Abstract
Common beans are a common staple food with valuable nutritional qualities, but their high contents in antinutritional factors (ANFs) can decrease the bioavailability of (i) fat-soluble micronutrients including carotenoids and (ii) minerals. Our objective was to select ANF-poor bean lines that would not interfere with carotenoid and mineral bioavailability. To achieve this objective, seeds of commercial and experimental Phaseolus vulgaris L. bean lines were produced for 2 years and the bean’s content in ANFs (saponins, phytates, tannins, total polyphenols) was assessed. We then measured carotenoid bioaccessibility and mineral solubility (i.e. the fraction of carotenoid and mineral that transfer into the aqueous phase of the digesta and is therefore absorbable) from prepared beans using in vitro digestion. All beans contained at least 200 mg/100 g of saponins and 2.44 mg/100 g tannins. The low phytic acid (lpa) lines, lpa1 and lpa12 exhibited lower phytate levels (≈ − 80%, p = 0.007 and p = 0.02) than their control BAT-93. However, this decrease had no significant impact on mineral solubility. HP5/1 (lpa + phaseolin and lectin PHA-E free) bean line, induced an improvement in carotenoid bioaccessibility (i.e., + 38%, p = 0.02, and + 32%, p = 0.005, for phytofluene bioaccessibility in 2021 and 2022, respectively). We conclude that decrease in the phytate bean content should thus likely be associated to decreases in other ANFs such as tannins or polyphenols to lead to significant improvement of micronutrient bioaccessibility.
Similar content being viewed by others
Introduction
The world's population is estimated to increase to 9.6 billion in 2050 in a context of climate change1. This alarming observation has pushed both scientists and industry to find sustainable solutions to improve nutrition. Increasing the proportion of pulses in our diet is widely recognized as a key factor in designing healthy sustainable diets for the future.
Phaseolus vulgaris L., or common bean, is a pulse commonly used as a staple food in seed form throughout the world. Due to the bean’s high protein content, common bean consumption can participate in reducing both meat consumption and cost of nutrition2. Common beans are rich in some B vitamins and minerals, but also contain bioactive compounds that can limit the assimilation of proteins, minerals, and vitamins. Due to these properties, these compounds are sometimes called antinutritional factors (ANFs)3. These ANFs include (i) phytates that can bind divalent cations (iron, magnesium, zinc) and proteins, limiting their digestion4, (ii) polyphenols and tannins that can also bind both cations and proteins5, (iii) saponins that can associate to lipid compounds such as cholesterol or to minerals and in turn reduce their intestinal absorption6, and (iv) the abundant seed storage protein lectin phytohemagglutinin PHA, which consists of 2 subunits (PHA-E and PHA-L, respectively), and which is known to interfere with iron absorption in animal studies7.
The presence of these ANFs in beans can thus decrease both mineral, fat-soluble vitamin and carotenoid bioavailability present in the same meal, as previously confirmed in vitro8,9.
Different treatments eliminate ANFs or reduce their activity (heat treatment, high pressure, hydration, fermentation) but these methods do not eliminate/inactivate the ANFs completely3,10,11. Decreasing bean ANF content by selecting optimized bean lines can be an interesting option to increase the bean’s nutritional properties. Beans with low phytic acid, i.e. lpa mutants12,13, or beans without phaseolin/PHA-E have previously been developed14. In this study, we used three groups of bean lines:
-
lpa mutants The lpa1 mutation15,16 was introduced in the BAT 93 background (CIAT, Cali, Colombia), as described by17. The lpa12, lpa2, lpa3 and lpa4 mutants were isolated as lines with a high inorganic phosphate content through a screening of an EMS mutagenized common bean collection, as described previously17. The lpa12 mutant was characterized as an lpa mutant allelic to lpa1 and described in details elsewhere17.
-
Phaseolin and/or lectin and/or lpa mutants Phaseolin, the most abundant protein in beans, is not considered an ANF. However, its low digestibility has encouraged researchers to reduce its content in bean lines to improve their nutritional quality14. In this group, BAT 881 hybrid, created by CIAT, Colombia18,19,20 was considered as a control as it contains phaseolin, PHA-L and PHA-E. Line 938 was obtained as previously described15 and contains phaseolin and PHA-L, but neither PHA-E nor α-amylase inhibitor (a lectin-like protein). Finally, HP5/1 is also a lpa mutant harboring the same mutation as lpa1 but in another genetic background14. This line contains PHA-L but neither phaseolin nor PHA-E and α-amylase inhibitor.
-
Commercial lines Meccano and Mercato bean varieties were purchased from Blumen (Piacenza, Italy) and selected because of their capacity to resist high temperatures21,22. PAN-123 and PAN-146 varieties were purchased from Pannar (South Africa).
The different bean lots were prepared in a recipe containing canned tomato pulp and red bell peppers. This recipe was chosen to mimic a meal rich in carotenoids. Our objective was to determine whether changes in bean ANF composition could lead to an improvement of the bioaccessibility/solubility of carotenoids and minerals ingested within the same meal using in vitro digestion.
