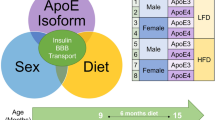
Abstract
There are genetic and environmental risk factors that contribute to the development of cognitive decline in Alzheimer’s disease (AD). Some of these include the genetic predisposition of the apolipoprotein E4 genotype, consuming a high-fat diet (HFD), and the female sex. Brain insulin receptor resistance and deficiency have also been shown to be associated with AD and cognitive impairment. Intranasal (INL) insulin enhances cognition in AD, but the response varies due to genotype, diet, and sex. We investigated here the combination of these risk factors in a humanized mouse model, expressing E3 or E4, following a HFD in males and females on cognitive performance and the brain distribution of insulin following INL delivery. The HFD had a negative effect on survival in male mice only, requiring sex to be collapsed. We found many genotype, diet, and genotype x diet effects in anxiety-related tasks. We further found beneficial effects of INL insulin in our memory tests, with the most important findings showing a beneficial effect of INL insulin in mice on a HFD. We found insulin distribution throughout the brain after INL delivery was largely unaffected by diet and genotype, indicating these susceptible groups can still receive adequate levels of insulin following INL delivery. Our findings support the involvement of brain insulin signaling in cognition and highlight continuing efforts investigating mechanisms resulting from treatment with INL insulin.
Similar content being viewed by others
Introduction
The insulin found in the brain is primarily transported from blood into brain by the blood–brain barrier (BBB)1,2,3. The receptors for insulin in the brain have a different structure and size compared to those expressed peripherally, consistent with a different evolutionary pathway that preserves more of the ancestral insulin’s effects on growth4,5. In the brain, insulin modulates metabolism, neurotransmitter release, neuronal growth and survival, synaptic plasticity, and cognition5,6,7,8.
Brain insulin action is impaired in Alzheimer’s disease (AD)9. AD individuals have a lower cerebrospinal fluid (CSF) to plasma insulin ratio with increased plasma insulin levels and decreased CSF insulin levels compared to those in cognitively healthy age-matched adults. Post-mortem brain studies have revealed decreases in key insulin signaling proteins in the brains of AD patients10,11 with an impaired response to insulin in post-mortem AD brain regions involved in cognition, including the cerebral cortex and hippocampus, compared to the response in cognitively healthy controls12. Thus, insulin activity in brain is decreased in AD by two mechanisms: a decrease in the level of insulin in the brain and a decrease in insulin receptor sensitivity. Obesity and diabetes also show deficiencies in insulin action in both peripheral and brain tissues. Similar to AD, obesity and peripheral insulin resistance are also associated with decreased insulin transport into the brain13,14. Further, CSF insulin levels are decreased in obese animals and humans and correlate with peripheral insulin sensitivity, supporting a link between obesity and central insulin dysregulation13,14,15,16. Obesity and consumption of a high-fat diet (HFD) can affect cognition17, possibly mediated by triglycerides18,19. Obesity during middle-age can reduce cognitive performance and is associated with an increased risk to develop mild cognitive impairment (MCI) later in life20. Consistent with human data, rodents fed a HFD show cognitive impairments7,21,22,23 and neuronal insulin resistance24.
Apolipoprotein E (apoE) plays an important role in the metabolism and redistribution of lipoproteins and cholesterol25. In the brain, apoE is primarily produced by astrocytes26, involved in neuronal maintenance and repair and BBB repair after injury27. In humans, there are three major isoforms of apoE: E2, E3, and E4. Compared to E3, E4 increases the risk to develop AD28 and cognitive injury following environmental challenges, including a HFD29. People with diabetes carrying E4 are more predisposed to developing AD than those without E430. In human apoE mice, E3 associates more with the insulin receptor than E431. E4 carriers may experience impairments in insulin signaling at an earlier stage than E3 carriers due to differences in interactions with key insulin signaling proteins. In addition, total apoE levels are reduced in the hippocampus of E3, but not E4 mice, fed a HFD for 5 weeks32.
Increasing brain insulin levels improves memory in young, healthy subjects33,34, suggesting that even those without central insulin resistance can exhibit the beneficial effects of enhancing brain insulin availability. Increasing brain insulin levels by delivering insulin via the intranasal (INL) route35 bypasses the BBB, limits the glucose regulatory effects of insulin as shown by unchanged glucose levels in healthy males33, can improve peripheral metabolism, decreases food intake in healthy men36,37, and improves cognition, including in early AD38.
In animal models of AD, INL insulin reaches the hippocampus39,40 and improves cognition in a murine brain insulin resistant model41,42. However, obese subjects show a different response to INL insulin43, which might relate to genetic factors such as the apoE isoform44. People with E4 are less likely to respond or even fare worse after INL insulin therapy compared to non-E4 carriers38,45,46. Whole brain insulin signaling is disrupted in female E4 mice compared to female E3 mice30 and E4 female mice are more susceptible than E3 female mice to the detrimental effects of a HFD on the hippocampal epigenome and metabolome22. These risk factors for AD have been investigated independently in terms of brain insulin and cognitive response. Therefore, in this current study we investigated the 3 risk factors together to identify if they interact. We delivered INL insulin to E3 and E4 mice on a HFD or control diet and assessed behavioral and cognitive performance and characterized the distribution of insulin following INL delivery to determine whether delivery was altered by these AD risk factors.
Results
Mortality of male mice
We behaviorally tested a total of 100 mice. We found that HFD in males, especially E4 males, was associated with increased mortality. Five (36%) E3 HFD males, 9 (56%) E4 HFD males and 2 (15%) E4 males on standard diet (STD) died before the end of behavioral testing. In comparison, there was no loss of females. There was significantly higher mortality of males than females for both the E3 (p = 0.0081, Fisher’s exact test) and the E4 (p = 0.0008, Fisher’s exact test) HFD groups.
We tested INL insulin distribution in a total of 109 mice. Nine (64%) E3 HFD males, 6 (38%) E4 HFD males, and 1 (6%) E3 HFD female died before the end of the 6 months diet period. There was significantly higher mortality of males than females for both the E3 HFD (p = 0.0014, Fisher’s exact test) and the E4 HFD (p = 0.0072, Fisher’s exact test) groups. There was no effect of genotype in HFD mice (females p = 0.485, males p = 0.272). For both the behavior and the insulin distribution studies, neither sex nor genotype had an effect on mortality in the control diet groups.
Elevated zero maze
In the elevated zero maze test, there was an effect of diet on the time the mice spent in the anxiety-provoking open areas of maze (F(1,91) = 9.6, p = 0.0026) and a trend towards a genotype x diet interaction (F(1,91) = 3.9, p = 0.05) (Fig. 1A). Mice on the HFD spent less time in the open areas of the maze and this was much more pronounced in E4 than E3 mice. For distance moved in the elevated zero maze, there was also an effect of diet (F(1,91) = 22.11, p < 0.0001), with reduced activity levels in the HFD mice (Fig. 1B).
