. As a result, it is critical to recognize that, while the ALSFRS-R is a useful tool for tracking disease progression and patient function, using a total score rather than individual subscale scores may introduce bias when assessing the effectiveness of specific interventions that it directly queries. By contrast, a recent study found that the ALSFRS-R bulbar subscale had poor to fair screening accuracy for detecting global pharyngeal dysphagia, while the ALSFRS-R swallowing item had poor overall screening accuracy for both global swallowing and safety status87. As a result, future research could benefit from using metrics that assess respiratory function independently of the ALSFRS-R. This results in a more accurate representation of the efficacy of interventions, lowering bias risks and increasing the clinical relevance of the findings.
Conclusions
To the best of our knowledge, we are the first to compare the effectiveness of the five non-pharmacologic interventions for ALS patients. We conducted a systematic review and NMA of 25 RCTs with a total of 1226 patients and showed that EI is more effective than the other interventions, but some benefits are unique to each intervention. The current study findings support a multimodal intervention strategy with an emphasis on EI for slowing disease progression in patients with ALS. At the same time, it is also critical to interpret these findings with the knowledge that the stage of disease may influence the feasibility and effectiveness of these interventions. Further research is required to determine the full scope of these interventions' applicability and to optimize intervention strategies for diverse patient profiles across the ALS spectrum.
Data availability
The datasets analyzed and generated during the current study are available from the corresponding author on reasonable request.
References
Zarei, S. et al. A comprehensive review of amyotrophic lateral sclerosis. Surg. Neurol. Int. 6, 171. https://doi.org/10.4103/2152-7806.169561 (2015).
Oliveira, A. S. B. & Pereira, R. D. B. Amyotrophic lateral sclerosis (ALS): Three letters that change the people’s life. For ever. Arquivos de Neuro-Psiquiatria 67, 750–782. https://doi.org/10.1590/S0004-282X2009000400040 (2009).
Thomsen, G. M., Gowing, G., Svendsen, S. & Svendsen, C. N. The past, present and future of stem cell clinical trials for ALS. Exp. Neurol. 262(Pt B), 127–137. https://doi.org/10.1016/j.expneurol.2014.02.021 (2014).
Cappella, M., Pradat, P. F., Querin, G. & Biferi, M. G. Beyond the traditional clinical trials for amyotrophic lateral sclerosis and the future impact of gene therapy. J. Neuromuscul. Dis. 8, 25–38. https://doi.org/10.3233/jnd-200531 (2021).
Corcia, P. et al. Causes of death in a post-mortem series of ALS patients. Amyotroph. Later. Scler. 9, 59–62. https://doi.org/10.1080/17482960701656940 (2008).
Andersen, P. M. et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)–revised report of an EFNS task force. Eur. J. Neurol. 19, 360–375. https://doi.org/10.1111/j.1468-1331.2011.03501.x (2012).
Abe, K. et al. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 16, 505–512 (2017).
Miller, R. G., Mitchell, J. D. & Moore, D. H. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD001447.pub3 (2012).
Aschenbrenner, D. S. New drug approved for ALS. AJN Am. J. Nurs. 123, 22–23 (2023).
Cruz, M. P. Edaravone (Radicava): A novel neuroprotective agent for the treatment of amyotrophic lateral sclerosis. P t 43, 25–28 (2018).
Inoue-Shibui, A. et al. Interstitial pneumonia and other adverse events in riluzole-administered amyotrophic lateral sclerosis patients: A retrospective observational study. BMC Neurol. 19, 72. https://doi.org/10.1186/s12883-019-1299-1 (2019).
Rashidan Shah, A. & Tatarenkova, I. RELYVRIO AS THE TREATMENT FOR PATIENTS WITH AMYOTROPHIC LATERAL SCLEROSIS (ALS). Издaeтcя пo peшeнию peдaкциoннo-издaтeльcкoгo coвeтa ФГБOУ BO КГMУ Mинздpaвa Poccии, 76 (2023).
Tsitkanou, S., Della Gatta, P., Foletta, V. & Russell, A. The role of exercise as a non-pharmacological therapeutic approach for amyotrophic lateral sclerosis: Beneficial or detrimental?. Front. Neurol. 10, 783 (2019).
Connolly, S., Galvin, M. & Hardiman, O. End-of-life management in patients with amyotrophic lateral sclerosis. Lancet Neurol. 14, 435–442 (2015).
