Abstract
The objective of this study was to investigate the effect of microencapsulated bioactive compounds from lemongrass mixed dragon fruit peel pellet (MiEn-LEDRAGON) supplementation on fermentation characteristics, nutrient degradability, methane production, and the microbial diversity using in vitro gas production technique. The study was carried out using a completely randomized design (CRD) with five levels of MiEn-LEDRAGON supplementation at 0, 1, 2, 3, and 4% of the total dry matter (DM) substrate. Supplementation of MiEn-LEDRAGON in the diet at levels of 3 or 4% DM resulted in increased (p < 0.05) cumulative gas production at 96 hours (h) of incubation time, reaching up to 84.842 ml/ 0.5 g DM. Furthermore, supplementation with 3% MiEn-LEDRAGON resulted in higher in vitro nutrient degradability and ammonia–nitrogen concentration at 24 h of the incubation time when compared to the control group (without supplementation) by 5.401% and 11.268%, respectively (p < 0.05). Additionally, supplementation with MiEn-LEDRAGON in the diet led to an increase in the population of Fibrobacter succinogenes at 24 h and Butyrivibrio fibrisolvens at 12 h, while decreasing the population of Ruminococcus albus, Ruminococcus flavefaciens, and Methanobacteriales (p < 0.05). Moreover, supplementation of MiEn-LEDRAGON in the diet at levels of 2 to 4% DM resulted in a higher total volatile fatty acids (VFA) at 24 h, reaching up to 73.021 mmol/L (p < 0.05). Additionally, there was an increased proportion of propionic acid (C3) and butyric acid (C4) at 12 h (p < 0.05). Simultaneously, there was a decrease in the proportion of acetic acid (C2) and the ratio of acetic acid to propionic acid (C2:C3), along with a reduction of methane (CH4) production by 11.694% when comparing to the 0% and 3% MiEn-LEDRAGON supplementation (p < 0.05). In conclusion, this study suggests that supplementing MiEn-LEDRAGON at 3% of total DM substrate could be used as a feed additive rich in phytonutrients for ruminants.
Similar content being viewed by others
Introduction
Ruminant production plays a crucial role in the food chain, which is extremely beneficial for providing meat and milk as high-protein food by efficiently converting fibrous biomass and by-products1. However, the production of ruminants is accompanied by considerable emissions of enteric methane (CH4), resulting in environmental consequences that contribute to 30% of the methane released into the atmosphere and account for 6% of the total global anthropogenic greenhouse gas emissions1,2,3,4. In terms of animal performance, ruminal CH4 production leads to an energy loss ranging from 3 to 10% of the animal's gross energy intake5. Consequently, there is a need to develop sustainable mitigation approaches for CH4 emissions to enhance forage conversion efficiency without negative impacts on animal health and the environment.
Currently, there is growing interest in local feed additives, especially those derived from herb and fruit peels by-product available within commercial canning facilities or on farms5,6. Most of herbs and fruit peels are abundant in phytogenic active compounds such as condensed tannins and saponins, as well as antioxidant contents—nutritional components present in plants that contribute to animal health and have the potential to improve rumen fermentation as well as enhance ruminant productivity while also decreasing CH4 emissions from ruminants5,6,7,8. Those secondary bioactive compounds of plants can reduce ruminal CH4 synthesis in the rumen by reducing the number of methanogens and protozoa7,9. Previous studies5,6,8,10,11,12,13,14 demonstrated that the tropical herbs and fruit peels could be used to improve rumen fermentation as feed additives, which is beneficial to ruminants.
Dragon fruit (Hylocereus undatus) is a tropical fruit, and its peel represents a substantial by-product arising from either fruit processing or fresh consumption15,16. Dragon fruit peel is a non-toxic and biologically safe alternative source of plant-containing antioxidants, particularly phenolic compounds, as well as condensed tannins and saponins11,15,17. Previous studies11,16,17 demonstrated that the addition of dragon fruit peel powder or pellet could improve ruminal fermentation characteristics, enhance fermentation end-product, lower number of protozoa, and CH4 production.
Lemongrass (Cymbopogon citratus) is a tropical medicinal herb that includes the essential oil citronella, which has been confirmed to improve ruminant digestibility, rumen fermentation, ruminal microbial population, and microbial protein synthesis as well as decrease CH4 production18,19. The bioactive compound extracted from it holds therapeutic and growth-promoting potential in animals18. It also contributes to vitamin A synthesis and influences the proliferation and metabolism of various bacterial types, including those found in the rumen19.