Baseline elevated zero maze and rotorod. (A) There was an effect of diet on the time the mice spent in the anxiety-provoking open areas of elevated zero maze. ***p = 0.0026 (ANOVA). (B) Mice on a HFD moved less than mice on a STD in the elevated zero maze. **** p < 0.0001 (ANOVA). (C) Mice on a HFD fell quicker of the rotating rod than mice on a STD. ****p < 0.0001 (ANOVA)). HFD: high fat diet; STD: standard diet. E3 males: n = 9 HFD and n = 12 STD; E3 females: n = 19 HFD and n = 13 STD; E4 males: n = 7 HFD and n = 11 STD; E4 females: n = 15 HFD and n = 14 STD.
Rotorod
In the rotorod test, there was an effect of diet on ability of the mice to stay on the rotating rod (fall latency) (F(1,201) = 129.2, p < 0.0001), with mice on the HFD showing a shorter fall latency (Fig. 1C).
Open field
When activity levels were assessed in the open field over two subsequent days, there was an effect of day (F(1,93) = 154.5, p < 0.001), with lower activity levels on day 2 than day 1 indicating spatial habituation that was not impaired by the HFD (Fig. 2A). There was also an effect of diet (F(193) = 30.744, p < 0.001), with lower activity levels in mice on a HFD than a STD (Fig. 2A). In addition, there was a day x diet interaction (F(1,201) = 5.159, p = 0.025) (Fig. 2A).
Open field and novel object. (A) All groups showed spatial habituation learning and moved less on day 2 than day 1 in the open field. ****p < 0.001 (ANOVA). Activity levels were lower in mice on a HFD than STD. ****p < 0.001 (ANOVA). (B) Mice on a HFD spent less time in the center of the open field than mice on a STD. ****p < 0.001 (ANOVA). (C) Mice on a HFD had lower activity levels than mice on a STD in the open field containing two identical objects on the object recognition training day. ****p < 0.001 (ANOVA). (D) Mice moved more in the open field containing two identical objects than on the previous day in the open field without object. ****p < 0.001 (ANOVA). There was also a diet × day interaction (F(1,95) = 14.182, ****p < 0.001 (ANOVA)). Mice on a STD showed a relatively larger increase in activity levels when two identical objects were introduced in the open field than mice on a HFD. (E) Mice on a HFD moved less than mice on a STD on the novel object recognition testing day. ****p < 0.001 (ANOVA). (F) In the object recognition test, E3 mice on a STD, treated with saline or insulin showed object recognition and spent more time exploring the novel than familiar object. *p < 0.05 versus the familiar object (paired t test). E3 and E4 mice on a HFD treated with saline spent a similar time exploring the two objects. However, E3 and E4 mice on a HFD treated with insulin and E4 mice on a STD treated with saline showed a trend towards exploring the novel object more than the familiar object. #p = 0.0586 (paired t test). HFD: high fat diet; STD: standard diet. E3 males: n = 9 HFD and n = 12 STD; E3 females: n = 19 HFD and n = 13 STD; E4 males: n = 7 HFD and n = 11 STD; E4 females: n = 15 HFD and n = 14 STD.
Time spent in the more anxiety-provoking center of the open field was analyzed. There was an effect of diet (F(1,95) = 12.965, p < 0.001) with mice on a HFD spending less time in the center than mice on a STD (Fig. 2B). In addition, there was a trend towards a genotype x diet interaction (F(1,95) = 3.456, p = 0.066).
Novel object recognition
This test assesses memory in that mice will spend more time with a novel object than a familiar one. Being able to distinguish between objects that are novel and those that are not demonstrates memory of the non-novel object. However, we first took advantage of measuring activity levels of mice during the first (training day) when mice were presented in the open field with two identical objects. There was an effect of diet with mice on a HFD showing lower activity levels than mice on a STD (F(1,95) = 27.853, p < 0.001) (Fig. 2C). We further assessed activity by comparing activity levels during open field testing (second day) to activity levels on the training (identical objects) day for object recognition. There was an effect of day (F(1,95) = 103.2, p < 0.001) with mice moving more on the object recognition training day (Fig. 2D). However, there was also a diet x day interaction (F(1,95) = 14.182, p < 0.001) (Fig. 2D). Mice on a STD showed a relatively larger increase in activity levels when two identical objects were introduced in the open field than mice on a HFD. When activity levels were analyzed on the novel object recognition testing day, there was also an effect of diet with mice on a HFD moving less than mice on a STD (F(1,91) = 30.436, p < 0.001) (Fig. 2E). Consistent with this result, the HFD mice also spent less time than STD mice in exploring the two objects on the novel object recognition testing day (t = 2.527, p = 0.0205, unpaired t test). There was no effect of insulin treatment on activity levels.
In the object recognition test, E3 mice on a STD, treated with saline (t = 1.951, p = 0.0398) or insulin (t = 2.236, p = 0.0247) spent more time exploring the novel than familiar object, demonstrating memory of the original object (Fig. 2F). E3 mice on a HFD treated with saline spent a similar time exploring the two objects, suggesting that they did not recognize the original object (Fig. 2F). In contrast, E3 mice on a HFD treated with insulin showed a trend towards exploring the novel object more than the familiar object (t = 1.756, p = 0.0586) (Fig. 2F). In E4 mice on a STD treated with saline, there also was a trend towards exploring the novel object more than the familiar object (t = 1.890, p = 0.0503) (Fig. 2F). This was not seen in E4 mice on a STD treated with insulin (Fig. 2F). E4 mice on a HFD treated with saline spent a similar time exploring the familiar and novel objects. In contrast, in E4 mice on a HFD treated with insulin, there was a trend towards more time spent exploring the novel object (t = 1.756, p = 0.0586) (Fig. 2F).
Water maze
INL insulin was administered following visible platform training. During visible platform training (task learning), there was an effect of diet (F(1,786) = 132.4, p < 0.0001) and diet x genotype interaction (F(1,786) = 3.995, p = 0.0460) for swim speeds (Fig. 3A). HFD mice swam slower than STD mice. While on a STD, the swim speeds were not different in E4 than E3 mice; on a HFD swim speeds were lower in E4 than E3 mice (t = 2.239, p = 0.0257).