Wills, A.-M. et al. Hypercaloric enteral nutrition in patients with amyotrophic lateral sclerosis: A randomised, double-blind, placebo-controlled phase 2 trial. Lancet 383, 2065–2072 (2014).
Rahmati, M. & Malakoutinia, F. Aerobic, resistance and combined exercise training for patients with amyotrophic lateral sclerosis: A systematic review and meta-analysis. Physiotherapy 113, 12–28 (2021).
Gibbons, C., Pagnini, F., Friede, T. & Young, C. A. Treatment of fatigue in amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD011005.pub2 (2018).
El Brooks, B. R. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J. Neurol. Sci. 124, 96–107. https://doi.org/10.1016/0022-510x(94)90191-0 (1994).
Miller, R. G., Munsat, T. L., Swash, M. & Brooks, B. R. Consensus guidelines for the design and implementation of clinical trials in ALS. World Federation of Neurology committee on Research. J. Neurol. Sci. 169, 2–12. https://doi.org/10.1016/s0022-510x(99)00209-9 (1999).
Pegoraro, V., Merico, A. & Angelini, C. MyomiRNAs dysregulation in ALS rehabilitation. Brain Sci. 9, 8. https://doi.org/10.3390/brainsci9010008 (2019).
Kitano, K. et al. Effectiveness of home-based exercises without supervision by physical therapists for patients with early-stage amyotrophic lateral sclerosis: A pilot study. Arch. Phys. Med. Rehabil. 99, 2114–2117. https://doi.org/10.1016/j.apmr.2018.02.015 (2018).
Zhu, Y. et al. Mixed comparison of different exercise interventions for function, respiratory, fatigue, and quality of life in adults with amyotrophic lateral sclerosis: Systematic review and network meta-analysis. Front. Aging Neurosci. 14, 919059. https://doi.org/10.3389/fnagi.2022.919059 (2022).
Flis, D. J. et al. Swim training modulates mouse skeletal muscle energy metabolism and ameliorates reduction in grip strength in a mouse model of amyotrophic lateral sclerosis. Int. J. Mol. Sci. 20, 233. https://doi.org/10.3390/ijms20020233 (2019).
Wilczak, N. & Keyser, J. Insulin-like growth factor system in amyotrophic lateral sclerosis. Endocr. Dev. 9, 160–169. https://doi.org/10.1159/000085764 (2005).
Sakowski, S. A., Schuyler, A. D. & Feldman, E. L. Insulin-like growth factor-I for the treatment of amyotrophic lateral sclerosis. Amyotroph. Later. Scler. 10, 63–73. https://doi.org/10.1080/17482960802160370 (2009).
Wallace, J. D. et al. The response of molecular isoforms of growth hormone to acute exercise in trained adult males. J. Clin. Endocrinol Metab. 86, 200–206. https://doi.org/10.1210/jcem.86.1.7129 (2001).
McCall, G. E. et al. Bed rest suppresses bioassayable growth hormone release in response to muscle activity. J. Appl. Physiol. 1985(83), 2086–2090. https://doi.org/10.1152/jappl.1997.83.6.2086 (1997).
McCall, G. E., Grindeland, R. E., Roy, R. R. & Edgerton, V. R. Muscle afferent activity modulates bioassayable growth hormone in human plasma. J. Appl. Physiol. 1985(89), 1137–1141. https://doi.org/10.1152/jappl.2000.89.3.1137 (2000).
Nindl, B. C., Kraemer, W. J. & Hymer, W. C. Immunofunctional vs immunoreactive growth hormone responses after resistance exercise in men and women. Growth Horm. IGF Res. 10, 99–103. https://doi.org/10.1054/ghir.2000.0146 (2000).
Ari, Z. et al. Serum testosterone, growth hormone, and insulin-like growth factor-1 levels, mental reaction time, and maximal aerobic exercise in sedentary and long-term physically trained elderly males. Int. J. Neurosci. 114, 623–637. https://doi.org/10.1080/00207450490430499 (2004).
Brooks, A. J. & Waters, M. J. The growth hormone receptor: Mechanism of activation and clinical implications. Nat. Rev. Endocrinol. 6, 515–525 (2010).
Nichols, N. L. et al. Intermittent hypoxia and stem cell implants preserve breathing capacity in a rodent model of amyotrophic lateral sclerosis. Am. J. Respir. Crit. Care Med. 187, 535–542 (2013).