Material and methods
Chemicals
Retinyl acetate, β-carotene, α-carotene, vitamin D3 (> 95% pure), deuterated vitamin D3, 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD), phytic acid, DMACA, vanillin, iron III, pancreatin, ammonium thiocyanate, catechins, α-amylase, pepsin, bile, ferric chloride and sodium carbonate were purchased from Sigma Aldrich (Saint-Quentin-Fallavier, France). Lutein, lycopene, phytoene and phytofluene were purchased from Extrasynthèse (Genay, France). Soyasaponin I was generously supplied by Stéphane Georgé, CTCPA Avignon. Canned tomato pulp, red bell peppers and Isio4 oil (Lesieur, Asnières, France) were purchased from a local supermarket (Marseille, France). Methanol, acetonitrile, hexane, dichloromethane, methyl-tert-butylether, acetone, ethanol absolute anhydrous, sulfuric acid, hydrochloric acid, nitric acid, formic acid, hydrogen peroxide were purchased from CarloErba Reagents (Peypin, France).
Bean production
The experimental and commercial bean seeds were germinated in greenhouses, then the 3–4-week-old seedlings were grown in open fields in Montanaso Lombardo (45°20′12″N 9°28′11″E, CREA GB, Italy), during Spring–Summer 2021 and 2022. Eight bean lines were produced in both 2021 and 2022 (BAT 93, lpa12, lpa4, BAT-881, HP5/1, Meccano, Mercato). Five additional bean lines were produced in 2022 (lpa1, lpa2, lpa3, PAN-126 and PAN-143). After harvest, the seeds were placed at – 20 °C for 3 weeks to prevent the action of Acanthoscelides obtectus (weevil), which spoils the seeds. Finally, the seeds were dried in an oven at 37 °C for five days.
Bean cooking
A first set of experiment was performed to determine bean cooking time. Briefly, samples of 20 seeds were soaked for 16 h in distilled water at room temperature. A Mattson pin drop cooker (Michigan State University Department of Physics and Astronomy Machine Shop) was then used to measure cooking times of soaked seeds23. The cooker containing 25 seeds (20 seeds from the same sample and 5 control seeds) from a single sample was placed in a glass beaker with boiling distilled water set on a hot plate. Individual beans were considered cooked when the piercing rod had passed through a seed. The sample cooking time was recorded when 80% of the pins pierced the beans. Two technical replicates were evaluated for each sample.
Bean cooking was then performed in an experimental kitchen (CREA AN, Italy). To this aim, approximately 300 g of dried beans were soaked for 17.5 h at 20 °C in 1 L of distilled water. After draining soaking water, beans were cooked in a pot with approximately 2 L of boiling distilled water, over moderate heat, to mimic home cooking. Each sample was cooked until beans felt tender when pierced with a fork, considering the reference cooking times previously established. Remaining cooking water was drained. Beans were then vacuum-sealed in plastic bags and were used withing 3 weeks.
Lyophilization and ANFs quantification
Once the vacuum-bags of beans were opened for in vitro digestion, a fraction of beans was lyophilized for 7 days in a Christ-alpha manifold 100400 freeze-dryer from Fischer Scientific. Then, beans were dry milled in a Pulverisette 2 (Fritsch, Idar-Oberstein, Germany) and stored in plastic tubes at room temperature until antinutrient analysis (saponins, phytates, tannins, and total polyphenols).
Saponins
Triterpene saponins were analyzed using a spectrophotometric method adapted from24. Briefly, 1 g of sample was weighed separately and mixed with 5 mL of 80% methanol diluted in ultrapure water at room temperature for 24 h. The mixture was then centrifuged for 10 min at 10 °C and 4500 rpm. The supernatant was recovered and placed in new tubes. The procedure was repeated twice, and the supernatant was recovered in the same tube and homogenized. The supernatant (0.125 mL) was mixed with 0.125 mL of 80% methanol and 0.250 mL of 8% vanillin diluted in pure ethanol absolute anhydrous. Tubes were placed in an ice bath, and 2.5 mL of 72% sulfuric acid diluted in water was added. After 15 min, the tubes were incubated for 10 min at 60 °C in a shaking incubator. Then the tubes were placed back in the ice bath. A calibration curve was obtained using a soyasaponin I standard, diluted in 80% methanol and treated in the same way as the samples. The absorbance was measured at 544 nm in a spectrophotometer (Shimadzu UV-1800) against a solution containing the same amounts of methanol, vanillin, and sulfuric acid as the samples. All samples were measured by triplicate.
Phytates
Phytate quantification was performed using a spectrophotometric method adapted from25. Briefly, 0.2 g of sample was mixed with 10 mL of 0.5 M hydrochloric acid (to achieve a high extraction percentage) at room temperature for 60 min. The tubes were then centrifuged for 10 min at 4 °C and 4500 rpm. The supernatant was extracted to another tube and refrigerated at 4 °C until measurement on the same day. The complex of iron(III) thiocyanate was prepared using: 25 mL of ferric chloride (300 μg/mL), 25 mL of ammonium thiocyanate (150 mg/mL), 0.2 mL of concentrated nitric acid, and water to complete 100 mL. The calibration curve was prepared using a solution of phytic acid 1 g/L, diluted with ultrapure water and 0.5 M hydrochloric acid (the same volume of the dilution to obtain a condition similar to the samples). A blank tube was prepared with 100 μL of 0.5 M hydrochloric acid and 900 μL of ultrapure water for the spectrophotometer method. 2 mL of the iron(III) thiocyanate complex was added to 0.1 mL of the supernatants, 0.9 mL of ultrapure water in tubes, the blank tube, and 1 mL of the calibration curve tubes. All tubes were stirred at 40 °C for 2 h and 30 min, then cooled to room temperature and centrifuged for 5 min at 4 °C and 4500 rpm. The absorbance was measured against the blank at 480 nm in a spectrophotometer (Shimadzu UV-1800). All samples were measured in triplicate.