Water maze latency learning curves. (A) During visible platform training, HFD mice swam slower than STD mice ****p < 0.0001 (ANOVA). There was also a diet × genotype interaction (F(1,786) = 3.995, p = 0.0460 (ANOVA)). On a STD, the swim speeds were not different in E4 than E3 mice. However, on a HFD swim speeds were lower in E4 than E3 mice. *p = 0.0257 (ANOVA). (B) During hidden platform training, there were effects of diet and genotype on swim speeds. Mice on a HFD swam faster than mice on a STD and E4 mice swam faster than E3 mice. ***p < 0.0001, **p = 0.008 (ANOVA). (C, D) When the time to reach the visible platform (latency) was analyzed, there were effects of genotype (F(1,380) = 7.138, p = 0.0079), diet (F(1,380) = 16.04, p < 0.0001), and a session x genotype interaction (F(3,380) = 3.992, p = 0.0081) (ANOVA). E4 mice performed poorer than E3 mice and HFD mice performed worse than mice on a regular diet. In E3 mice, the effect of HFD was most pronounced in the first two sessions. ***p = 0.0006 (ANOVA), on the first day of hidden platform training. In contrast, in E4 mice the effects of HFD were most pronounced in the third session, the first session on the second day of hidden platform training. **p = 0.0024 (ANOVA). (E–H) When the time to reach the hidden platform (latency) was analyzed, there was a session x treatment interaction (F(5,455) = 2.262, p = 0.047 (ANOVA)). The beneficial effects of insulin were most pronounced in the first and third hidden platform sessions. In the first hidden session, insulin-treated mice reached the hidden platform faster than saline-treated mice. *p = 0.047 (ANOVA). In the third hidden session, there was a genotype × diet × treatment interaction (F(1,91) = 4.075, p = 0.046 (ANOVA). There was a trend towards insulin improving the performance of E3 mice on a STD and E4 mice on a HFD. (I) When spatial memory retention was assessed in the probe trial by analyzing the time required to first swim to the platform was analyzed as performance measure, there was a genotype x diet x treatment interaction (F(1,91) = 11.146, p = 0.001) (ANOVA). In mice on a STD, there was an effect of genotype (F(1,37) = 112, p < 0.0001), an effect of insulin (F(1,37) = 4.227, p < 0.0469), and a treatment × genotype interaction (F(1,36) = 4.212, p = 0.0473) (ANOVA). In mice on a HFD, there was an effect of genotype (F(1,36) = 107.2, p < 0.0001), and trends towards an effect of insulin (F(1,36) = 4.039, p = 0.0520) and a trend towards a treatment × genotype interaction (F(1,36) = 4.045, p = 0.0518) (ANOVA). In E3 mice on a HFD and in E4 mice on a STD, insulin improved cognitive performance. **p = 0.0042, *p = 0.0496 (t test). In contrast, no beneficial effect of insulin on spatial memory retention was seen in E4 mice on a HFD or E3 mice on a STD. HFD: high fat diet; STD: standard diet. E3 males: n = 9 HFD and n = 12 STD; E3 females: n = 19 HFD and n = 13 STD; E4 males: n = 7 HFD and n = 11 STD; E4 females: n = 15 HFD and n = 14 STD.
When the time to reach the visible platform (latency) was analyzed, all groups improved their performance over the four sessions (effect of session: (F(3,380) = 128.1, p < 0.0001) (Fig. 3C, D). There were effects of genotype (F(1,380) = 7.138, p = 0.0079), diet (F(1,380) = 16.04, p < 0.0001), and a session × genotype interaction (F(3,380) = 3.992, p = 0.0081). E4 mice performed poorer than E3 mice (Fig. 3C, D). HFD mice performed worse than mice on a STD (Fig. 3C, D). In E3 mice, the effect of HFD was most pronounced in the first two sessions (F(1,190) = 6.01, p = 0.0006) on the first day of hidden platform training, while in E4 mice the effects of HFD were most pronounced in the third session, the first session on the second day of hidden platform training (t = 3.213, p = 0.0024) (Fig. 3E–H).
During hidden platform training (spatial learning), there was an effect of diet (F(1,786) = 79.337, p < 0.0001), genotype (F(1,786) = 7.014, p = 0.008) and a trend towards a diet × treatment interaction (F(1,786) = 3.0.21, p = 0.082) for swim speeds (Fig. 3B). Mice on a HFD swam faster than mice on a STD, while E4 mice swam faster than E3 mice.
When the time to reach the hidden platform (latency) was analyzed, all groups improved their performance over the six sessions (effect of session: (F(5,455) = 39.552, p < 0.0001) (Fig. 3E–H). In addition, there was a session × treatment interaction (F(5,455) = 2.262, p = 0.047). Manual inspection indicated that the beneficial effects of insulin were most pronounced in the first and third hidden platform sessions. Therefore, we also analyzed these sessions separately. In the first hidden session, there was an effect of treatment (F(1,91) = 4.068, p = 0.047), with insulin-treated mice reaching the hidden platform faster than saline-treated mice (Fig. 3E–H). In the third hidden session, there was a trend towards an effect of diet (F(1,91) = 3.478, p = 0.065) and a genotype x diet x treatment interaction (F(1,91) = 4.075, p = 0.046) (Fig. 3E–H). This interaction seemed driven by a trend towards insulin improving the performance of E3 mice on a STD and E4 mice on a HFD. Overall, these data indicate that both E3 and E4 mice show improved cognitive performance the morning following insulin treatment.
As effects of diet on swim speeds can affect the latency performance measure, we also analyzed the distance moved during the visible and hidden platform training sessions. When distance moved during the visible platform sessions was analyzed, all groups improved their performance over the four sessions (effect of session: (F(3,380) = 93.74, p < 0.0001) (Figs. S1B, C). There was a trend towards an effects of genotype (F(1,380) = 3.349, p = 0.0680), a trend towards a session x genotype interaction (F(3,380) = 2.207, p = 0.0868) and a trend towards a session x genotype x diet interaction (F(3,380) = 2.184, p = 0.0895) (Figs. S1B, C).
When distance moved during the hidden platform sessions was analyzed, all groups improved their performance over the six sessions (effect of session: (F(5,455) = 24.411, p < 0.0001) (Fig. S1D–G). In addition, there was a trend towards an effect of diet (F(1,91) = 3.047, p = 0.084), a trend towards a session × treatment interaction (F(5,455) = 1.950, p = 0.085), and a trend towards a session × genotype × diet interaction (F(1,91) = 3.053, p = 0.084). In the first hidden platform session, there was a trend towards an effect of treatment (F(1,91) = 3.664, p = 0.059). In the third hidden platform session, there was a trend towards an effect of genotype (F(1,91) = 3.502, p = 0.065) and a trend towards a genotype × diet × treatment interaction (F(1,91) = 2.935, p = 0.090) (Fig. S1D–G).
Finally, spatial memory retention was assessed in the probe trial (Fig. 3I). The time required to first swim to the platform was analyzed as a performance measure. There was a genotype × diet × treatment interaction (F(1,91) = 11.146, p = 0.001). When time to first swim to the platform location was analyzed in mice on a STD, there was an effect of genotype (F(1,37) = 112, p < 0.0001), an effect of insulin (F(1,37) = 4.227, p = 0.0469), and a treatment x genotype interaction (F(1,36) = 4.212, p = 0.0473). In mice on a HFD, there was an effect of genotype (F(1,36) = 107.2, p < 0.0001), and trends toward effects of insulin (F(1,36) = 4.039, p = 0.0520) and a treatment x genotype interaction (F(1,36) = 4.045, p = 0.0518). In E3 mice on a HFD, insulin improved cognitive performance (t = 3.140, p = 0.0042). This was not seen in E4 mice on a HFD. E4 mice, but not E3 mice, on a STD showed improved performance when treated with insulin (t = 2.068, p = 0.0496). Overall, these data indicate that E3 mice on a HFD and E4 mice on a STD show improved spatial memory retention following insulin treatment.