Nano and microencapsulation techniques are employed to enhance stability, increase bio-accessibility, offer controlled release characteristics, and enhance the convenience of storage and handling for target components acting as active core materials enclosed within polymer walls or carrier materials20. These techniques could enhance the stability of core active ingredients, preserving them against negative environmental conditions including high temperatures, exposure to light, changes in pH, and the presence of oxygen, which may greatly affect the chemical and physical properties of the product15,21. Phupaboon et al.20 achieved microencapsulation to encapsulate high-quality bioactive substances extracted from Mitragyna speiosa, Cannabis indica, and Cannabis sativa, which was effective in preserving elevated concentrations of bioactive components. Recently, Matra et al.22 demonstrated that microencapsulated phytonutrients from Mitragyna leaf extracts have the potential to improve ruminal degradability, enhance fermentation end-products, and decrease methane production in an in vitro study. It is a new challenge to apply the microencapsulation process to increase the efficiency of the use of bioactive compounds in the feed industry.
However, research on microencapsulated-phytonutrients from lemongrass mixed dragon fruit peel pellet (MiEn-LEDRAGON) has not been reported. The study’s hypothesis was that MiEn-LEDRAGON could be effectively retained as active in the in vitro inoculum and improve fermentation characteristics, degradability, and microbial diversity, as well as mitigate CH4 production. Therefore, the objective of this study was to determine the effect of MiEn-LEDRAGON supplementation on fermentation characteristics, degradability, methane production, and microbial diversity using in vitro gas production technique.
Materials and methods
All cattle donors involved in this study received approval from the Animal Ethics Committee of Khon Kaen University (record no. IACUC-KKU-110/66). Our study confirmed that all the methodologies employed in this research adhered to the pertinent guidelines and regulations. Moreover, strict adherence to the ARRIVE guidelines was maintained throughout the entire study.
Microencapsulation of phytonutrients product pellet preparation
The phytonutrients product pellet from lemongrass mixed dragon fruit peel (LEDRAGON) – contains lemon grass powder (50%), dragon fruit peel powder (45%), molasses (3%), and cassava powder (2%) on a dry matter basis. All feed ingredients were meticulously combined and mixed with water, then processed through a pelleting machine to form the LEDRAGON pellets. Subsequently, the pellets were sun-dried for 48 h to achieve a moisture level of at less than 10%. These pellets were stored in a sealed container and utilized throughout the entire experimental duration. Subsequently, the LEDRAGON pellet was ground to powder for plant secondary bioactive substance extraction, modifying the procedure outlined by Phupaboon et al.20. Specifically, 5 g of LEDRAGON pellet powder was mixed with 100 mL of deionized water and subjected to microwave extraction at 100 W for 10 min (final temperature ≤ 60 °C)20. The resulting bioactive extract juice was encapsulated using a technique involving ionic gelation in combination with surfactant ingredients, utilizing 10% cricket-extracted protein (w/v) in phosphate buffer (PPB) at pH 5.5. The bioactive extract juice was stirred overnight at room temperature with surfactant ingredients in a 1:1 ratio. The bioactive substance was microencapsulated using the spray-drying technique with a Bǚchi B-191 mini spray dryer. The dried microencapsulated-LEDRAGON powders (MiEn-LEDRAGON) were gathered, tightly packed, sealed, and kept at a temperature of − 20 °C until used in the in vitro investigation.