Tannins
The tannin assay was adapted from26 and10. Briefly, 0.12 g of samples were extracted in 12 mL of ultrapure water, with agitation for 30 min. After agitation, the samples were centrifuged for 10 min at 4 °C and 4500 rpm. The supernatants were transferred to new tubes. The calibration curve was prepared from a catechin solution at a concentration of 500 mg/L, diluted in pure methanol and water 1:1 (v/v). DMACA was prepared the day of the experiment from a solution of 2% (w/v) DMACA in pure methanol and 6 M hydrochloric acid 1:1 (v/v). DMACA solution (9 mL) were required and then diluted in pure methanol to a total volume of 25 mL. In new tubes, 4 mL of the sample extract (homogenized), calibration, and reference solutions were added individually, followed by 1 mL of DMACA solution and 2 mL of 50% methanol diluted in water 1:1 (v/v) to all tubes. All tubes were mixed for 20 min at room temperature. The absorbance was measured at 640 nm. All samples were measured in triplicate.
Total polyphenols
Total polyphenols were measured using the Folin-Ciocalteu method, optimized and adapted to plant-derived products27. Samples (1 g) were extracted with a 7:3 (v/v) solution of acetone and water for 30 min. The supernatants were filtered and placed into new tubes (raw extracts). The acetone content of the mixture was reduced to 7% using distilled water, then 2 mL of the mixture were placed on an Oasis cartridge and filtered (washing extracts). A fraction of the washing extracts was then incubated for 2 h at 85 °C in an oil bath (heated washing extracts).
The raw, washing and heated washing extracts were individually added to 2.5 mL of Folin-Ciocalteu reagent diluted 1:10 (v/v) in water and incubated for 2 min at room temperature. Then 2 mL of sodium carbonate (75 g/L) was added. All tubes were incubated for 15 min at 50 °C. After incubation, the tubes were cooled in an ice bath and the absorbance was measured briefly at 750 nm by HPLC (gradient of 0.5% water, formic acid, and acetonitrile). The results were finally expressed in gallic acid equivalents.
In vitro digestion
Fresh red bell peppers were first cooked in a pan for 45 min with a spoon of water. Beans were then added to canned tomato pulp and cooked red bell peppers. Isio4 oil was supplemented with vitamin D3 at a concentration of 0.14 mg/100 mg of oil and was added just before the beginning of the digestion assay. Each test meal contained 3 g of cooked beans, 1.5 g of cooked red bell peppers, 1.5 g of canned tomato pulp and 100 µL of oil. In vitro digestions were conducted by mimicking an oral, a gastric and a duodenal step as previously described28, except that all volumes were divided by two. The digesta obtained at the end of the duodenal phase was centrifuged at 2500 rpm for 1 h at 10 °C to isolate the aqueous phase of the digesta, which contained the absorbable compounds (fat-soluble compounds included in mixed micelles and soluble minerals). Aliquots of the digestas and aqueous phases were frozen at – 80 °C until analysis. In vitro digestions were performed in quadruplicate for each sample.
Carotenoid extraction and quantification by HLPC
A double hexane extraction was performed to extract carotenoids from each sample (digesta or aqueous phase). Briefly, 500 μL aliquots of the samples were mixed with 500 μL of retinyl acetate in ethanol (internal standard). Hexane (2 mL) were added and the mixture was vortexed for 10 min. Then, all samples were centrifuged for 10 min at 2500 rpm, 4 °C. The hexane phase was carefully removed and transferred to another tube. The process was repeated twice and the second extracted phase was pooled with the first one. Once dried, the residue was recovered with 200 μL of methanol and dichloromethane (65/35, v/v). The mixture was vortexed and transferred to a vial for HPLC analysis. The high-performance liquid chromatography (HPLC) analysis was carried out on a Thermo Scientific UltiMate 3000 with a C30 column at 40 °C according to a previously published protocol29. A volume of 50–80 μL was injected. Identification of carotenoids was performed with Chromeleon 7.2 (ThermoFischer Scientific). Quantification was done by comparing the peak areas with standard curves.
Carotenoid bioaccessibility was defined as the percentage of the amount of carotenoids found in the aqueous phase divided by the amount of carotenoids found in the digesta.