INL insulin distribution
In a separate set of mice from our behavior testing, we identified what brain regions insulin was reaching after INL delivery and whether there were differences due to genotype, diet, and/or sex. In this separate cohort of mice from our behavioral set of mice, we measured body weights every month during the diet paradigm. To increase the probability of dietary effects and to match our previous studies investigating insulin BBB transport in the similarly aged strains of mice treated with the same diet regimen30, we elected to compare HFD to a low fat diet (LFD), rather than to STD. Both females and males gained significant weight on the HFD compared to mice on a LFD (Fig. 4A, B). In females, there was a significant effect of not only diet (F(1, 58) = 514, p < 0.0001), but also genotype (F(1, 58) = 4.467, p = 0.0389) in the percent of baseline weight gained over the 6-month period (Fig. 4C). In males, there was also a significant effect of diet (F(1, 37) = 175.4, p < 0.0001) and genotype (F(1, 37) = 9.983, p = 0.0031) in the percent of baseline weight (Fig. 4D).
Weight gain over study period for INL insulin distribution. Weights were recorded each month. Both (A) female and (B) male mice gained weight on a HFD (ANOVA). The total weight gained after 6 months, as expressed as a percent of the baseline, was statistically significant for (C) females and (D) males and there were significant differences due to apoE genotype for both sexes (ANOVA). Tukey post hoc: *p < 0.05 as marked. LFD: low-fat diet, HFD: high-fat diet. Females: n = 14 E3 LFD, n = 15 E3 HFD, n = 17 E4 LFD, n = 16 E4 HFD; Males: n = 11 E3 LFD, n = 4 E3 HFD, n = 16 E4 LFD, n = 10 E4 HFD.
As genotype, diet, and sex can impact the size of brain regions and impact atrophy, we assessed whether the combination of these risk factors had an impact on weight of each brain region (Table 1). We a priori planned for a sex-dependent effect and therefore analyzed the sexes separately. Outliers identified by the ROUT method (Q = 1%) within each region and group were removed (female CB, n = 1; male cortex, n = 1). There were 3 regions, plus the whole brain (F(1,57) = 4.199, p = 0.0451), that were impacted by diet in females compared to just one region in males. A HFD significantly reduced the brain weight of the cortex (F(1,57) = 3.22, p = 0.0392) and cerebellum (F(1,56) = 7.05, p = 0.0103), with a trend in reduced weight of the frontal cortex (F(1,57) = 3.912, p = 0.0528) and in females and the cortex in males (F(1,37) = 5.466, p = 0.0249). Alternatively, there were 5 regions impacted by genotype in females compared to a trend in 3 regions for males. The hypothalamus (F(1,57) = 4.340, p = 0.0417), parietal cortex (F(1,57) = 6.618, p = 0.0127), and cerebellum (F(1,57) = 4.229, p = 0.0444) were significantly impacted by genotype in females, with trends in the cortex (F(1,57) = 3.216, p = 0.0782) and pons/medulla (F(1,57) = 3.851, p = 0.0546). For males, there was a trend for genotype in the whole brain (F(1,38) = 2.942, p = 0.0945) and frontal cortex (F(1,38) = 3.272, p = 0.0784) and a trend in genotype × diet interaction in the thalamus (F(1,38) = 3.488, p = 0.0696) (see Table 1). For the distribution analysis presented next, the weight of each brain region is factored into the %Inj/g, eliminating any effect of diet or genotype due to differences in region weight.
Due to the increased mortality in males on a HFD and in order to assess effects on distribution due to genotype and diet, we combined sexes. The level of %Inj/g 125I-insulin reaching each brain region at the various time points are listed in Table 2. Outliers were removed by the ROUT (Q = 1%) method (WB E4 LFD: t5 n = 2, t30 n = 1; Ctx E4 LFD: t5 n = 2, t30 n = 1; OB E3 HFD: t30 n = 1; Str E4 LFD: t15 n = 1; FC E3 LFD: t15 n = 2, E4 LFD: t5 n = 1, t30 n = 1; Hy E4 LFD: t5 n = 1; Hi E3 LFD: t15 n = 1, t30 n = 1; Thal E4 LFD: t30 n = 1; Oc E3 LFD: t15 n = 1, E3 HFD: t30 n = 1, E4 LFD: t5 n = 1; Pc E4 LFD: t5 n = 2, t30 n = 1; CB E4 LFD: t5 n = 2; MB E3 HFD: t30 n = 1, E4 LFD: t5 n = 1; Po E3 HFD: t5 n = 1, E4 LFD: t5 n = 1). There was a significant effect of time in the whole brain (F(3,84) = 3.425, p = 0.028), in many brain regions, and serum levels (%Inj/ml, F(3,87) = 6.694, p = 0.0004), indicating 125I-insulin was distributed over time. Figure 5 shows the distribution pattern among the groups (E3 and E4, respectively) for the whole brain, select brain regions important for either cognition (olfactory bulb, cortex, hippocampus) or metabolism (hypothalamus), and into serum. There were no statistical differences due to genotype or diet but there was significant distribution over time.
125I-insulin distribution after IN delivery over time in E3 and E4 mice. The %Inj/g average for (A) whole brain, (B) olfactory bulb, (C) cortex, (D) hypothalamus, and (E) hippocampus or (F) %Ing/ml average for serum at each time point in E3 mice is presented. The %Inj/g average for (G) whole brain, (H) olfactory bulb, (I) cortex, (J) hypothalamus, and (K) hippocampus or (L) %Ing/ml average for serum at each time point in E4 mice is presented. Two-way ANOVA showed significant change over time for all regions except cortex. n = 3–9/timepoint/group (see Table 2 for n per group and timepoint). %Inj/g: percent injected insulin per gram of tissue.
Levels of 125I-insulin tended to peak at 30 min for most brain regions. Figure 6 shows a representative heat map for the brain wide distribution for 125I-insulin at this time point for females and males within each group. We also analyzed whether there were differences at this single time point between the groups (Suppl. Fig. S2). There were no statistical differences due to genotype or diet in either females (Fig. S2A–F) or males (Fig. S2G–L). Due to lack of diet differences, we further investigated whether there were genotype differences when the diets were collapsed (Suppl. Fig. 3). There was a genotype effect in the olfactory bulb in females at 30 min, with higher levels in E4 than E3 mice (Suppl. Fig. S3B). Overall, the distribution data indicate 125I-insulin following INL delivery spreads throughout the brain over time, but sex, genotype, and diet do not largely impact distribution, except for the olfactory bulb, at 15 months of age.
Female and male 125I-insulin brain distribution summary at 30 min following IN delivery. The average %Inj/g at 30 min for each group is represented by the heat map. %Inj/g: percent injected insulin per gram of tissue, LFD: low-fat diet, HFD: high-fat diet, OB: olfactory bulb, Str: striatum, FC: frontal cortex, Hy: hypothalamus, Hi: hippocampus, Th: thalamus, PC: parietal cortex, OC: occipital cortex, CB: cerebellum, MB: midbrain. Females: E3 LFD n = 5, E3 HFD n = 4, E4 LFD n = 4, E4 HFD n = 4. Males: E3 LFD n = 4, E3 HFD n = 2, E4 LFD n = 4, E4 HFD n = 2.
Discussion
We present for the first time the influences and interactions of apoE status, diet (HFD vs STD), and sex on the cognitive effects of insulin administered by the INL route. In separate mice, we also examine the effects of apoE status, diet (HFD vs LFD), and sex on the distribution within brain of insulin given by the INL route. However, our analysis of sex was hampered due to the increased mortality in males on a HFD. The excess male mortality seen in this study might be related to higher mortality in males than females following a high fat intake with regard to all-cause mortality47. The excess male mortality might also be related to potential cardiovascular disease, which is associated with higher mortality in males than females48. Typically, males show higher mortality rates than females under challenging environmental conditions, including not only high fat intake but also famine49.
