Abstract
The release of sterilized insects to control pest populations has been used successfully during the past 6 decades, but application of the method in vertebrates has largely been overlooked or met with failure. Here, we demonstrate for the first time in fish, that a small population of sea lamprey (Petromyzon marinus; Class Agnatha), arguably one of the most impactful invasive fish in the world, can be controlled by the release of sterilized males. Specifically, the release of high numbers of sterile males (~ 1000's) into a geographically isolated population of adult sea lamprey resulted in the first multiyear delay in pesticide treatment since treatments began during 1966. Estimates of percent reduction in recruitment of age-1 sea lamprey due to sterile male release ranged from 7 to 99.9% with the precision of the estimate being low because of substantial year-to-year variability in larval density and distribution. Additional monitoring that accounts for recruitment variability in time and space would reduce uncertainty in the degree to which sterile male release reduces recruitment rates. The results are relevant to vertebrate pest control programs worldwide, especially as technical opportunities to sterilize vertebrates and manipulate sex ratios expand.
Similar content being viewed by others
Introduction
Controlling or eradicating insect pests through the release of sterilized conspecifics was independently conceived by three researchers in disparate parts of the world during the 1940s1. Success of the technique relies on at least four conditions: (1) sterilized animals are equally competitive in mating as wild individuals, (2) sterile individuals are released during the time and at the location wild individuals are mating, (3) production of offspring decreases in a predictable way when the effective number of reproducing individuals decrease (population growth is density dependent), and (4) sterilized individuals are released at great enough numbers to overwhelm mating among wild individuals (overflooding ratio)2. These conditions have been met for many pest insects like screw worm (Cochliomyia hominivorax), the Mediterranean fruit fly (Ceratitis capitata), and pink bollworm (Pectinophora gossypiella; (see3 for a full review)) and accordingly, numerous populations have been suppressed, eradicated, contained, or establishment prevented through coordinated production and release of sterile individuals4.
Sterile male release may be relevant for the control of 1000's of other species of pest animals5,6, but the approach is infrequently considered for vertebrates given challenges related to sterilization. Efforts to develop sterilization methods that do not reduce the reproductive vigor of an individual have been investigated in amphibians7, crustaceans8, and mammals9,10, but, to date, have not been applied at management-relevant scales. To our knowledge, in fishes (vertebrates with fins that live in water), the only species where a sterilization method has been validated and sterilized individuals have been released to control an invasive population has been the sea lamprey (Petromyzon marinus).
Integrated control of sea lamprey in the North American Great Lakes (Great Lakes) has been desired since 198011, but this vision has largely not been realized12. Sea lamprey are cartilaginous agnathan fish native to the American and European Coasts of the Atlantic Ocean where they provide valued ecological services in streams; sea lamprey clean substrate when constructing nests in gravel and provide nutrients when they die after spawning13. Sea lamprey larvae filter feed for several years prior to transforming to juveniles and migrating to the Ocean to parasitize fish13. Sea lamprey invaded the Great Lakes in the 1930s using canals built for commerce vessels14. Juvenile sea lamprey that parasitize freshwater fish are destructive because each sea lamprey consumes more than 1 kg of blood in the 12–18 month transition to adulthood and most freshwater fishes in the Great Lakes are too small to survive that magnitude of blood loss15,16. Therefore, an international program to control sea lamprey-induced mortality on valued fishes has been in place since the 1950s17 and reduced sea lamprey populations by 90% across all the Great Lakes18. However, a critical vulnerability of the control program is its complete dependence on two complementary tactics: (1) barriers that block adult sea lamprey migration thereby aggregating larval sea lamprey populations into stream segments where (2) a pesticide selective for lamprey (lampricide) can be applied17.
Male sea lamprey build nests along with females, males often mate with multiple females, and both sexes die after spawning19. Therefore, tactics that exploit the unique physiology and mating system14 of sea lamprey may be able to supplement the use of barriers and lampricide12. Following the conceptual model developed by Knipling20, a method for sterilizing adult male sea lamprey through injection of a chemosterilant in a laboratory was developed in 1970s by Lee Hanson21. Subsequent laboratory and field tests in the 1980s, 1990s, and 2000s, demonstrated that sterilized males completed sexual maturation, arrived to spawning habitat at rates consistent with the number released, attracted and mated with females, and that embryo survival was reduced in places where sterile males spawned22. However, despite the experimental release of over 100,000 sterile males in Lake Superior tributaries from 1991 to 1996 and the release of ~ 410,000 sterile males in the St. Marys River from 1991 to 2011 (~ 20,000 per year), a direct connection between sterile male release, a reduction in larval sea lamprey recruitment to age-1, and a reduction in lampricide treatment was not made23. Given the equivocal outcomes and opportunities to expand lampricide control options, the sterile male release technique was discontinued during 201123. Furthermore, a field test evaluating the efficacy of sterile female release also did not document a reduction in sea lamprey recruitment24.