Vitamin D extraction and quantification by LC-MS2
Samples were stored at – 80 °C before analysis. The protocol was adapted from30 and 500 μL of sample was required. A solution of deuterated cholecalciferol in ethanol (20 μL) at a concentration of 0.4 ng/μL was also required. The extracted cholecalciferol was derivatized with a solution of PTAD in acetonitrile at a concentration of 4 mg/mL. PTAD (50 μL) were added to the dry extract, mixed and incubated for 10 min. This step was repeated and stopped at 5 min when 20 μL of ultrapure water was added, mixed and incubated again for 5 min. The mixture was evaporated under nitrogen. The dry residue was diluted in 100 μL of acetonitrile and transferred to a vial for analysis by liquid chromatography coupled to tandem mass spectrometry (LC–MS2) using a Thermo Fischer Scientific instrument (Illkirch, France). Chromeleon 7.2 ThermoFischer Scientific software was applied to set up, directly control, and process data. Cholecalciferol was quantified as the ratio of the peak area and the retention time to the result of the deuterated solution.
Vitamin D bioaccessibility was defined as the percentage of the amount of vitamin D found in the aqueous phase divided by the amount of vitamin D found in the digesta.
Mineral extraction and quantification by ICP-OES
Iron, zinc, magnesium, and copper were extracted from individual ingredients (canned tomato pulp, red bell peppers and beans), digestas, and aqueous phases. Briefly, 0.45 g of solid samples, or 1 mL of digestas or aqueous phases (0.1 g of dry matter), were placed in adequate teflon containers. A solution of 69.5% nitric acid and 30% hydrogen peroxide was added in the following proportions: 7:1 for solid samples and 1.75:0.25 for micro-extractions. After 30 min, the containers were closed and the mixtures were microwave-digested/mineralized using the ETHOS™ EASY Milestone at 1200 Watts for 1 h 15 min (including cooling time). The contents were transferred to plastic tubes, and ultrapure water was added to reach a final volume of 25 mL for solid samples and 6 mL for digestas/aqueous phases, respectively. The final solutions were analyzed with the Agilent Technologies 5100 inductively coupled plasma optical emission spectrometry (ICP-OES) system. All replicates of digestas and aqueous phases were measured once and were calculated as the mean ± SEM of a sample. Ingredients were measured once. ICP-OES carried out three measurements before averaging the results.
Solubility of minerals was calculated as the percentage of the amount of minerals found in the aqueous phase of the digesta divided by the amount of minerals in the digesta.
Zinc bioavailability estimation
For comparison with the zinc solubility in the digesta, predicted zinc absorption was calculated according to31.
For this, we used:
where TAZ = total absorbed zinc (mmol), TDZ: total dietary zinc (mmol), TDP: total dietary phytates (mmol). For this purpose, we used the zinc content and phytates content measured in our samples. TAZ was estimated for a bean portion of 200 g. Then zinc absorption (ZA) was calculated as the ratio TAZ/TDZ:
Statical analysis
Carotenoid bioaccessibility and mineral solubility obtained with BAT 93 were set to 100% and all other bioaccessibility and solubility values were expressed relative to BAT 93 condition.
After verifying the result's nature, the Kruskal–Wallis test was applied to test the differences between genotypes in groups of more than 3 samples and a Wilcoxon-Mann Whitney test for groups with only 2 samples. If a significant difference was found, a Nemenyi test was applied as a post hoc test. Results were expressed as means ± SEM. Values were considered significant when p < 0.05 (alpha 5%, IC = 95).
For all comparations, each genotype was tested against its control, and commercial lines were tested against BAT 93. Principal component analysis (PCA) was applied to summarize and visualize the dataset, and then identify correlations between the variables. R Studio version 4.3.2 was used for the analysis.
Results
Nutritional profiles of the ingredients for in vitro digestion
Table 1 shows the ANF, carotenoid and mineral contents of both ingredients (canned tomato pulp, red bell peppers) and cooked bean samples.
The lpa mutant bean lines had less phytates than their control for a given year. lpa1 and lpa12 lines had the lowest phytate amount (≈ − 80% compared to BAT-93, p = 0.007 and p = 0.02). HP5/1 displayed a lower content in phytates than BAT-881 (> − 50% compared to BAT-881, p = 0.09). Finally, in the group of commercial bean lines, Mercato beans contained significantly less phytates and saponins than BAT-93-T beans in 2021 (− 54.1% and − 48.0% for phytates and saponins, respectively, p = 0.02, p = 0.02), but these differences were not found in 2022. ANF were not measured in tomato pulp and red bell peppers.
The canned tomato pulp and the red bell peppers used for the in vitro digestion in 2021 and 2022 were from the same lots stored at – 80 °C. Carotenoid analysis showed that canned tomato pulp was rich in lycopene, phytoene and phytofluene (486.8 μg/100 g, 1064.3 μg/100 g, and 187.2 μg/100 g, respectively), and also contained β-carotene and lutein (52.3 μg/100 g and 32.5 μg/100 g, respectively). Red bell peppers contained large amount of β-carotene and phytoene (195.8 μg/100 g and 419.7 μg/100 g, respectively), as well as α-carotene, lutein and phytoene (22.4 μg/100 g, 11.9 μg/100 g, and 55.0 μg/100 g, respectively). Regarding the bean lines, BAT 93 contained a minor amount of β-carotene (0.01 μg/100 g). All the beans contained trace amounts of lutein (from 0.05 to 0.91 μg/100 g).