Seven, Y. B. et al. Compensatory plasticity in diaphragm and intercostal muscle utilization in a rat model of ALS. Exp. Neurol. 299, 148–156. https://doi.org/10.1016/j.expneurol.2017.10.015 (2018).
Seven, Y. B. & Mitchell, G. S. Mechanisms of compensatory plasticity for respiratory motor neuron death. Respir. Physiol. Neurobiol. 265, 32–39. https://doi.org/10.1016/j.resp.2019.01.001 (2019).
Tabor, L. C. et al. Respiratory training in an individual with amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 3, 819–823. https://doi.org/10.1002/acn3.342 (2016).
Vicente-Campos, D. et al. POWERbreathe(®) inspiratory muscle training in amyotrophic lateral sclerosis. J. Clin. Med. 11, 6655. https://doi.org/10.3390/jcm11226655 (2022).
Macpherson, C. E. & Bassile, C. C. Pulmonary physical therapy techniques to enhance survival in amyotrophic lateral sclerosis: A systematic review. J. Neurol. Phys. Ther. 40, 165–175. https://doi.org/10.1097/npt.0000000000000136 (2016).
Dung, H. Acupuncture: An Anatomical Approach (CRC Press, 2013).
Liang, S., Christner, D., Du Laux, S. & Laurent, D. Significant neurological improvement in two patients with amyotrophic lateral sclerosis after 4 weeks of treatment with acupuncture injection point therapy using enercel. J. Acupunct. Meridian Stud. 4, 257–261. https://doi.org/10.1016/j.jams.2011.09.017 (2011).
Chiò, A., Mora, G. & Lauria, G. Pain in amyotrophic lateral sclerosis. Lancet Neurol. 16, 144–157 (2017).
Hemerková, P. & Vališ, M. Role of oxidative stress in the pathogenesis of amyotrophic lateral sclerosis: Antioxidant metalloenzymes and therapeutic strategies. Biomolecules 11, 437. https://doi.org/10.3390/biom11030437 (2021).
Goncharova, P. S. et al. Nutrient effects on motor neurons and the risk of amyotrophic lateral sclerosis. Nutrients 13, 3804. https://doi.org/10.3390/nu13113804 (2021).
Rosenfeld, J. et al. Creatine monohydrate in ALS: Effects on strength, fatigue, respiratory status and ALSFRS. Amyotroph. Later. Scler. 9, 266–272. https://doi.org/10.1080/17482960802028890 (2008).
Piquet, M. A. Nutritional approach for patients with amyotrophic lateral sclerosis. Rev. Neurol. (Paris) 162, 4s177-174s187 (2006).
Limousin, N. et al. Malnutrition at the time of diagnosis is associated with a shorter disease duration in ALS. J. Neurol. Sci. 297, 36–39. https://doi.org/10.1016/j.jns.2010.06.028 (2010).
Dorst, J. et al. Fat-rich versus carbohydrate-rich nutrition in ALS: A randomised controlled study. J. Neurol. Neurosurg. Psychiatry 93, 298–302 (2022).
Palmieri, A. et al. Efficacy of hypnosis-based treatment in amyotrophic lateral sclerosis: A pilot study. Front. Psychol. 3, 465. https://doi.org/10.3389/fpsyg.2012.00465 (2012).
Pagnini, F., Phillips, D., Bosma, C. M., Reece, A. & Langer, E. Mindfulness, physical impairment and psychological well-being in people with amyotrophic lateral sclerosis. Psychol. Health 30, 503–517 (2015).
Shay, J. W. Telomeres and aging. Curr. Opin. Cell Biol. 52, 1–7. https://doi.org/10.1016/j.ceb.2017.12.001 (2018).
Anitha, A., Thanseem, I., Vasu, M. M., Viswambharan, V. & Poovathinal, S. A. Telomeres in neurological disorders. Adv. Clin. Chem. 90, 81–132. https://doi.org/10.1016/bs.acc.2019.01.003 (2019).
Smith, E. M., Pendlebury, D. F. & Nandakumar, J. Structural biology of telomeres and telomerase. Cell. Mol. Life Sci. 77, 61–79. https://doi.org/10.1007/s00018-019-03369-x (2020).
Kaliman, P. et al. Rapid changes in histone deacetylases and inflammatory gene expression in expert meditators. Psychoneuroendocrinology 40, 96–107 (2014).
Zarei, S. et al. A comprehensive review of amyotrophic lateral sclerosis. Surg. Neurol. Int. 6, 171 (2015).