Experimental design and dietary treatments
This study utilized the gas production technique at various incubation intervals. The design of experiment was a completely randomized design (CRD) with three replications run. The dietary treatments were basal diet (rice straw + concentrate diet) with a 60:40 of roughage to concentrate (R:C) ratio supplemented with MiEn-LEDRAGON at 0, 1, 2, 3, and 4% DM of total substrate. The experimental dietary samples were dried in an oven at 72°C, ground to pass through a 1-mm sieve using a Cyclotech Mill (Tecator, Sweden), and then analyzed for chemical composition as well as in the in vitro gas production test. The experimental diets, comprising rice straw, concentrate diet, dragon fruit peel, lemon grass, pellet product from lemongrass mixed dragon fruit peel (LEDRAGON), and MiEn-LEDRAGON, underwent analysis for dry matter (DM, ID 967.03) and ash (ID 492.05) content, employing the standard procedures of AOAC23. The amount of nitrogen (N) components was determined using the Nitrogen Analyzer (Leco FP828, LECO Corporation, Saint Joseph, MI, USA) to evaluate the crude protein (CP) content. The content of neutral detergent fiber (NDF) and acid detergent fiber (ADF) was determined following the standard method outlined by Van Soest et al.24. Total phenolic content was assessed using the Folin–Ciocalteu reagent through absorbance measurement at 765 nm25. The total flavonoid content was evaluated by measuring colorimetric changes using a 10% aluminium chloride solution and reading at 415 nm26. The analyses were conducted in triplicate, and the outcomes were presented as mg of gallic acid equivalents (mg GAE) per g of DM and mg of quercetin equivalents (mg QUE/g DM), following the description of Phupaboon et al.20 The assessment of antioxidative capacities involved three distinct methods: 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging method27, 2, 2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) radical scavenging activity28, and ferric reducing ability power (FRAP) method29. The analyses were conducted in triplicate, and the outcomes were presented as the % of radical scavenging inhibition and mmol of Trolox equivalents (mmol TROE/g DM), following the description of Phupaboon et al.20. The compositions and ingredients of the experimental diets, comprising rice straw, concentrate diet, dragon fruit peel, lemon grass, LEDRAGON, and MiEn-LEDRAGON are shown in Table 1. All concentrate diets being formulated to be at 14.6% DM of CP, which was recommend for beef cattle.
Animal donors and preparation of ruminal inoculums
Four, male 4-year-old, Thai-native steers with 380 ± 10.0 kg of body weight (BW) were used as ruminal liquor donors. The experimental donor cattle were given ad libitum access to rice straw and provided with 0.5% of their body weight (BW) daily for the concentrate diet (145 g/kg CP and 810 g/kg TDN), twice a day (6:00 am and 4:00 pm). All experimental donors were housed in individual pens, and free available was made for clean water and mineral blocks. The cattle were provided with the diet for a period of 21 days before the collection of rumen liquor. For each incubation run, ruminal fluid was collected from each donor animal for 300 mL via oral suction using a vacuum pump and then transferred to an Erlenmeyer flask before the morning feeding. Ruminal fluid was filter strained through four layers of cheesecloth into pre-warmed thermos containers before being transferred to the laboratory. The medium preparation adhered to the procedures outlined by Makkar et al.30, where 2400 mL of ruminal buffer mixed medium was combined with 1200 mL of ruminal fluid obtained from animal donors and stirred at 39 °C under CO2-flushed conditions. Ruminal fluid mixture (40 mL) was moved into each experimental bottle and incubated at 39 °C in a water bath.
In vitro gas production and kinetics of gas
Gas productions were promptly recorded using the modified procedures described by Suriyapha et al.31 immediately after incubation at 0, 0.5, 1, 2, 4, 6, 8, 12, 18, 24, 48, 72, and 96 h. The gas kinetics were analyzed by fitting curves using the models proposed by Ørskov and McDonald32 as follows:
where a = soluble fraction from gas production, b = insoluble fraction from gas production, c = rate of gas production constant for the insoluble fraction (b), t = incubate time, (|a|+ b) = the potential extent of gas production, and y = gas produced at time ‘t’.