No significant differences between the lpa mutant bean lines and their respective control were found for mineral contents in 2021 and 2022. In the low phaseolin/lectin/phytate line (HP5/1), a lower iron and magnesium content was found when compared to BAT-881 (− 46% and − 39% in 2023, respectively, p = 0.02, p = 0.03). Mercato beans contained less iron (− 49%, p = 0.02) and less zinc (− 44%, p = 0.02) than BAT-93 in 2021, but these differences were not observed in 2022. PAN-146, which was only characterized in 2022, had more zinc (+ 92%, p = 0.049), magnesium (+ 39%, p = 0.01) and copper (+ 90%, p = 0.01) than BAT-93.
Bioaccessibility of carotenoids and vitamin D
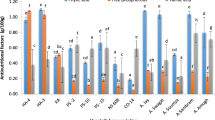
In 2021, Mercato beans were associated with a higher bioaccessibility of lycopene when compared to BAT-93 (p = 0.02, Fig. 1c). However, this was not found in 2022, where Mercato beans were associated with a lower bioaccessibility of β-carotene (p = 0.03, Fig. 1b), phytoene (p = 0.002, Fig. 1f), phytofluene (p = 0.002, Fig. 1h) compared to its control.
Carotenoid bioaccessibility from test meals containing the different beans lines. Test meals were constituted of cooked beans prepared with oil, tomato pulp and red bell peppers. In vitro digestion was performed to mimic the oral, gastric and duodenal steps. Carotenoid bioaccessibility was defined as the percentage of the amount of carotenoid found in mixed micelles divided by the amount found in the digesta. Data are the mean ± SEM, n = 3 to 4. Significant differences with the control BAT 93 are represented by (#). Significant differences with the control BAT-881 are represented by (¤). For both symbols (#, ¤): *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Compared to BAT-881, HP5/1 was associated with a higher bioaccessibility of phytofluene (+ 38%, p = 0.02, Fig. 1g) in 2021, and a higher bioaccessibility of β-carotene (+ 169%, p = 0.02,
Fig. 1a), phytoene (+ 32%, p = 0.005, Fig. 1f) and phytofluene (+ 36%, p = 0.005, Fig. 1h) in 2022.
No difference was found regarding vitamin D bioaccessibility (data not shown).
Solubility of minerals
In 2021, HP5/1 was associated with a lower solubility of iron (− 14%, p = 0.02, Fig. 2a) and copper (− 4%, 0.02, Fig. 2g) but a higher solubility of magnesium (+ 29%, p = 0.02, Fig. 2e) compared to its control, while test meals containing Mercato beans had a higher zinc solubility (+ 34, p = 0.02, Fig. 2c) compared to test meals containing BAT-93. The only effect confirmed in 2022 was the lower iron solubility (− 29%, p = 0.049, Fig. 2b) observed for test meals containing HP5/1 compared to its control.
Mineral solubility from test meals containing the different beans lines. Test meals were constituted of cooked beans prepared with oil, tomato pulp and red bell peppers. In vitro digestion was performed to mimic the oral, gastric and duodenal stages. Mineral solubility was defined as the percentage of the amount of mineral found in the aqueous phase divided by the amount found in the digesta. Data are the mean ± SEM, n = 3 to 4. Significant differences with the control BAT-93 are represented by (#). Significant differences with the control BAT-881 are represented by (¤). For both symbols (#, ¤): *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
The prediction of total absorbed zinc (TAZ) was significantly higher for lpa12 mutants than for its control BAT-93 (+ 50%, p = 0.02) in 2021 and 2022. Compared to their control BAT-93, zinc absorption (ZA) was higher in lpa12 and Mercato beans (p = 0.02, p = 0.02) in 2021 and lpa1 (p = 0.008) in 2022 (Table 2).
Correlation between ANF content and bioaccessibility/solubility of fat-soluble compounds and minerals.
For 2021, the first and the second principal components of the PCA (Fig. 3a,b) explained 26% and 21.5% of the total variance, respectively. The first axis shows that total polyphenol contents are inversely correlated with the solubility of magnesium, iron and cupper. The second axis shows that tannin and saponin contents are inversely correlated with lycopene, phytoene and β-carotene bioaccessibility.
Relationship between carotenoid bioaccessiblity/ mineral solubility and bean content in minerals, phytates, saponins and tannins in 2021. A principal component analysis (PCA) was conducted on all samples to analyze the relationship between the presence of phytates, saponins, tannins and carotenoid bioaccessibility and mineral content/solubility (b). Correlation values are given in panel (a).
For 2022 (Fig. 4a,b), the first and the second principal components of the PCA explained 26.1% and 20.6% of the total variance, respectively. The first axis also shows an inverse correlation between the solubility of minerals or mineral contents (except for copper) and the bean content in total polyphenols, tannins and saponins.
Relationship between carotenoid bioaccessiblity/ mineral solubility and bean content in minerals, phytates, saponins and tannins in 2022. A Principal Component Analysis (PCA) was conducted on all samples to analyze the relationship between the presence of phytates, saponins, tannins and carotenoid bioaccessibility and mineral content/solubility (b). Correlation values are given in panel (a).
Discussion
This study aimed at evaluating the impact of the ANF content of prepared beans on carotenoid bioaccessibility and mineral solubility.