Ortega-Hombrados, L. et al. Systematic review of therapeutic physical exercise in patients with amyotrophic lateral sclerosis over time. Int. J. Environ. Res. Public Health 18, 1074 (2021).
de Almeida, J. L., Silvestre, R., Pinto, A. & De Carvalho, M. Exercise and amyotrophic lateral sclerosis. Neurol. Sci. 33, 9–15 (2012).
Dal Bello-Haas, V., Kloos, A. D. & Mitsumoto, H. Physical therapy for a patient through six stages of amyotrophic lateral sclerosis. Phys. Ther 78, 1312–1324 (1998).
Drory, V. E., Goltsman, E., Goldman Reznik, J., Mosek, A. & Korczyn, A. D. The value of muscle exercise in patients with amyotrophic lateral sclerosis. J. Neurol. Sci. 191, 133–137. https://doi.org/10.1016/S0022-510X(01)00610-4 (2001).
Lunetta, C. et al. Strictly monitored exercise programs reduce motor deterioration in ALS: Preliminary results of a randomized controlled trial. J. Neurol. 263, 52–60. https://doi.org/10.1007/s00415-015-7924-z (2016).
Braga, A. C. M., Pinto, A., Pinto, S. & De Carvalho, M. The role of moderate aerobic exercise as determined by cardiopulmonary exercise testing in ALS. Neurol. Res. Int. 2018, 1–10. https://doi.org/10.1155/2018/8218697 (2018).
Merico, A. et al. Effects of combined endurance and resistance training in Amyotrophic Lateral Sclerosis: A pilot, randomized, controlled study. Eur. J. Transl. myol. 28, 7278. https://doi.org/10.4081/ejtm.2018.7278 (2018).
Ferri, A. et al. Tailored exercise training counteracts muscle disuse and attenuates reductions in physical function in individuals with amyotrophic lateral sclerosis. Front. Physiol. https://doi.org/10.3389/fphys.2019.01537 (2019).
van Groenestijn, A. C. et al. Aerobic exercise therapy in ambulatory patients with ALS: A randomized controlled trial. Neurorehabil. Neural Repair 33, 153–164. https://doi.org/10.1177/1545968319826051 (2019).
Kalron, A. et al. Effects of a 12-week combined aerobic and strength training program in ambulatory patients with amyotrophic lateral sclerosis: A randomized controlled trial. J. Neurol. 268, 1857–1866. https://doi.org/10.1007/s00415-020-10354-z (2021).
Tandan, R. et al. A controlled trial of amino acid therapy in amyotrophic lateral sclerosis: I. Clinical, functional, and maximum isometric torque data. Neurology 47, 1220–1226 (1996).
Desnuelle, C., Dib, M., Garrel, C. & Favier, A. A double-blind, placeho-controlled randomized clinical trial of alpha-tocopherol (vitamin E) in the treatment of amyotrophic lateral sclerosis. Amyotroph. Later. Scler. Motor Neuron Disord. 2, 9–18. https://doi.org/10.1080/146608201300079364 (2001).
Kaufmann, P. et al. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify phase III. Ann. Neurol. 66, 235–244. https://doi.org/10.1002/ana.21743 (2009).
de Carvalho Silva, L. B. et al. Effect of nutritional supplementation with milk whey proteins in amyotrophic lateral sclerosis patients. Arquivos De Neuro-Psiquiatria 68, 263–268. https://doi.org/10.1590/s0004-282x2010000200021 (2010).
Beghi, E. et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for ALS. Amyotroph. Later. Scler. Frontotemp.l Degener. 14, 397–405. https://doi.org/10.3109/21678421.2013.764568 (2013).
Wills, A.-M. et al. Hypercaloric enteral nutrition in patients with amyotrophic lateral sclerosis: A randomised, double-blind, placebo-controlled phase 2 trial. Lancet 383, 2065–2072. https://doi.org/10.1016/s0140-6736(14)60222-1 (2014).
Di Lazzaro, V. et al. Repetitive transcranial magnetic stimulation for ALS. A preliminary controlled study. Neurosci. Lett. 408, 135–140. https://doi.org/10.1016/j.neulet.2006.08.069 (2006).
Zanette, G., Forgione, A., Manganotti, P., Fiaschi, A. & Tamburin, S. The effect of repetitive transcranial magnetic stimulation on motor performance, fatigue and quality of life in amyotrophic lateral sclerosis. J. Neurol. Sci. 270, 18–22. https://doi.org/10.1016/j.jns.2008.01.011 (2008).