In vitro degradability, fermentation characteristics and microbial DNA analysis
The analysis of in vitro dry matter degradability (IVDMD) and in vitro organic matter degradability (IVOMD) was conducted following 12 and 24 h of incubation, in accordance with the methods outlined by Tilley and Terry33. At 12 and 24 h, the gas that was in the empty area in the headspace of the bottle was retrieved using a 10 mL syringe and transferred into the vial to collect methane. The concentration of methane was assessed utilizing high-purity methane as a reference standard under the gas chromatography (GC) system (Nexis GC-2030: SHIMADZU, Shimadzu Corp., Kyoto, Japan) equipped with a standard column (SH-Rt-Q-BOND 30 m, 0.53 mm, 20 μm, Shimadzu Co., Tokyo, Japan) following the description of Kaewpila et al.34. The ruminal pH parameters were measured at 12 and 24 h of incubation using a digital pH meter (HANNA Instrument (HI) 8424 microcomputer, Singapore). The ruminal liquor incubated bottles were distributed into two parts. The first part (20 mL) was kept into 5 mL of 1 M H2SO4 and stored at – 20 °C for ammonia nitrogen (NH3–N) analysis using a spectrophotometer (UV/VIS spectrophotometer, PG Instruments LtD., London, United Kingdom) based on the methods of Fawcett and Scott35, while the measurement of in vitro volatile fatty acids (VFAs) was conducted using the GC system (Nexis GC-2030), which included the molecular sieve 13X, 30/60 mesh column (Alltech Associates Inc., Deerfield, IL, USA), following the description of So et al.36. The second part (10 mL) was collected into a plastic bottle and stored at – 20 °C for microbial extraction. The procedure described by Koike and Kobayashi37 was utilized for the extraction of community DNA from rumen fluid. DNA purification was performed using the QIAgen DNA Mini Stool Kit columns (QIAGEN, Valencia, CA, USA). Specific primers, as described by Matra et al.22, were applied to quantify the microbial populations of Fibrobacter succinogenes, Ruminococcus albus and Ruminococcus flavefaciens37, Butyrivibrio fibrisolvens38, Megasphaera elsdenii39, and Methanobacteriales40. The Chromo 4TM system from Bio-Rad (Hercules, CA, USA) was employed for real-time PCR amplification and detection, following the guidelines for DNA analysis37.
Statistical analysis
The investigation results were statistically analyzed as a completed randomized design (CRD) statistical run with Proc. Mixed procedure in SAS software41 (Version 9.4):
where Yij = each observations for a given variable, µ = overall mean, τi = the fixed effect of treatment (the MiEn-LEDRAGON levels at 0, 1, 2, 3, and 4% DM of total substrate), δij = the random effect of the replication run, and εijk = the residual random term. Results are presented as mean values with the standard error of the mean. Differences between treatment means were determined by the Duncan’s New Multiple Range Test42, and differences of p < 0.05 were considered to represent statistically significant differences.
Result
Kinetics and cumulative gas production
The effect of MiEn-LEDRAGON supplementation levels on gas kinetics and cumulative gas production is shown in Table 2. The soluble fraction of gas production (a) was decreased (p < 0.05) when supplemented with MiEn-LEDRAGON (1 to 4% DM in the diet). The insoluble fraction of gas production (b) value increased significantly (p < 0.05) upon supplementation with MiEn-LEDRAGON, reaching its highest value at the 3 or 4% MiEn-LEDRAGON supplementation level. The rate of gas production value (c) exhibited a decrease upon supplementation with MiEn-LEDRAGON in the diet (p < 0.05). Furthermore, the potential extent of gas (|a|+ b) value and cumulative gas production were increased with MiEn-LEDRAGON supplementation at 3 or 4% DM in the diet (p < 0.05), and cumulative gas production ranged from 64.95 to 84.84 mL/0.5g DM of substrate (Fig. 1).
In vitro degradability, pH value and ammonia–nitrogen concentration
Table 3 shows the effect of MiEn-LEDRAGON supplementation levels on in vitro dry degradability, pH value, and concentration of ammonia–nitrogen (NH3–N). There were no changes in IVDMD, IVOMD, and NH3–N at 12 h of incubation time (p > 0.05). Whereas, the greater IVDMD, IVOMD, and NH3–N at 24 h of incubation time were shown in MiEn-LEDRAGON supplementation at 3 or 4% DM in the diet (p < 0.05). However, the pH value did not change (p > 0.05) when MiEn-LEDRAGON was supplemented in the diet.
In vitro microbial dynamics
The effect of MiEn-LEDRAGON supplementation levels on in vitro microbial populations is shown in Table 4. The supplementation of MiEn-LEDRAGON had no impact on the Fibrobacter succinogenes population at 12 h of incubation (p > 0.05), whereas a greater number of Fibrobacter succinogenes were observed at 24 h of supplementation with MiEn-LEDRAGON in the diet at levels of 3 or 4% DM (p < 0.05). In addition, the number of Butyrivibrio fibrisolvens at 12 h of incubation was higher (p < 0.05) when MiEn-LEDRAGON was added to the diet at 3 or 4% DM, nevertheless, there was no change for the Butyrivibrio fibrisolvens population at 24 h (p > 0.05). Meanwhile, the populations of Ruminococcus albus and Ruminococcus flavefaciens at 12 h and 24 h were decreased (p < 0.05) when supplemented with MiEn-LEDRAGON (1 to 4% DM in the diet). Moreover, the supplementation of MiEn-LEDRAGON at 2 to 4% DM showed a lower Methanobacteriales population at 12 h and 24 h (p < 0.05). However, there was no change for the Megasphaera elsdenii population by dietary treatment (p > 0.05).