The ANF contents given are those found in cooked beans. As soaking and cooking can reduce soluble ANF levels10, these contents are certainly lower than in raw beans.
This study was conducted with beans produced in 2021 and in 2022, as the nutritional and antinutritional composition of beans can vary from one year to another. For instance, Mercato beans contained less phytates and less saponins than BAT 93 in 2021, but not in 2022. These differences can partly be explained by the growth conditions and the stability of the bean profiles. The 2022 cropping season was characterized by high temperatures and low rainfall. Although plants were bred in experimental fields and subjected to controlled irrigation, dry years such as 2022 may influence the chemical composition of bean seeds. It is therefore not surprising to observe significant differences in the nutritional characteristics of seeds obtained in 2021 and 2022. However, despite these variations, the amount of ANFs determined generally confirmed the different nutritional profile of each bean variety. All lpa mutant beans had reduced phytate amounts. Some of these differences were not significant, but this might possibly be due to the number of samples analyzed.
The lpa mutant beans have been further developed for several reasons. The first reason was to improve mineral absorption. Indeed, phytic acid can bind to divalent cations like iron, zinc, and calcium, reducing their absorption in the digestive tract. The lpa beans may thus facilitate the absorption of these essential minerals. The negative effect of phytates has also been found for other micronutrients. We previously showed that phytates can decrease both vitamin D and K bioaccessibility and their uptake by enterocytes8,9. This can be particularly deleterious for the nutrition of populations in regions where beans are staple foods and where dietary deficiencies in minerals are common32.
Phaseolin and lectin variants were further studied because of their nutritional interest. Indeed, these variant beans had a lower content of the antinutrients lectins that can interfere with iron absorption7 and the absence of phaseolin can improve protein digestibility33. The HP5/1 line also had a reduced phytate content33.
Since bean ANFs cannot only impact on the bean’s micronutrient bioavailability but also on the bioavailability of micronutrient coming from the same meal, beans were also included in a micronutrient-rich recipe to study their effect on both mineral solubility and carotenoid bioaccessibility. Beans were associated to tomato and red bell pepper, two vegetables particularly rich in carotenoids34,35. Vitamin D-enriched oil was also added to the test meals. All test meals were digested using an in vitro digestion model previously validated to evaluate both fat-soluble compound bioaccessibility and mineral solubility9. Despite their low phytate content, lpa mutant beans did not improve the mineral solubility and carotenoid/vitamin D bioaccessibility. This result can have several explanations. The first one is that lpa mutant lines still contain phytates at a concentration that may be sufficient to have a negative effect on mineral solubility. The second reason might be that the lpa lines, as shown in our study, contain significant amounts of tannins, polyphenols and saponins. Both tannins, polyphenols and saponins have the ability to chelate or bind to minerals, forming complexes that are often insoluble, which consequently decrease mineral bioavailability36. Some tannins and polyphenols can also inhibit digestive enzymes, which can interfere with the proper digestion and absorption of micronutrients in the gastrointestinal tract37,38. Saponins further limit the incorporation of lipids into mixed micelles produced during digestion39 and haver a highly significant negative effect on vitamin K bioaccessibility, at least in vitro8. Finally, we did not quantify fibers in our study. These fibers are not ANFs, but fibers can impair the bioaccessibility of both minerals8,10 and fat-soluble micronutrients such as carotenoids40.
Our results differ from a previous clinical trial showing an increased iron bioavailability of common bean lpa1 mutants in human volunteers41. In this study, the phytate content of the lpa1 beans was 90% lower when compared to a control, while the polyphenol content remained similar. In our study, only lpa1 showed a comparable decrease in phytates. However, even if lpa1 mutant content in polyphenols was similar to the control bean line, lpa1 saponin content was significantly higher than in the control bean line, which may impact on micronutrient bioaccessibility. Interestingly, an increased carotenoid bioaccessibility was obtained with HP5/1 beans when compared to their respective control BAT-881. This may be due to their low content in phytates in addition to low phaseolin and lectin. However, mineral solubility in the presence of HP5/1 beans was not improved compared to the control condition.
We then estimated zinc absorption by using a prediction equation31. Such prediction was not performed with iron as iron prediction equation requires to know the iron status of the individuals to be calculated31. When estimating zinc absorption from a portion of 200 g of lpa beans, it was predicted zinc increased twofold compared to its control. This increase that was not observed in vitro but we only estimated the solubility (i.e. the mineral found in the aqueous phase of the digesta after digestion) while the equation is supposed to predict the amount of zinc after digestion and absorption by the intestine. Such discrepancy can also derive from the fact that the zinc bioavailability equation does not consider polyphenols and tannins from the diet.
However, the PCA further confirmed a negative correlation between ANFs and both carotenoid bioaccessibility and mineral solubility, according to previous PCA analysis8.
Overall, our study indicates that the total ANF content of the mutated bean lines is still not sufficiently optimized. Further agronomical research is thus needed to improve the phytate content in bean seeds but also of other ANFs.
Additionally, evaluating the fiber content of beans, and to finely characterize in greater detail all polyphenols present in each bean line would be interesting and will be, therefore, a target for future studies.