Di Lazzaro, V. et al. Motor cortex stimulation for ALS: A double blind placebo-controlled study. Neurosci. Lett. 464, 18–21. https://doi.org/10.1016/j.neulet.2009.08.020 (2009).
Peng, J., Li, Y. Q., Deng, C. N. & Jiang, J. J. Application of continuous nursing combined with massage in nursing care of amyotrophic lateral sclerosis patients. J. Sichuan Tradit. Chin. Med. 37, 210–212 (2019).
Feng, W., Yang-pu, Z. & Qun, S. A. Effect of Thunder-fire Moxibustion and Medicine on Respiratory Function in Patients with Amyotrophic Lateral Sclerosis. Shanghai J. Acupuncture Moxibustion 40 (2021).
Herrmann, C. et al. Pharyngeal electrical stimulation in amyotrophic lateral sclerosis: A pilot study. Ther. Adv. Neurol. Disord. 15, 175628642110683. https://doi.org/10.1177/17562864211068394 (2022).
Pagnini, F. et al. Meditation training for people with amyotrophic lateral sclerosis: a randomized clinical trial. Amyotroph. Later. Scler. Frontotemp. Degener. 17, 45. https://doi.org/10.1080/21678421.2016.1231971/0064 (2016).
Pagnini, F. et al. An online non-meditative mindfulness intervention for people with ALS and their caregivers: A randomized controlled trial. Amyotroph. Later. Scler. Frontotemp. Degener. 23, 116–127. https://doi.org/10.1080/21678421.2021.1928707 (2022).
Cheah, B. C. et al. INSPIRATIonAL–INSPIRAtory muscle training in amyotrophic lateral sclerosis. Amyotroph. Later. Scler. 10, 384–392. https://doi.org/10.3109/17482960903082218 (2009).
Pinto, S., Swash, M. & de Carvalho, M. Respiratory exercise in amyotrophic lateral sclerosis. Amyotroph. Later. Scler. 13, 33–43. https://doi.org/10.3109/17482968.2011.626052 (2012).
Plowman, E. K. et al. Impact of expiratory strength training in amyotrophic lateral sclerosis: Results of a randomized, sham-controlled trial. Muscle Nerve 59, 40–46. https://doi.org/10.1002/mus.26292 (2019).
Plowman, E. K. et al. Respiratory strength training in amyotrophic lateral sclerosis: A double-blind, randomized, multicenter, sham-controlled trial. Neurology 100, e1634–e1642. https://doi.org/10.1212/WNL.0000000000206830 (2023).
Núñez-Núñez, M. et al. Research integrity in randomized clinical trials: A scoping umbrella review. Int. J. Gynecol. Obstetr. 162, 860–876 (2023).
Monaghan, T. F. et al. Blinding in clinical trials: seeing the big picture. Medicina 57, 647 (2021).
Kaufmann, P. et al. The ALSFRSr predicts survival time in an ALS clinic population. Neurology 64, 38–43. https://doi.org/10.1212/01.Wnl.0000148648.38313.64 (2005).
Cedarbaum, J. M. et al. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. J. Neurol. Sci. 169, 13–21 (1999).
Pinto, S., Oliveira Santos, M., Gromicho, M., Swash, M. & de Carvalho, M. Respiratory phenotypes in amyotrophic lateral sclerosis as determined by respiratory questions on the Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised and their relation to respiratory tests. Eur. J. Neurol. 30, 1594–1599. https://doi.org/10.1111/ene.15765 (2023).
Chapin, J. L. et al. Diagnostic utility of the amyotrophic lateral sclerosis Functional Rating Scale-Revised to detect pharyngeal dysphagia in individuals with amyotrophic lateral sclerosis. PLoS ONE 15, e0236804. https://doi.org/10.1371/journal.pone.0236804 (2020).
Author information
Authors and Affiliations
Contributions
Conceptualization, Zhao Li and Hyunsik Kang; methodology, Zhao Li and Hyunsik Kang; validation, Zhao Li and Hyunsik Kang; investigation, Zhao Li; data curation, Zhao Li and Hyunsik Kang; writing-original draft preparation, Zhao Li and Hyunsik Kang. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Z., Kang, H. Efficacy of non-pharmacological interventions for individuals with amyotrophic lateral sclerosis: systematic review and network meta-analysis of randomized control trials. Sci Rep 14, 11365 (2024). https://doi.org/10.1038/s41598-024-62213-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62213-w
- Springer Nature Limited