E3 and E4 mice on a HFD showed reduced activity levels in the elevated zero maze and open field, as well as reduced swim speeds during visible platform training in the water maze (task learning). This is consistent with reduced activity in C57BL/6J and DBA/2 wild-type mice on a HFD in the open field50. E3 and E4 mice on a HFD showed increased anxiety levels in the elevated zero maze and open field. Consistent with these findings, insulin resistance is associated with increased anxiety levels51,52 and metformin treatment can reduce anxiety levels of mice on a HFD as measured in the elevated plus maze52,53. Mice with loss of hippocampal insulin receptor signaling show increased anxiety levels in the elevated plus maze as well as depressive-like behaviors54. Neuronal morphology is altered in the hippocampus of these mice, indicating insulin receptor signaling is necessary for dendrite integrity. While this anxiety effect of the HFD was more pronounced in E4 mice in the elevated zero maze, it was more pronounced in E3 mice in the open field. This result highlights that distinct anxiety measures are detected in the elevated plus maze and open field52,55. E3 and E4 mice on a HFD also showed impaired sensorimotor function on the rotorod, consistent with motor impairments in patients with diabetes56. Impaired sensorimotor function is E3 and E4 mice on a HFD is also consistent with the reduced activity levels in the open field and the exercise recommendation as guideline to treat diabetes57.
INL insulin differentially impacted cognitive performance in E3 and E4 mice on a HFD. Although activity levels were lower in HFD than STD mice and the HFD spent less time exploring both objects than STD mice, the main comparison is the percent time spent exploring the novel vs the familiar object, which likely abrogates the decreased activity. In addition, although insulin did not affect activity levels, there was a trend towards improved cognitive performance in this test, suggesting that reduced activity levels in general are not necessarily associated with reduced cognitive performance.
In the object recognition test, insulin was administered the day prior to the novel object testing. E3 and E4 mice on a HFD treated with saline spent a similar time exploring the two objects, but when treated with insulin, the E3 and E4 mice on a HFD showed a trend towards exploring the novel object more than the familiar object. In the water maze test, insulin was administered the day before hidden platform training and six days before assessment of spatial memory retention in the probe trial. In the first hidden platform session, there was a beneficial effect of treatment, with insulin-treated mice reaching the hidden platform faster than saline-treated mice. Thus, the treatment effect was similar in both genotypes when insulin was administered the day before the cognitive testing. However, when spatial memory retention was assessed in the water maze, insulin improved cognitive performance in E3 but not in E4 mice on a HFD. Conversely, insulin improved performance in E4 but not in E3 mice on a STD. Thus, the beneficial treatment effect of insulin on spatial memory retention in the water maze probe trial in E3 and E4 mice was not similar but also diet-dependent. Based on the difference in time interval between insulin administration and memory testing in the object recognition, first hidden platform session of the water maze and water maze probe trial test, we cannot exclude that there might be similar beneficial effects of insulin on cognitive performance in E3 and E4 mice on a HFD in the probe trial if insulin would be administered the day prior to the probe trial. Also, as only one dose of insulin (1 µg) was used in the current study, it is conceivable that a different dose of insulin is required for E3 than for E4 to reveal beneficial cognitive effects 6 days following administration. This is consistent with the finding in humans that those expressing apoE3 often react to drugs differently than those expressing apoE458. We observed significant differences within each sex in the weight of the brain regions following 6 months of HFD and due to genotype. Female brain regions were more affected by these risk factors than male brain regions. These brain region weight differences are similar to our previous report in cohorts of male and female E3 and E4 mice following the same diet regimen30. There have been clinical reports on E4 and obese individuals with AD exhibiting greater cortical atrophy59,60.
Insulin is distributed throughout the entire brain following INL delivery, spreading over a 1 h period and peaking around 30 min. Here, peak levels of 125I-insulin reaching the brain were on average 0.213%Inj/g. This is in contrast to the olfactory bulb, which contained nearly twofold more at approximately 0.39%Inj/g. The olfactory bulb is near the site of delivery and previous results have shown a similar increase in the amount of 125I-insulin present in this region40. Brain distribution did not differ due to genotype, diet, or sex, except when diet was collapsed in females at the 30 min time point, revealing a genotype difference in the olfactory bulb. These results suggest that any differences in the response to INL insulin due to apoE isoform, diet, or sex, are largely not due to the kinetics of delivery. The distribution pattern of INL insulin and lack of differences in a mouse model of AD compared to controls is similar to what we have found in SAMP8 mice40. One limitation of the current distribution study is that we did not investigate degradation, which could affect the overall amount of insulin delivered to a site of action. However, our previous report in the SAMP8 mice identified there was no group effect on insulin degradation40.
Multiple clinical studies have shown differences in the response to INL insulin. In one study, brain insulin responsiveness is negatively associated with peripheral insulin sensitivity and that this association is stronger in men, than women61. Men respond to chronic INL insulin compared to placebo controls, where women do not36. Additionally, people with E4 are less likely to respond or even fare worse after INL insulin therapy compared to non-E4 carriers45,46,62. The results from our distribution study indicate that the differences reported in the clinical literature and the behavioral effects of INL insulin observed in the current study are likely not due to group differences in the ability for insulin to reach the target sites in the brain, but rather due to differences in the ability to respond to the delivered insulin. Our behavioral data support the differences in the ability to respond to the INL insulin.
Unfortunately, we lost many male mice on the HFD. Remarkably, we did not see this in female mice on the HFD. Consistent with this pattern, we observed in an independent study an increased mortality in 6 month old male mice that remained on a HFD for 5 months when we were not allowed to visit the campus due to COVID-19-related modified operations63. What is striking is that on a HFD, we do not see a sex difference in final body weights, pointing towards an alternative cause of death than body weight gain. Consistent with this pattern, we have also seen in C57BL/6J wild-type mice that males are more susceptible to HFD-induced type II diabetes than females64. The potential reason for the remarkable sex difference for mortality and susceptibility to diabetes is a topic for future work.
Long-term INL insulin treatment normalized glucose tolerance and utilization and improved insulin signaling in the liver and thereby reduced insulin resistance65. The differential effects of E3 and E4 on lipid biosynthesis pathways might contribute to the distinct effects of INL insulin in E3 and E4 mice on different diets. Future work is needed to identify these lipid biosynthesis pathway changes taking place throughout the brain, with a focus on those regions critical for memory. These findings will further elucidate the pathway changes taking place in the brain due to apoE genotype, and diet, which may help explain our behavioral findings in these groups following INL insulin delivery since distribution is largely unaffected. Our work finds that INL insulin can enhance memory in some groups due to apoE genotype and diet and importantly, identifies those groups at risk for developing cognitive impairments (E4, obese, and/or female individuals) may still increase brain insulin levels through INL delivery.