Release of sterilized individuals for pest control has been most successful when population density is low, populations are isolated, and populations are overwhelmed with sterile individuals4. A numeric model of a closed sea lamprey population where 30% of the adult population was trapped with a subsequent release of a 10:1 ratio of sterile to wild males resulted in a nearly 90% reduction in abundance in a single generation25. However, these idealized modeled conditions were not met when sterile males were released in tributaries of Lake Superior and the St. Marys River; ratios of sterile to wild males ranged from 1:1 to 1:5 and populations were open to immigration or emigration from non-treated tributaries23.
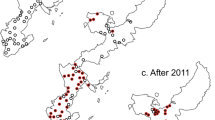
In 2013, a fish biologist informed us of a population of sea lamprey that may complete its lifecycle within a tributary to Lake Huron, USA (Cheboygan River watershed). By 2016, we characterized this population that completes its lifecycle by spawning in the Pigeon, Sturgeon, and Maple Rivers and parasitizing fishes in Burt and Mullett Lakes (Michigan, U.S.A., Fig. 1;26). By contrast, a typical population of sea lamprey would migrate to the Great Lakes to feed on fish. The Cheboygan River population has been partially landlocked since the 1960s and has been treated with lampricide about every 4 years since 196627. Although the larval population of sea lamprey can approach 1,000,000, the total adult spawning population in these three tributaries combined was estimated at less than 200 from 2013 to 201626. Accordingly, the Pigeon, Sturgeon, and Maple Rivers emerged as a model system to experimentally evaluate the sterile male release technique given that the adult population was small and had low immigration rates23.
Average lampricide treatment frequency (number of years between applications) in the Pigeon, Sturgeon, and Maple Rivers during a reference period prior to sterile male release (2000–2016) and after the initiation of sterile male release (2017–2022). Sterile male release did not occur in 2020. The Sturgeon and Maple Rivers flow into Burt Lake while the Pigeon River flows into Mullett Lake. The entire watershed drains into northern Lake Huron.
Here, our goal was to release sterile males in the Pigeon, Sturgeon, and Maple Rivers over multiple years at overflooding rates prescribed for the sterile insect technique (i.e. exceeding 35:1 sterile to non-sterile ratio)28 to determine how (1) the ratio of sterile to non-sterile males influenced the need for reoccurring pesticide treatment and (2) quantify reductions in age-1 larval sea lamprey recruitment attributed to the release of sterile males. The outcomes have direct implications for one of the largest aquatic invasive species control programs in the world and relevance for a diverse array of pest control programs worldwide.
Results
Sterile male sea lamprey were released during 2017–2019 and 2021–2022 in the Pigeon, Sturgeon, and Maple Rivers. Ratios mostly exceeded 35 sterile males to every non-sterile male (wild male) as determined by the observed ratio of sterile to non-sterile males captured in assessment traps (Table 1; Supplemental Tables 1 and 2). However, in the Pigeon River, ratios of sterile to non-sterile males were 6:1 during 2021 and 10:1 during 2022. The lower ratios were a result of increased abundance of non-sterile males and because fewer sterile males were released (Table 1). Sterile males were not released prior to 2017 and were not released during 2020 due to the global pandemic.
The number of viable eggs in the study streams was estimated to decrease 83–99% during years sterile males were released. The number of viable eggs was calculated given the number of sterile males released and estimates of adult sea lamprey abundance, sex ratio, and fecundity in assessment traps. Abundance estimates of non-sterile adult female and male sea lamprey in the Pigeon, Sturgeon, and Maple Rivers were consistently less than 60 per stream each year (Table 1). The exception was for the Pigeon River where abundance estimates were about 100 during 2021 and 2022 (Table 1). As reported in a previous publication27, males represented 50% of the wild population and average female fecundity was 51,000 eggs (range 3100–72,000). Therefore, the estimated total number of viable eggs in each stream during years when sterile males were not released was between 127,500 and 2,800,000 (Table 1). During years when sterile males were released, the total estimated number of viable eggs after accounting for sterile males and assuming completely random mating was between 32 and 383,500 (83–99% reduction in viable eggs) .