Finally, we previously also showed that the ANF content of bean seeds can be decreased by optimizing their preparation process10. This decrease was, however, insufficient to improve fat-soluble micronutrient bioavailability. We, therefore, think that combining seed improvement with an optimized processing method can ultimately contribute to a significant improvement of micronutrient bioavailability from common beans.
Data availability
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
ONU. ONU : La Population Mondiale Devrait Atteindre 9,6 Milliards en 2050|Nations Unies. United Nations. https://www.un.org/fr/desa/un-report-world-population-projected-to-reach-9-6-billion-by-2050 (2023).
Carbas, B., Machado, N. & Oppolzer, D. Nutrients, antinutrients, phenolic composition, and antioxidant activity of common bean cultivars and their potential for food applications. Antioxidants 9, 186 (2020).
Cominelli, E., Sparvoli, F. & Lisciani, S. Antinutritional factors, nutritional improvement, and future food use of common beans: A perspective. Front. Plant Sci. 13, 169 (2022).
Lolas, G. M. & Markakis, P. Phytic acid and other phosphorus compounds of beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 23, 13–15 (1975).
Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 56, 317–333 (1998).
Francis, G., Kerem, Z., Makkar, H. P. S. & Becker, K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 88, 587–605 (2002).
Hisayasu, S. et al. Soybean protein isolate and soybean lectin inhibit iron absorption in rats. J. Nutr. 122, 1190–1196 (1992).
Margier, M., Antoine, T. & Siriaco, A. The presence of pulses within a meal can alter fat-soluble vitamin bioavailability. Mol. Nutr. Food Res. 63, e1801323 (2019).
Antoine, T. et al. Evaluation of vitamin D bioaccessibility and mineral solubility from test meals containing meat and/or cereals and/or pulses using in vitro digestion. Food Chem. 347, 128621 (2021).
Antoine, T., Georgé, S. & Leca, A. Reduction of pulse “antinutritional” content by optimizing pulse canning process is insufficient to improve fat-soluble vitamin bioavailability. Food Chem. 370, 131021 (2022).
Margier, M., Georgé, S. & Hafnaoui, N. Nutritional composition and bioactive content of legumes: Characterization of pulses frequently consumed in france and effect of the cooking method. Nutrients 10, 1668 (2018).
Wang, W. et al. Genetic control of seed phytate accumulation and the development of low-phytate crops: A review and perspective. J. Agric. Food Chem. 70, 3375–3390 (2022).
Colombo, F. et al. MRP transporters and low phytic acid mutants in major crops: Main pleiotropic effects and future perspectives. Front. Plant Sci. 11, 301 (2020).
Giuberti, G., Tava, A. & Mennella, G. Nutrients’ and antinutrients’ seed content in common bean (Phaseolus vulgaris L.) lines carrying mutations affecting seed composition. Agronomy 9, 317 (2019).
Campion, B. et al. Isolation and characterisation of an lpa (low phytic acid) mutant in common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 118, 1211–1221 (2009).
Panzeri, D. et al. A defective ABC transporter of the MRP family, responsible for the bean lpa1 mutation, affects the regulation of the phytic acid pathway, reduces seed myo‐inositol and alters ABA sensitivity. Panzeri: 2011: New Phytologist (Wiley, 2011). https://doi.org/10.1111/j.1469-8137.2011.03666.x.
Cominelli, E., Confalonieri, M. & Carlessi, M. Phytic acid transport in Phaseolus vulgaris: A new low phytic acid mutant in the PvMRP1 gene and study of the PvMRPs promoters in two different plant systems. Plant Sci. 270, 1–12 (2018).
CIAT. Fighting Drought and Aluminum Toxicity : Integrating Functional Genomics Phenotypic Screening and Participatory Evaluation with Women and Small-Scale Farmers to Develop Stress-Resistant Common Bean and Brachiaria for the Tropics: Final Report, Reporting Period April 2006-March 2010. 235 https://cgspace.cgiar.org/handle/10568/71265 (2010).
CIAT. Annual Report 2002-Project SB-2: Assessing and Utilizing Agrobiodiversity Through Biotechnology. http://ciat-library.ciat.cgiar.org/articulos_ciat/annual_report_2002/biotechn_2002.pdf (2002).
Gebeyehu, S. Physiological Response to Drought Stress of Common Bean (Phaseolus vularis L.) Genotypes Differing in Drought Resistance (Cuvillier, 2006).
Blumen Vegetable Seeds. Sementi d’eccellenza: MECCANO. Blumen Vegetable Seeds. https://www.blumenvegetableseeds.it/prodotti/meccano/?lang=en (2023).
Blumen Vegetable Seeds. Sementi d’eccellenza: MERCATO. Blumen Vegetable Seeds. https://www.blumenvegetableseeds.it/prodotti/mercato/?lang=en (2023).
Wang, N. & Daun, J. K. Determination of cooking times of pulses using an automated Mattson cooker apparatus. J. Sci. Food Agric. 85, 1631–1635 (2005).
Li, J., Zu, Y.-G. & Fu, Y.-J. Optimization of microwave-assisted extraction of triterpene saponins from defatted residue of yellow horn (Xanthoceras sorbifolia Bunge.) kernel and evaluation of its antioxidant activity. Innov. Food Sci. Emerg. Technol. 11, 637–643 (2010).