Methods
Animals
Male and female human apoE targeted replacement (TR) mice, models originally generated by Dr. Patrick Sullivan66,67,68 were used in this study. All mice were on a standard chow diet (PicoLab Rodent Diet 20, no. 5053; PMI Nutrition International, St. Louis, MO, USA) prior to being placed on the specialty diets. Mice had ad libitum access to diets and water and were maintained on a 12 h light cycle. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at OHSU and the Puget Sound VA and were in compliance with all Federal regulations.
Experimental design
For cognitive assessment, female and male homozygous human E3 and E4 TR mice (n = 100 mice) were fed a HFD (60% kcal from fat, Research Diets D12492) or a remained on a STD (PicoLab Rodent Diet 20) for 6 months beginning at 9 months of age (tested at 15 months of age) (E3 males: n = 9 HFD and n = 12 STD; E3 females: n = 19 HFD and n = 13 STD; E4 males: n = 7 HFD and n = 11 STD; E4 females: n = 15 HFD and n = 14 STD). Mice were tested for behavioral performance in the elevated zero maze (week 1, day 1, morning), rotorod (week 1, days 1 and 2, afternoon), open field (week 1, days 2 and 3, morning), novel object recognition (week 1, days 4 and 5), and water maze (week 2, days 1–5, week 3, day 1) tests as described below (Fig. 7).
Behavior testing and INL delivery paradigm. Behavioral testing took place for 2 weeks after the completion of the 6-month diet regimen, beginning with the elevated zero maze, rotorod, and open field. Following completion of baseline testing in the novel object task, INL insulin (1 µg) or vehicle was administered, and mice were retested the following day. The next week, mice were tested in the water maze. After learning the task, mice were administered INL insulin or vehicle and retested for the hidden platform the following day. INL: intranasal. Generated using Biorender.com software.
For INL insulin distribution, mice were placed on an ingredient matched LFD (10% kcal from fat, Research Diets D12450Bi) or HFD. Final mouse numbers included (E3 males: n = 7 HFD and n = 10 LFD; E3 females: n = 15 HFD and n = 14 LFD; E4 males: n = 10 HFD and n = 15 LFD; E4 females: n = 16 HFD and n = 17 LFD).
INL insulin injections
A 10 μl Multi-flex tip (Thermo Fisher Scientific, Waltham, MA) was inserted to the surface of the cribriform plate at 4 mm depth level in a single nares for the 1 µl INL injection. Mice remained in the supine state for a duration of at least 30 s before being placed onto the right side. For behavior studies, INL insulin or saline was administered, under isoflurane anesthesia, at 1 µg/µl insulin in a total volume of 1 µl following the behavioral testing in the open field containing two identical object and prior to the novel object recognition test day and following visible platform training and prior to the first hidden platform training day in the water maze test (Fig. 7). For distribution studies, mice were anesthetized with 40% urethane prior to INL injection of 1 × 106 cpm/mouse 125I-insulin.
Elevated zero maze
Measures of anxiety were assessed in the elevated zero maze as previously described.
The elevated zero maze consisted of two open and two closed areas (each 35.5 cm in length; Hamilton-Kinder, Poway, CA). The closed areas were surrounded by opaque walls (15 cm tall). Mice were placed into the maze into one of the open areas and allowed to explore for a single 10-min trial. Distance moved and the percentage of time spent in the open areas were analyzed with Motor Monitor software (Kinder Scientific, Poway, CA). The zero maze was cleaned with 0.5% acetic acid between trials.
Rotorod
Sensorimotor function was assessed using the rotorod. Mice were placed on a rotating rod (diameter: 3 cm, elevated: 45 cm; Rotamex-5, Columbus Instruments, Columbus, OH,
USA). Rotation speed started at 5 rpm and accelerated 1.0 rpm every 3 s. Latency to fall (s) was recorded using photo beams located below the rod. Mice received three subsequent trials.
Open field and novel object recognition
The mice were habituated to an open field enclosure (16 × 16 inches, Kinder Scientific, Poway, CA) for 10 min on two subsequent days. The next day the mice were exposed to the open field containing two identical objects (orange wooden blocks in the shape of hexagonal prisms) for a 15-min trial. The objects were placed 10 cm apart and 15 cm from the adjacent walls of the arena. The following day, one object was replaced with a novel object (green wooden block in the shape of a triangular prism) and mice were allowed to explore for 15 min. During object recognition trials, objects were affixed to the floor of the arena using masking tape. The arenas and objects were cleaned with 0.5% acetic acid between trials. Physical interaction with the object in the form of sniffing within a 2 cm proximity was coded as object exploration by hand scoring videos acquired with Noldus Ethovision software (version 17, Wageningen, The Netherlands). A discrimination index was calculated. The time spent exploring the familiar object was subtracted from the time exploring the novel object, and the resulting number was divided by the total time spent exploring both objects.
Water maze
Hippocampus-dependent spatial learning and memory was assessed in the water maze. The maze consisted of a circular pool (diameter 140 cm) filled with opaque water (24 °C). Mice were first trained to locate an “escape” platform (plexiglass circle, 6 cm radius) submerged 2 cm below the surface of the water and made visible by the use of a cue (a colored cylinder, 2.5 cm radius, 8 cm height) during the “visible” trials (days 1 and 2).
For the visible and hidden platform training days, there were two daily sessions, morning and afternoon, which were separated by an intersession interval of 2 h. Each session with the visible or hidden platform consisted of two trials, with 10-min inter-trial intervals. Mice were placed into the water facing the edge of the pool in one of nine randomized locations (consistent for each mouse). A trial ended when the mouse located the platform. Mice that failed to locate the platform within 60 s were led to the platform by placing a finger in front of their swim path. Mice were taken out of the pool after they remained on the platform for a minimum of 10 s. The maze was divided conceptually into four quadrants. During the visible platform sessions (days 1 and 2), the location of the platform was moved between each of the four quadrants to avoid procedural biases in task learning. Subsequently, mice were trained to locate a hidden platform (days 3–5), requiring the mice to rely on extra maze cues for spatial reference and orientation.
Seventy-two hours after the last hidden platform training trial, spatial memory retention of the mice was assessed in the probe trial. During the probe trials, mice were placed into the water in the quadrant opposite of the target quadrant. The time required to first swim over the platform location, distance moved, and swimming speeds were analyzed using Noldus Ethovision video tracking software (Ethovision 17, Noldus Information Technology, Wageningen, Netherlands) set at ten samples/s.
Radioactive labeling of human insulin
Human insulin (Sigma-Aldrich, St. Louis, MO, USA) (ten microgram) was iodinated using the chloramine-T method as previously described30.
Radioactive insulin distribution
Following INL injection, samples were collected at 5, 15, 30, and 60 min. Whole blood was collected from the carotid artery and the whole brain and olfactory bulbs removed. The whole brain was dissected on ice into multiple regions including the frontal cortex (FC), striatum (Str), hypothalamus (Hy), hippocampus (Hi), thalamus (Th), parietal cortex (PC), occipital cortex (OC), cerebellum (CB), midbrain (MB), and pons (Po) by the method of Glowinski and Iversen69. Each region was weighed, and the amount of radioactivity (counts per minute, CPM) was measured in an automatic gamma counter (Wizard 2, Perkin Elmer). Blood was centrifuged for 3200 xg for 10 min to collect serum. Aliquots (50 µl) of serum were counted for radioactivity as well.