We surveyed sea lamprey spawning locations in the Pigeon, Sturgeon, and Maple Rivers and observed sterile males and their nests throughout historically infested reaches, indicating that sterile males moved upstream and occupied spawning habitat similar to non-sterile sea lamprey. Specifically, sea lamprey spawning habitat was surveyed22 throughout the Pigeon, Sturgeon, and Maple Rivers during the 2017 and 2018 spawning season. Only sterile males were observed on nests in the Pigeon, Sturgeon, and Maple Rivers. No viable embryos or recently hatched larvae were collected when nests were disturbed and plankton nets were placed immediately downstream of the nest22. In the Pigeon River, a total of 38 sea lamprey nests were located, 12 sterile males were observed on those nests, and no sea lamprey embryos were recovered. In the Sturgeon River, a total of 10 sea lamprey nests were located, six sterile males were observed on those nests, and no sea lamprey embryos were recovered. In the Maple River, 287 sea lamprey nests were located, 172 sterile males were observed on those nests, and sea lamprey embryos were recovered from four nests, but were dead (number of embryos in each nest = 2, 3, 12, 24).
During the years when sterile males were released only the Pigeon River was selected for lampricide treatment by the Great Lakes Fishery Commission because estimates of the abundance of larval sea lamprey over 100 mm exceeded economic thresholds for treatment (Fig. 1)29. During the 17-year period prior to the release of sterile males (2000–2016) and using the same treatment thresholds, the Pigeon, Sturgeon, and Maple Rivers were selected for lampricide treatment 6, 5, and 5 times, respectively (described in27; treatments occurring roughly every 3 years).
While we found that sterile male release coincided with a decline in the abundance of larvae below an economic threshold requiring lampricide treatment, we also wanted to investigate if the abundance and distribution of age-1 sea lamprey also decreased predictably the year after sterile males were released. To do so, age-1 sea lamprey larval densities were monitored using standard electrofishing practices during years prior to and concurrent with sterilized male release in our study streams. A Bayesian statistical model was developed to investigate if changes in age-1 abundance were correlated to the number of sterile males released.
Importantly, the ability to document reductions in recruitment of age-1 larval sea lamprey during years sterile males were released had limited statistical power and estimates of sterile male release effect size had low precision. Recruitment of age-1 larvae varied substantially from year-to-year, independent of whether sterile males were released, and larvae were spatially clustered (non-independent; Figs. 2 and 3, Supplemental Table 3). Releasing 1000 sterile males (roughly what was needed to exceed 35:1 ratio most years) was associated with a 67% reduction in age-1 recruitment in the Pigeon River (95% credible interval: 9–99.9%), a 50% reduction in the Sturgeon River (95% credible interval: 7–98%), and a 52% reduction in the Maple River (95% credible interval: 7–99.9%; Fig. 4).
Average observed age-1 larval density (color-coded diamond symbols) and individual observations (grey circles) by year and stream. Color indicates the number of sterile male sea lamprey released during the corresponding recruitment year of the observed age-1 year class (for example—data listed under 2018 are larval that were spawned in 2017 and recruited to age-1 in 2018).
The mean number of age-1 larval sea lamprey per m2 of habitat surveyed (Mean age-1 density) during years with (yes) and without (no) the release of sterile male adult sea lamprey. Point color illustrates the number of surveys completed each year and is relative indicator of the confidence in the density estimate.
Estimated effect of sterile-male-release technique on age-1 recruitment in the Pigeon, Sturgeon, and Maple Rivers. The prior probability distribution (estimated effect size assigned as input into our Bayesian analysis; broken line with unshaded area-under-curve) and posterior probability distributions (output from our Bayesian analysis; shaded area-under-curve with solid lines) for expected percent reduction in age-1 recruitment associated with releasing 1000 sterile males are depicted. Expected percent reduction is derived from model parameter q as 100 * [1 − e−qE].
Discussion
To our knowledge, this is the first invasive fish population in which the abundance of offspring was documented to decrease over multiple years after the release of sterilized males. In our case, the outcome was delayed lampricide treatment in the Pigeon, Sturgeon, and Maple Rivers because the number of juvenile sea lamprey that would be targeted did not justify the cost of lampricide treatment27. The postponement of lampricide treatments in the Pigeon, Sturgeon, and Maple Rivers had the added benefits of allowing resources to be diverted to control sea lamprey in other priority areas in the Great Lakes12, thereby collectively benefiting the entire program. The results were not surprising because sterile males were released at ratios of 35:1 or greater on average, immigration rates of new conspecifics were low, and sterile males survived, migrated to spawning habitat, and competed for mates2,4,23—a scenario which previous modeling approaches predicted large theoretical reductions in recruitment25,30,—and a scenario previous field tests of sterile sea lamprey release were unable to achieve23.