In vitro volatile fatty acids and methane production
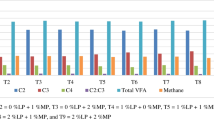
Table 5 shows the effect of MiEn-LEDRAGON supplementation levels on in vitro fermentation end-products (VFA) and production of methane (CH4). Even though the total VFA at 12 h was similar across all dietary groups (p > 0.05), the supplementation of MiEn-LEDRAGON in the concentrate diet at levels of 2 to 4% DM resulted in a greater total VFA (p < 0.05) at 24 h after incubation than the control group. The proportion of acetic acid (C2) at 12 h and 24 h were decreased (p < 0.05) with the MiEn-LEDRAGON supplemented levels of 2 to 4% DM in the diet. Meanwhile, there was an increased proportion of propionic acid (C3) (p < 0.05) when supplemented with MiEn-LEDRAGON in the diet at levels of 2 to 4% DM in the diet. Furthermore, the supplementation of MiEn-LEDRAGON in the concentrate diet showed a higher proportion of butyric acid (C4) at 12 h (p < 0.05) when compared with the control group, whereas there was no change (p > 0.05) in the C4 proportion at 24 h. The ratio of acetic acid to propionic acid (C2:C3) at 12 h and 24 h were decreased (p < 0.05) with the MiEn-LEDRAGON supplemented levels of 2 to 4% DM in the diet. Furthermore, the in vitro CH4 production at 12 h decreased (p < 0.05) with the supplementation of MiEn-LEDRAGON (1 to 4% DM) in the diet. Additionally, there was an observed lower CH4 production at 24 h with the MiEn-LEDRAGON supplementation levels ranging from 2 to 4% DM in the diet (p < 0.05).
Discussion
In this study, the gas production from the immediately soluble fraction (a) was shown to have a negative value. Several studies31,43,44,45,46 indicate that when mathematical models were used to match with gas output kinetics, negative values were recorded for a variety of substrates and microbial activity. This occurred due to the delayed establishment of ruminal microbe growth on the substrates in the initial phase of incubation31,43,45. This situation indicates the presence of a gap time and delay period after the ingestion of the soluble portion of the substrate, prior to the fermentation of the cell walls44,45,46. Therefore, the application of the absolute value of 'a' (|a|) has been recognized as a means to describe the optimum fermentation of the soluble fraction31,44,45. In this study, higher gas production of (|a|) was observed in the MiEn-LEDRAGON supplementation group (1 to 4% DM). This increase could be attributed to the elevated levels of oligosaccharides in dragon fruit peel, particularly fructooligosaccharides (FOS), known as a soluble dietary fraction47,48,49, which was extracted in MiEn-LEDRAGON. The soluble fraction facilitates binding with rumen microbes, contributing to a higher production of gas31,43,46.
The present study observed an increased gas production of (b) with rising MiEn-LEDRAGON levels, possibly attributed to the protein-based polymers constituting the polymer walls or carrier materials of MiEn-LEDRAGON. Generally, proteins are one type of carrier material for microencapsulation that are in their native state within the environment, as they are typically insoluble or bound without undergoing degradation in most instances, but it is soluble in diluted acids or bases in minute quantities50. Therefore, the elevated insoluble content could be a contributing factor to the higher gas production of (b). In a recent study, Matra et al.22 demonstrated that the supplementation of microencapsulated Mitragyna leaves extract at 4 to 6% DM in the diet led to increased gas (b) in an in vitro study.
Typically, a higher soluble fraction is associated with a rapid gas production rate (c)31,44,46,51. However, contrary to this expectation, our study found a lower rate of gas production (c) in the MiEn-LEDRAGON supplemented group. This observation may be attributed to the increased presence of the insoluble fraction derived from the polymer wall materials of MiEn-LEDRAGON. Chumpawadee et al.45 revealed that a higher insoluble fraction in feed affects slow microbial degradation. The breakdown of the insoluble fraction occurred at a rate approximately four times slower than the breakdown of the soluble fraction during anaerobic digestion52. This leads to a decline in the microbial degradation efficiency in the rumen, causing a gradual reduction or slowdown in the degradation process, which is to achieve higher and longer stability of the core substrate within the ruminal inoculum53,54. Similarly, Ibrahim and Hassen55 demonstrated that the slow release provided by microencapsulated tannin effectively protects the bioactive compound, leading to a slow degradation rate until it reaches the target site.