The percentage of the injected dose present in a ml of serum (%Inj/ml) was calculated by:
where Inj is CPM administered (measured by injection checks throughout the study) and CPM/ml is the amount of radioactivity in a ml of serum. The percentage of injected dose taken up per gram of brain region tissue (%Inj/g) was calculated at each time point by:
where W is the weight of the brain region in grams, and CPM is the amount of radioactivity present in each brain region.
Statistical analyses
All data are reported as mean ± standard error of the mean and were analyzed using SPSS v.22 (IBM, Armonk, NY, USA) or GraphPad v.8 (La Jolla, CA, USA) software.
For analysis of survival, contingency table and Fisher’s exact tests were used. Genotype (i.e., E3 or E4), diet (i.e., STD/LFD or HFD, and treatment (i.e., insulin or saline), or genotype and diet (in behavioral testing prior to insulin or saline treatment), were included as factors in analysis of variance (ANOVAs) as indicated. Due to the high mortality of male mice, we did not use sex as a factor for the analysis of the behavioral data. We used sex as a variable for body and brain weights due to sufficient n in each group. Additionally, to graphically represent insulin distribution after INL delivery at the 30 min time point (Fig. 6), we show sex separately. However, similar to the behavior data, due to the high mortality of male mice, we did not use sex as a factor for the analysis of the INL insulin distribution data. Repeated-measures were used when appropriate. Statistical significance was considered as p < 0.05. When sphericity was violated (Mauchly’s test), Greenhouse–Geisser corrections were used. For each study, the mice were tested in four separate cohorts, each containing mice of all experimental groups. For behavior, all researchers were blinded to genotype and treatment and the code was only broken after the data were analyzed. In the distribution study, we a priori planned for sex-dependent differences in body and brain weights and therefore analyzed the sexes separately. Outliers were removed by the ROUT method (Q = 1%).
Statement of approval
All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at OHSU and the Puget Sound VA and were in compliance with all Federal regulations and followed the ARRIVE guidelines.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information file.
References
Margolis, R. & Alszuler, N. Insulin in the cerebrospinal fluid. Nature 215, 1375–1376 (1967).
Woods, S. & Porte, D. Relationship between plasma and cerebrospinal fluid insulin levels of dogs. Am. J. Physiol. 233, E331-334 (1977).
Banks, W. et al. Transport of insulin across the blood-brain barrier: Saturability at euglycemic doses of insulin. Peptides 18, 1423–1429 (1997).
Chan, S. et al. Structure and evolution of insulin and insulin-like growth factors in chordates. Prog. Brain Res. 92, 15–24 (1992).
Banks, W., Owen, J. & Erickson, M. Insulin in the brain: There and back again. Pharmacol. Ther. 136, 82–93 (2012).
Banks, W. The source of cerebral insulin. Eur. J. Pharmacol. 490, 5–12 (2004).
Stranahan, A. et al. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 18, 1085–1088 (2008).
Infante-Garcia, C. et al. Long-term central pathology and cognitive impairment are exacerbated in a mixed model of Alzheimer’s disease and type 2 diabetes. Psychoneuroendocrinology 65, 15–25 (2015).
Freiherr, J. et al. Intranasal insulin as a treatment for Alzheimer’s disease: A review of basic research and clinical evidence. CNS Drugs 27, 505–514 (2013).
Liu, Y. et al. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J. Pathol. 225, 54–62 (2011).
Steen, E. et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease–is this type 3 diabetes?. J. Alzheim. Dis. 7, 63–80 (2005).
Talbot, K. et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Invest. 122, 1316–1338 (2012).
Kaiyala, K. et al. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes 49, 1525–1533 (2000).
Heni, M. et al. Evidence for altered transport of insulin across the blood-brain barrier in insulinresistant humans. Acta Diabetol. 51, 679–681 (2014).
Baskin, D. et al. Genetically obese Zucker rats have abnormally low brain insulin content. Life Sci. 36, 627–633 (1985).
Kern, W. et al. Low cerebrospinal fluid insulin levels in obese humans. Diabetalogia 49, 2790–2792 (2006).
Whitmer, R. A. et al. Obesity in middle age and future risk of dementia: A 27 year longitudinal population based study. Br. Med. J. Clin. Res. Ed. 330, 1360 (2005).
Banks, W. A. et al. Triglycerides cross the blood-brain barrier and induce central leptin and insulin receptor resistance. Int. J. Obes. (Lond.) 42, 391–397. https://doi.org/10.1038/ijo.2017.231 (2018).
Farr, S. A. et al. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology 149, 2628–2636. https://doi.org/10.1210/en.2007-1722 (2008).
Pricket, C., Brennan, L. & Stolwyk, R. Examining the relationship between obesity and cognitive function: A systematic literature review. Obes. Res. Clin. Pract. 9, 93–113 (2015).
Johnson, L. et al. Apolipoprotein E4 mediates insulin resistance-associated cerebrovascular dysfunction and the post-prandial response. J. Cereb. Blood Flow Metab. 39, 770–781 (2017).
Johnson, L. A., Torres, E. R., Impey, S., Stevens, J. F. & Raber, J. Apolipoprotein E4 and insulin resistance interact to impair cognition and alter the epigenome and metabolome. Sci. Rep. 7, 43701 (2017).
Johnson, L. et al. Amelioration of metabolic syndrome-associated cognitive impairmens in mice via a reduction in dietary fat content or infusion of non-diabetic plasma. EBioMedicine 3, 26–42 (2016).
Pratchayasakul, W. et al. Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sci. 88, 619–627 (2011).
Mahley, R. W. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science 240, 622–630 (1988).
Strittmatter, W. J. & Roses, A. D. Apolipoprotein E and Alzheimer’s disease. Annu. Rev. Neurosci. 19, 53–77 (1996).
Jackson, R. et al. APOE4 derived from astrocytes leads to blood–brain barrier impairment. Brain 145, 3582–3593 (2022).
Farrer, L. A. et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. J. Am. Med. Assoc. 278, 1349–1356 (1997).
Winkler, K. et al. Competition of Abeta amyloid peptide and apolipoprotein E for receptor-mediated endocytosis. J. Lipid Res. 40, 447–455 (1999).
Rhea, E. et al. Effects of apolipoprotein E isoform, sex, and diet on insulin BBB pharmacokinetics in mice. Sci. Rep. 11, 18636 (2021).
Zhao, N. et al. Apolipoprotein E4 impairs neuronal insulin signaling by trapping insulin receptor in the endosomes. Neuron 96, 115–129 (2017).
Lane-Donovan, C. & Herz, J. High-fat diet changes hippocampal apolipoprotein E (ApoE) in a genotype- and carbohydrate-dependent manner in mice. PLOS One 11, e0148099 (2016).
Ruiz, A. J. & Kullmann, D. M. Ionotropic receptors at hippocampal mossy fibers: Roles in axonal excitability, synaptic transmission, and plasticity. Front. Neural Circ. 6, 112. https://doi.org/10.3389/fncir.2012.00112 (2012).
Benedict, C. et al. Intranasal insulin improves memory in humans. Psychoneuroendocrinology 29, 1326–1334 (2004).