Documenting a precise and mechanistic understanding of how sterile male release influenced sea lamprey recruitment was challenging here and could be for other studies. In our case, statistical power was limited (Fig. 4) by the unstructured method by which larval recruitment data were collected in years prior to the release of sterile males, the uncertainty with length-based age assignment in larval sea lamprey31, changing stream habitat (removal of two dams in the system)26, and the inherent variability in fish recruitment relationships (including sea lamprey)32. What is especially unclear is if age-1 recruitment rate is density dependent33, a key condition for consistent reductions in recruitment after sterile male release2. To address these uncertainties, we initiated an adaptive assessment monitoring plan in 202034 that leverages systematic annual assessments for larval sea lamprey coupled with pedigree analysis to estimate the effective number of spawners each year and accurately age larval sea lamprey35. The adaptive assessment plan will mature by 2031 through continued surveys in the Pigeon, Sturgeon, and Maple Rivers as well as ten other streams in the Great Lakes where sterile males may be released. A primary learning objective is to reduce uncertainty around sea lamprey stock-recruitment dynamics34.
Integrating pest control tools that are effective on high density populations (pesticides) with those that are effective on low-density populations (sterilization and pheromone-based) may help alleviate limitations in procuring individuals for sterilization. An immediate and obvious limitation of sterile male release for vertebrate pest control is an inability to propagate or capture the large number of sterile individuals needed to achieve overflooding rates. Here, about 1000 sterile males were released per year into each study stream. Over 300 tributaries to the Great Lakes require regular lampricide treatment29 and roughly 20,000 adult males are captured in the Great Lakes each year36. Therefore, sole reliance on sterile male release as a control tool has exceptionally limited geographic applicability for sea lamprey control.
Sterile male release may find broader application for sea lamprey control and other vertebrate pests if used in combination with tactics that are effective against high density populations (pesticides) and tactics that are effective against low density populations such as removal in traps and pheromone-based approaches12. In our study, regular lampricide treatment prior to sterile male release reduced adult sea lamprey density in the Pigeon, Sturgeon, and Maple Rivers27 and allowed overwhelming rates of 35:1 to be achieved. Combining sterile male release with additional tactics that remove sea lamprey, would again lower the number of sterile males needed. For example, if 80% of the adult sea lamprey were removed in traps, only 200 sterile males would be needed to reach overflooding rates in the Pigeon River. If 20,000 males can be sterilized per year23 and 200 sterile males are needed per stream, integrated approaches to controlling low density populations may be applicable to as many as 30% of sea lamprey producing tributaries.
Therefore, control tools developed for a pest species could be viewed as complementary ‘courses’ in a complete ‘meal’ when deployed sequentially; first with tools that are highly effective at controlling dense populations and then, those that are effective at controlling low density populations12. In the case of sea lamprey control, tools effective for high density populations are the only tools currently used (concrete barriers are designed to block 100% of adult populations; lampricides kill 95% of larval populations), leaving opportunities where tools that are effective on low density populations like trapping37, chemosensory cues38, and seasonal non-physical barriers39 could be combined with sterile male release. While these ideas are not new11, they have often been overlooked in sea lamprey control during the past 20 years12—meanwhile being deployed and shown effective for insect pests such as the Mediterranean fruit fly, screw worm, and tsetse flies40.
These findings are timely since the technological and financial hurdles to developing new methods to sterilized vertebrates may diminish in coming years41,42,43. Sea lamprey are currently chemosterilized in a contained laboratory using highly specialized protective equipment20, which limits the geographic scope of its deployment, increases labor, facility, and animal transportation costs, and applicability to other pests. RNA interference-based (RNAi) methods for sterilizing are quickly developing for insect pests44 and may also be applicable for the relatively small group of fishes that die after spawning like sea lamprey and semelparous salmonids45. RNAi-based methods can be species-specific and target specific genes, and therefore, may pose minimal risk to people or the environment46. RNAi has already been tested in the laboratory as a next generation sea lamprey pesticide47, and similar work could develop the next generation sterilant used to sterilize sea lamprey streamside14. The same concept may be applicable to a broad range of vertebrate pests depending on their life history and mating system.