Dost, K. & Tokul, O. Determination of phytic acid in wheat and wheat products by reverse phase high performance liquid chromatography. Anal. Chim. Acta 558, 22–27 (2006).
Li, Y.-G., Tanner, G. & Larkin, P. The DMACA-HCl protocol and the threshold proanthocyanidin content for bloat safety in forage legumes. J. Sci. Food Agric. 70, 89–101 (1996).
Georgé, S., Brat, P. & Alter, P. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 53, 1370–1373 (2005).
Malapert, A., Tomao, V. & Margier, M. β-cyclodextrin does not alter the bioaccessibility and the uptake by caco-2 cells of olive by-product phenolic compounds. Nutrients 10, 1653 (2018).
Gleize, B., Steib, M., Andre, M. & Reboul, E. Simple and fast HPLC method for simultaneous determination of retinol, tocopherols, coenzyme Q(10) and carotenoids in complex samples. Food Chem. 134, 2560–2564 (2012).
Bonnet, L. et al. Simultaneous quantification of cholecalciferol, 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D in adipose and brain tissue using LC-MSMS method. Nutrients 11, 1977 (2018).
Miller, L. V., Krebs, N. F. & Hambidge, K. M. A mathematical model of zinc absorption in humans as a function of dietary zinc and phytate 1,2. J. Nutr. 137, 135–141 (2007).
Gregorio, G. B. Progress in breeding for trace minerals in staple crops. Preprint https://doi.org/10.1093/jn/132.3.500S (2022).
Cominelli, E., Rodiño, P., Ron, A. & Sparvoli, F. Genetic approaches to improve common bean nutritional quality: Current knowledge and future perspectives. in Quality Breeding in Field Crops, 109–138 (2019). https://doi.org/10.1007/978-3-030-04609-5_5.
Martí, R., Roselló, S. & Cebolla-Cornejo, J. Tomato as a source of carotenoids and polyphenols targeted to cancer prevention. Cancers 8, 58 (2016).
Nadeem, M., Anjum, F. M., Khan, M. R., Saeed, M. & Riaz, A. Antioxidant potential of bell pepper (Capsicum annum L.): A review. J. Nutr. 21, 1–10 (2011).
Champ, M. M. Non-nutrient bioactive substances of pulses. Br. J. Nutr. 88(Suppl 3), S307–S319 (2002).
Griffiths, D. W. The inhibition of digestive enzymes by polyphenolic compounds. Adv. Exp. Med. Biol. 199, 509–516 (1986).
Gu, Y., Hurst, W. J., Stuart, D. A. & Lambert, J. D. Inhibition of key digestive enzymes by cocoa extracts and procyanidins. J. Agric. Food Chem. 59, 5305–5311 (2011).
Chavez-Santoscoy, R. A., Gutierrez-Uribe, J. A. & Serna-Saldivar, S. O. Effect of flavonoids and saponins extracted from black bean (Phaseolus vulgaris L.) seed coats as cholesterol micelle disruptors. Plant Foods Hum. Nutr. 68, 416–423 (2013).
Riedl, J., Linseisen, J., Hoffmann, J. & Wolfram, G. Some dietary fibers reduce the absorption of carotenoids in women. J. Nutr. 129, 2170–2176 (1999).
Petry, N., Egli, I., Campion, B., Nielsen, E. & Hurrell, R. Genetic reduction of phytate in common bean (Phaseolus vulgaris L.) seeds increases iron absorption in young women. J. Nutr. 143, 1219–1224 (2013).
Acknowledgements
This work was funded by the Era-net FOSC (BIO-Belief project, #288). Ángela Bravo-Núñez competitive postdoctoral contract “Margarita Salas” was funded by the government of Spain (Ministerio de Universidades) and by the European Union (NextGeneration-EU). Authors are grateful to Mark Pretzel Zumaraga (C2VN, Marseille, France) and Sarah Gervais (CTCPA, Avignon), for their valuable technical help.
Author information
Authors and Affiliations
Contributions
ER, KK, AL, KAR, LF, TS, ElC, FS, EmC, SL and SM contributed to the design of the project. ER supervised this work. ElC and FS developed lpa beans. AL, TS and LF maintained and multiplied HP5/1 and raised the plants of all genotypes to produce the beans. CF and CP determined cooking parameters. SL, SM and EmC cooked the beans. KAR and MM worked on the selection of the recipe, statistical analysis, and data treatment. KAR, ABN and CH prepared the protocols and performed antinutritional factor assays, in vitro digestions, HPLC and LS-MS2 analysis to determine fat-soluble micronutrient bioaccessibility. SG performed polyphenol assay. KAR, CIV and CMR determined mineral solubility. KAR and ER wrote the main manuscript text and KAR prepared figures. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alvarado-Ramos, K., Bravo-Nunez, Á., Halimi, C. et al. Improving the antinutritional profiles of common beans (Phaseolus vulgaris L.) moderately impacts carotenoid bioaccessibility but not mineral solubility. Sci Rep 14, 11908 (2024). https://doi.org/10.1038/s41598-024-61475-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-61475-8
- Springer Nature Limited