Rhea, E. M., Salameh, T. S. & Banks, W. A. Routes for the delivery of insulin to the central nervous system: A comparative review. Exp. Neurol. 313, 10–15 (2019).
Hallschmid, M. et al. Intranasal insulin reduces body fat in men but not in women. Diabetes 53, 3024–3029 (2004).
Benedict, C., Kern, W., Schultes, B., Born, J. & Hallschmid, M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J. Clin. Endocrinol. Metabol. 93, 1339–1344 (2008).
Reger, M. et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 70, 440–448 (2008).
Steen-Jensen, C. et al. Patients with Alzheimer’s disease who carry the APOE ε4 allele benefit more from physical exercise. Alzheim. Dement. 5, 99–106 (2019).
Rhea, E. M. et al. Intranasal insulin transport is preserved in aged SAMP8 mice and is altered by albumin and insulin receptor inhibition. J. Alzheim. Dis. 57, 241–252 (2017).
Salameh, T. S. et al. Central nervous system delivery of intranasal insulin: Mechanisms of uptake and effects on cognition. J. Alzheim. Dis. 47, 715–728 (2015).
Chen, Y. et al. Intranasal insulin restores insulin signaling, increases synaptic proteins, and reduces Abeta level and microglia activation in the brains of 3xTg-AD mice. Exp. Neurol. 261, 610–619 (2014).
Wagner, L. et al. Sex differences in central insulin action: Effect of intranasal insulin on neural food cue reactivity in adults with normal weight and overweight. Int. J. Obes. (Lond.) 46, 1662–1670 (2022).
Nijssen, K., Mensink, R. & Joris, P. Effects of intranasal insulin administration on cerebral blood flow and cognitive performance in adults: A systematic review of randomized, placebo-controlled intervention studies. Neuroendocrinology 113, 1–13 (2023).
Reger, M. A. et al. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J. Alzheim. Dis. 13, 323–331 (2008).
Rosenbloom, M. H. et al. A single-dose pilot trial of intranasal rapid-acting insulin in apolipoprotein E4 carriers with mild-moderate Alzheimer’s disease. CNS Drugs 28, 1185–1189 (2014).
Tamura, T. et al. Dietary carbohydrate and fat intakes and risk of mortality in the japanese population: The japan multi-institutional collaborative cohort study. J. Nutr. 153, 2352 (2023).
Beltran-Sanchex, H., Finch, D. & Crimmins, E. Twentieth century surge of excess adult male mortality. Proc. Natl. Acad. Sci. U. S. A. 112, 8993–8998 (2015).
Zarulli, V., Jones, B., Oksuzyan, A. & Vaupel, J. Women live longer than men even during severe famines and epidemics. Proc. Natl. Acad. Sci. U. S. A. 115, E832–E840 (2017).
Totten, M., Wallace, C., Pierce, D., Fordahl, S. & Erikson, K. The impact of a high-fat diet on physical activity and dopamine neurochemistry in the striatum is sex and strain dependent in C57BL/6J and DBA/2J mice. Nutr. Neurosci. 25, 2601–2615 (2022).
Li, C. et al. Diabetes and anxiety in US adults: Findings from the 2006 Behavioral Risk Factor Surveillance System. Diabet. Med. 25, 878–881 (2008).
Kundu, P. et al. Serum levels of -klotho are correlated with cerebrospinal fluid levels and predict measures of cognitive function. J. Alzheimer’s Dis. 86, 1471–1481 (2022).
Juliane, Z. et al. Metformin promotes anxiolytic and antidepressant-like responses in insulin-resistant mice by decreasing circulating branched-chain amino acids. J. Neurosci. 39, 5935–5948 (2019).
Reagan, L. P. et al. Hippocampal-specific insulin resistance elicits behavioral despair and hippocampal dendritic atrophy. Neurobiol. Stress 15, 100354. https://doi.org/10.1016/j.ynstr.2021.100354 (2021).
Figueiredo-Carquera, M. et al. Comparative analysis between Open Field and Elevated Plus Maze tests as a method for evaluating anxiety-like behavior in mice. Heliyon 9, e14522 (2023).
Murametsu, K. Diabetes mellitus-related dysfunction of the motor system. Int. J. Mol. Sci. 21, 7485 (2020).
Zahalka, S. et al. The role of exercise in diabetes. Endotext www.endotext.org (2023).
Hanson, A. J., Craft, S. & Banks, W. A. The APOE genotype: Modification of therapeutic responses in Alzheimer’s disease. Curr. Pharm. Des. 21, 114–120. https://doi.org/10.2174/1381612820666141020164222 (2015).
Wolk, D. A., Dickerson, B. C., Alzheimer’s Disease Neuroimaging, I. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 107, 10256–10261 (2010).
Ho, A. J. et al. Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiol. Aging 31, 1326–1339 (2010).
Wagner, L. et al. Brain insulin responsiveness is linked to age and peripheral insulin sensitivity. Diabetes Obes. Metab. 25, 2171–2180 (2023).
Reger, M. A. et al. Effects of intranasal insulin on cognition in memory-impaired older adults: Modulation by APOE genotype. Neurobiol. Aging 27, 451–458 (2006).
Kundu, P. et al. ApoE Isoform- and sex-dependent effects of xanthohumol on high fat diet-induced cognitive impairments and hippocampal metabolic pathways. Front. Pharmacol. 13, 954980 (2022).
Kundu, P. et al. Xanthohumol improves cognition in farnesoid X receptor-deficient mice on a high-fat diet. Dis. Model. Mech. 15, 049820 (2022).
Derkach, K. et al. Intranasal insulin restores metabolic parameters and insulin sensitivity in rats with metabolic syndrome. Bull. Exp. Biol. Med. 163, 184–189 (2017).
Sullivan, P. M., Mezdour, H., Quarfordt, S. H. & Maeda, N. Type III hyperlipoproteinemia and spontaneous atherosclerosis in mice resulting from gene replacement of mouse Apoe with human Apoe*2. J. Clin. Invest. 102, 130–135 (1998).
Sullivan, P. M. et al. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J. Biol. Chem. 272, 17972–17980 (1997).
Sullivan, P., Mace, B., Maeda, N. & Schmechel, D. Marked regional diffeences of brain human apolipoprotein E expression in targeted replacement mice. Neuroscience 124, 725–733 (2004).
Glowinski, J. & Iversen, L. L. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J. Neurochem. 13, 655–669 (1966).
Funding
The National Institute of Health (RF1AG059088 to JR, WAB) provided funding for this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceived and designed the analysis: WAB, JR; Collected the data: AC, EMR, AO, AB, RW, SP, WAB, JR; Contributed data or analysis tools: AC, EMR, AO, AB, RW, SP, WAB, JR; Performed the analysis: AC, EMR, AO, AB, RW, SP, WAB, JR; Wrote the paper: JR, EMR, WAB.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chaklai, A., Rhea, E.M., O’Niel, A. et al. Effects of a high-fat diet on cognition and brain distribution of intranasal insulin in E3 and E4 male and female mice. Sci Rep 14, 18641 (2024). https://doi.org/10.1038/s41598-024-62053-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62053-8
- Springer Nature Limited