Methods
Demographics of adult sea lamprey in the Pigeon, Sturgeon, and Maple Rivers
The abundance, size, fecundity, and migration timing of adult sea lamprey in the Pigeon, Sturgeon, and Maple Rivers 2011–2019 have been previously described26,27. Here, using the same methods, adult sea lamprey abundance, size, fecundity, and migration timing in the Pigeon, Sturgeon, and Maple Rivers 2020–2022 was estimated. Notable changes to the study system during the decade-long study include the removal of two dams. The Song of the Morning Dam was removed from the Pigeon River during 2015 and increased habitat available for sea lamprey to occupy by 35 km27. The Lake Kathleen Dam was removed from the Maple River during 2018 and increased the habitat sea lamprey could occupy by 34 km27.
Procurement and release of sterile males
Male sea lamprey to be sterilized were procured during their upstream migration from traps located on the lower Cheboygan River, Paradise Lake (Carp Lake) outlet, Ocqueoc River, Manistique River, Peshtigo River, and Thessalon River, which are tributaries to northern Lakes Huron or Michigan. Males were sexually immature (pre-spermiated) when sterilized and were distinguished from females based on their swollen dorsal ridge48. Males were sterilized by U.S. Fish and Wildlife Service as described in49 according to the animal use and care protocols therein. While other sterilization approaches have been investigated50, chemosterilization is the only method available for sterilizing sea lamprey at large scale. Experimental protocols involving the handling of fishes were carried out in accordance with United States federal guidelines for care and use of animals and were in accordance with American Fisheries Society, ARRIVE guidelines, and the U.S. Animal Welfare Act of 197051. Sterile males were fin-clipped prior to release so they could be distinguished from non-sterile males (wild) and released into the Pigeon River at East Mullett Lake Road, the Sturgeon River at Burt Lake State Park, and the Maple River at Brutus Road (within 3 km of their confluence with Burt or Mullett Lakes). Since the sterilization program was decommissioned between 2012 and 2016, we used laboratory assays to verify the sterilization method was still effective when this project started in 2017. Sterility was found to be 100% in 2017 and 2018 and 94% in 2021 using procedures outlined in49,52 (Supplemental Table 4; staffing limitations precluded sterility checks in 2019, 2022), where a single sterile male and single wild male were used to fertilize 100–500 eggs of a single female and the percent of sea lamprey embryos fertilized by each male surviving to stage-12 was determined53.
The observed ratio of sterile males and wild males on sea lamprey nests in the Pigeon, Sturgeon, and Maple Rivers was assessed in 2017 and 2018 according to procedures described in23. Specifically in this study, each year search effort was deployed over about 30 days and 6 h per day during the spawning season and in locations throughout the watershed where spawning habitat was present (less than 1 m in depth, rock substrate 2–5 cm in diameter, water velocity around 0.5 m/s) and larval sea lamprey were found historically. Two field staff would walk upstream and when a sea lamprey spawning nest was identified, would visually determine if sea lamprey were present. Regardless of if sea lamprey were present in the nest, the nest was probed and any embryos or recently hatched larvae present were collected using a drift net set downstream of the nest, counted, and then assessed for viability as described in23. While we could not be sure we investigated all possible areas of spawning, we were confident that the sampling covered a representative sample of spawning habitat at times of the year sea lamprey were spawning.
Estimates of sea lamprey recruitment to age-1 and lampricide treatment decision process
Backpack electrofishing deployed by trained U.S. Fish and Wildlife Service staff was used to capture larval sea lamprey following ranking survey methods described in54 with the assumed effectiveness described in55. Age-1 and older larvae were targeted because they are easier to see and capture than age-0 larvae, therefore, minimizing inter-personnel differences in catch rates. Differences in water conductivity and depth were assumed not to vary enough with each stream to change electrofishing effectiveness across years55. Captured sea lamprey were measured in length and larvae were assumed to have been spawned the previous year (age-1) if they were 20–40 mm and captured in May and June, were 40–60 mm and captured in July and August, and were 60–80 mm and captured in September and October31. Lampricide treatments were prescribed for the Pigeon, Sturgeon, and Maple Rivers using the methods in29 where treatments only occur when the cost to kill a larval sea lamprey over 100 mm exceeds an economic threshold.
The age-1 recruitment process model was a white noise time series (uncorrelated random variables with a mean of zero) on the log scale with instantaneous mortality proportional to the number of sterile males released (Supplemental Table 3).
The log-scale expected recruitment index for stream s in year yr (Ayr,s) is the sum of the average log-scale recruitment rate for stream s (as) and the deviation from the mean recruitment for year yr (a′yr,s) less the instantaneous mortality rate q multiplied by the sterile male release control effort (E = number of sterile males released/1000). Mortality rate was interpreted on the finite scale and converted to annual percent reduction in recruitment (100 * [1 − e−qE]). Recruitment index observations (measurements of age-1 density * 1000 rounded to the nearest integer) were assumed to follow a negative binomial distribution, with overdispersion parameter θ allowing for variance to be greater than the expected mean.
Model parameters were assigned low information priors (Supplemental Table 3).
Posterior distributions of model parameters were generated through Markov Chain Monte Carlo (MCMC) sampling implemented in Stan56 using the rstan package57 and R version 4.0.258. Four MCMC chains were run for 5000 iterations with a warmup of 2000 iterations. R-hat statistics calculated for each model parameter (min 1.0, max 1.01) and zero flagged divergent transitions after warmup indicated that saved iterations after the burn-in period were sampling from the posterior distribution and had achieved stationarity.
Data availability
All data generated or analyzed during this study are included in this published article and its Supplemental Information files.
References
Klassen, W., Curtis, C. F. & Hendrichs, J. History of the sterile insect technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management 1–44 (CRC Press, 2021).
Dyck, V. A., Hendrichs, J. & Robinson, A. S. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management (Taylor and Francis, 2021).
Hendrichs, J., Enkerlin, W. R. & Pereira, R. Invasive insect pests: Challenges and the role of the sterile insect technique in their prevention, containment, and eradication. In Sterile Insect Technique 885–922 (CRC Press, 2021).
Hendrichs, J., Vreysen, M. J. B., Enkerlin, W. R. & Cayol, J. P. Strategic options in using sterile insects for area-wide integrated pest management. In Sterile Insect Technique 841–884 (CRC Press, 2021).
Havel, J. E., Kovalenko, K. E., Thomaz, S. M., Amalfitano, S. & Kats, L. B. Aquatic invasive species: Challenges for the future. Hydrobiologia 750, 147–170 (2015).
Keller, R. P., Geist, J., Jeschke, J. M. & Kühn, I. Invasive species in Europe: Ecology, status, and policy. Environ. Sci. Eur. 23, 1–17 (2011).
Descamps, S. & De Vocht, A. Bisazir as a chemosterilant to control invasive vertebrates: Ecotoxicity and efficacy to induce male sterility in Lithobates catesbeianus. Manag. Biol. Invasions 13(4), 769 (2022).
Aquiloni, L. et al. Managing invasive crayfish: Use of X-ray sterilisation of males. Freshw. Biol. 54, 1510–1519 (2009).
Gonçalves da Silva, A., Kolokotronis, S. O. & Wharton, D. Modeling the eradication of invasive mammals using the sterile male technique. Biol. Invasions 12, 751–759 (2010).
Campbell, K. & Donlan, C. J. Feral goat eradications on islands. Conserv. Biol. 19(5), 1362–1374 (2005).
Sawyer, A. J. Prospects for integrated pest management of the sea lamprey (Petromyzon marinus). Can. J. Fish. Aquat. Sci. 37(11), 2081–2092 (1980).
Siefkes, M. J., Johnson, N. S. & Muir, A. M. A renewed philosophy about supplemental sea lamprey controls. J. Great Lakes Res. 47, 742–752 (2021).
Maitland, P. S., Renaud, C. B., Quintella, B. R., Close, D. A. & Docker, M. F. Conservation of native lampreys. Lampreys Biol. Conserv. Control 1, 375–428 (2015).
Wilkie, M. P., Johnson, N. S. & Docker, M. F. Invasive species control and management: The sea lamprey story. Fish Physiol. 39(B), 489–579 (2022).
Madenjian, C. P., Cochran, P. A. & Bergstedt, R. A. Seasonal patterns in growth, blood consumption, and effects on hosts by parasitic-phase sea lampreys in the Great Lakes: An individual-based model approach. J. Great Lakes Res. 29, 332–346 (2003).
Jorgensen, J. C. & Kitchell, J. F. Growth potential and host mortality of the parasitic phase of the sea lamprey (Petromyzon marinus) in Lake Superior. Can. J. Fish. Aquat. Sci. 62(10), 2343–2353 (2005).
Gaden, M., Brant, C. O. & Lambe, R. Why a great lakes fishery commission? The seven-decade pursuit of a Canada–US fishery treaty. J. Great Lakes Res. 47, 11–23 (2021).
Robinson, K. F., Miehls, S. M. & Siefkes, M. J. Understanding sea lamprey abundances in the Great Lakes prior to broad implementation of sea lamprey control. J. Great Lakes Res. 47, 328–334 (2021).
Johnson, N. S., Buchinger, T. J. & Li, W. Reproductive ecology of lampreys. Lampreys Biol. Conserv. Control 1, 265–303 (2015).
Knipling, E. F. Sterile-male method of population control: Successful with some insects, the method may also be effective when applied to other noxious animals. Science 130(3380), 902–904 (1959).
Hanson, L. H. & Manion, P. J. Sterility method of pest control and its potential role in an integrated sea lamprey (Petromyzon marinus) control program. Can. J. Fish. Aquat. Sci. 37(11), 2108–2117 (1980).
Bergstedt, R. A. & Twohey, M. B. Research to support sterile-male-release and genetic alteration techniques for sea lamprey control. J. Great Lakes Res. 33, 48–69 (2007).
Bravener, G. & Twohey, M. Evaluation of a sterile-male release technique: A case study of invasive sea lamprey control in a tributary of the Laurentian Great Lakes. N. Am. J. Fish. Manag. 36(5), 1125–1138 (2016).
Symbal, M. S., Twohey, M. T. & Bergstedt, R. A. Effectiveness of Sterile-Female Release in Reducing Sea Lamprey Production in the Trout River 20 (Great Lakes Fishery Commission, 2011).
Klassen, W., Adams, J. V. & Twohey, M. B. Modeling the suppression of sea lamprey populations by the release of sterile males or sterile females. J. Great Lakes Res. 30(4), 463–473 (2004).
Johnson, N. S. et al. Evidence that sea lampreys (Petromyzon marinus) complete their life cycle within a tributary of the Laurentian Great Lakes by parasitizing fishes in inland lakes. J. Great Lakes Res. 42(1), 90–98 (2016).
Johnson, N. S. et al. A case study of sea lamprey (Petromyzon marinus) control and ecology in a microcosm of the Great Lakes. J. Great Lakes Res. 47, 492–505 (2021).
Mastrangelo, T., Kovaleski, A., Botteon, V., Scopel, W. & Costa, M. D. L. Z. Optimization of the sterilizing doses and overflooding ratios for the South American fruit fly. PLoS ONE 13(7), e0201026 (2018).
Jubar, A. K. et al. Prioritizing lampricide treatments in Great Lakes tributaries and lentic areas during 2000–2017. J. Great Lakes Res. 47, S238–S246 (2021).
Erickson, R. A. et al. A spatially discrete, integral projection model and its application to invasive carp. Ecol. Modell. 387, 163–171 (2018).
Dawson, H. A., Higgins-Weier, C. E., Steeves, T. B. & Johnson, N. S. Estimating age and growth of invasive sea lamprey: A review of approaches and investigation of a new method. J. Great Lakes Res. 47, 570–579 (2021).
Dawson, H. A. & Jones, M. L. Factors affecting recruitment dynamics of Great Lakes sea lamprey (Petromyzon marinus) populations. J. Great Lakes Res. 35(3), 353–360 (2009).
Jones, M. L. et al. Compensatory mechanisms in Great Lakes sea lamprey populations: Implications for alternative control strategies. J. Great Lakes Res. 29, 113–129 (2003).
Lewandoski, S. A., Brenden, T. O., Siefkes, M. J. & Johnson, N. S. An adaptive management implementation framework for evaluating supplemental sea lamprey (Petromyzon marinus) controls in the Laurentian Great Lakes. J. Great Lakes Res. 47, 753–763 (2021).
Weise, E. M. et al. Pedigree analysis and estimates of effective breeding size characterize sea lamprey reproductive biology. Evol. Appl. 15(3), 484–500 (2022).
Adams, J. V., Barber, J. M., Bravener, G. A. & Lewandoski, S. A. Quantifying Great Lakes sea lamprey populations using an index of adults. J. Great Lakes Res. 47, 335–346 (2021).
Miehls, S. et al. Where you trap matters: Implications for integrated sea lamprey management. J. Great Lakes Res. 47, 320–327 (2021).
Fissette, S. D. et al. Progress towards integrating an understanding of chemical ecology into sea lamprey control. J. Great Lakes Res. 47, 660–672 (2021).
Johnson, N. S., Snow, B., Bruning, T. & Jubar, A. A seasonal electric barrier blocks invasive adult sea lamprey (Petromyzon marinus) and reduces production of larvae. J. Great Lakes Res. 47, 310–319 (2021).
Feldmann, U. & Hendrichs, J. Integrating the Sterile Insect Technique as a Key Component of Area-wide Tsetse and Trypanosomiasis Intervention (No. 3) (Food & Agriculture Org, 2001).
Ferreira-Martins, D., Champer, J., McCauley, D. W., Zhang, Z. & Docker, M. F. Genetic control of invasive sea lamprey in the Great Lakes. J. Great Lakes Res. 47, 764–775 (2021).
Thresher, R. E. Autocidal technology for the control of invasive fish. Fisheries 33(3), 114–121 (2008).
Ying, Y. A. N., Aumann, R. A., Haecker, I. & Schetelig, M. F. CRISPR-based genetic control strategies for insect pests. J. Integr. Agric. 22(3), 651–668 (2023).
Hashiro, S. & Yasueda, H. RNA interference-based pesticides and antiviral agents: Microbial overproduction systems for double-stranded RNA for applications in agriculture and aquaculture. Appl. Sci. 12(6), 2954 (2022).
Horak, K. E. RNAi: Applications in vertebrate pest management. Trends Biotechnol. 38(11), 1200–1202 (2020).
Vogel, E., Santos, D., Mingels, L., Verdonckt, T. W. & Broeck, J. V. RNA interference in insects: Protecting beneficials and controlling pests. Front. Physiol. 9, 1912 (2019).
Heath, G., Childs, D., Docker, M. F., McCauley, D. W. & Whyard, S. RNA interference technology to control pest sea lampreys—A proof of concept. Plos one 9(2), e88387 (2014).
Chung-Davidson, Y. W. et al. A thermogenic secondary sexual character in male sea lamprey. J. Exp. Bio. 216(14), 2702–2712 (2013).
Twohey, M. B. et al. The sterile-male-release technique in Great Lakes sea lamprey management. J. Great Lakes Res. 29, 410–423 (2003).
Sower, S. A. The endocrinology of reproduction in lampreys and applications for male lamprey sterilization. J. Great Lakes Res. 29, 50–65 (2003).
Jenkins, J. A. et al. Guidelines for the Use of Fishes in Research (American Fisheries Society, 2014).
Hanson, L. H. Sterilization of sea lampreys (Petromyzon marinus) by immersion in an aqueous solution of bisazir. Can. J. Fish. Aquat. Sci. 38(10), 1285–1289 (1981).
Piavis G. W. Embryological stages in the sea lamprey and effects of temperature on development, Fishery Bulletin 182, Volume 61, U.S. Fish and Wildlife Service (1961).
Hansen, G. J. & Jones, M. L. A rapid assessment approach to prioritizing streams for control of Great Lakes sea lampreys (Petromyzon marinus): A case study in adaptive management. Can. J. Fish. Aquat. Sci. 65(11), 2471–2484 (2008).
Steeves, T. B., Slade, J. W., Fodale, M. F., Cuddy, D. W. & Jones, M. L. Effectiveness of using backpack electrofishing gear for collecting sea lamprey (Petromyzon marinus) larvae in Great Lakes tributaries. J. Great Lakes Res. 29, 161–173 (2003).
Carpenter, B. et al. Stan: A probabilistic programming language. J. Stat. Softw. 76(1), 1–32 (2017).
Stan Development Team. RStan: the R interface to Stan. R package version 2.21.2. http://mc-stan.org/ (2020).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/ (2020).
Acknowledgements
Funding was provided by the Great Lakes Fishery Commission (2017_JOH_54061) and the Michigan Invasive Species Grant Program (IS16-2003). We thank the Northern Inland Lakes Citizens Fishery Advisory Committee for supporting experimental control of sea lamprey with sterile males. Skye Fissette collected sterile male nesting observations on the Maple River. Sofia Fall conducted the sterility assays in 2017. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. This manuscript was written in memory of David Keffer who was a member of the larval sampling crew and mentor and friend to the authors.
Author information
Authors and Affiliations
Contributions
All authors conceptualized the study and commented on the manuscript draft. M.S., J.B., B.S., and G.B. coordinated the procurement of sterilization of male sea lamprey. N.J. coordinated assessment of adult sea lamprey populations and sterilization quality assurance. A.J. coordinated assessment of larval sea lamprey populations. S.L. led statistical analysis of larval populations and created the figures. N.J. wrote the first draft of the manuscript and coordinated the overall project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Johnson, N.S., Lewandoski, S.A., Jubar, A.K. et al. A decade-long study demonstrates that a population of invasive sea lamprey (Petromyzon marinus) can be controlled by introducing sterilized males. Sci Rep 14, 12689 (2024). https://doi.org/10.1038/s41598-024-61460-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-61460-1
- Springer Nature Limited