In this study, a greater potential extent of gas production (|a|+ b) for the MiEn-LEDRAGON supplemented group could be due to a higher mathematical result from the sum of (|a|) and (b), which is primarily the consequence of the carbohydrate fermentation into VFA31,51. The present study, the observed increase in production of cumulative gas at 96 h after incubation with rising MiEn-LEDRAGON levels (3 or 4%) may be attributed to the phytonutrient content of MiEn-LEDRAGON. This includes total phenolic and flavonoid contents, as well as antioxidative substances, which likely have a beneficial ability to interact with fiber and protein contents, leading to enhanced ruminal fermentation and gas production6,16,22,56. Matra et al.16 suggested that the supplementation of dragon fruit peel at 4% of total substrate could increase the gas production and ruminal fermentation. Similarly, Matra et al.22 revealed that the use of microencapsulated phytonutrients from Mitragyna leaf extracts could enhance gas production and ruminal fermentation.
In this study, the similarity in both in vitro degradability of IVDMD and IVOMD at 12 h after incubation could be attributed to the deceleration and increased stability of the core substrate facilitated by the microencapsulated wall of MiEn-LEDRAGON in the in vitro degradation process53,54. This effect may lead to a temporary slowdown in the efficiency of microbial decomposition, resulting in similar outcomes for a short period55. Consequently, this might lead to similarities in cumulative gas production for the first 12 h and in vitro degradability at 12 h. While the present study revealed a greater in vitro degradability (IVDMD and IVOMD) at 24 h after incubation for added MiEn-LEDRAGON at 4 or 6% DM of total substrate. This might result from the phytonutrients, particularly flavonoids and phenolics, present in MiEn-LEDRAGON, which exhibit various biological effects that can influence ruminal microbes, ultimately enhancing the degradation of feed in the rumen22,57,58. In addition, this might be a result of the balanced concentrations of condensed tannins and saponin derived from the dragon fruit peel in MiEn-LEDRAGON, influencing rumen fermentation22,59. Moreover, it could be attributed to the vital nutrients promoting microbial activity sourced from the dragon fruit peel in MiEn-LEDRAGON, potentially enhancing the degradability of nutrients. Matra et al.16 demonstrated that the supplementation of dragon fruit peel at 4% of total substrate could enhance the in vitro degradability. Similarly, Matra et al.22 indicated that microencapsulated phytonutrients from Mitragyna leaf extracts could promote the in vitro degradability.
The present study indicates that the dietary treatments did not affect rumen pH within the normal range of 6.85 to 6.93. Wanapat et al.60 revealed that pH levels between 6.5 and 7.0 are considered optimal for ruminal fermentation, microbial activity, and microbial growth. Furthermore, the previous studies8,61 revealed that the incorporation of feed ingredients rich in phenolic compounds can improve rumen fermentation by maintaining the ruminal pH.
In this study, NH3–N concentration at 24 h after incubation was enhanced with MiEn-LEDRAGON supplementation at 4 or 6% DM. This may be attributed to the high CP level in MiEn-LEDRAGON (264 g/kg of CP), which is formulated with a cricket protein extract as a polymer wall carrier material. Furthermore, this is probably due to the capability of the plant bioactive extract to augment the proteolysis process22. According to Ahmed et al.62, the addition of plant-derived bioactives leads to an elevation in ruminal NH3–N concentration. Similarly, Matra et al.22 demonstrated that the microencapsulated phytonutrients from Mitragyna leaf extracts supplementation could enhance in vitro NH3–N concentration.
This study's results demonstrated a lower population of both cellulolytic Ruminococcus species (R. albus and R. flavefaciens) when MiEn-LEDRAGON was added to the diet at 3 or 4% DM. This could be attributed to the susceptibility of bio-active compounds namely phenolic and flavonoid compounds rich in MiEn-LEDRAGON1,63. This finding aligns with Kim et al.63, who reported a decrease in the population of R. albus and R. flavefaciens when flavonoid plant extract was included in the diet compared to the control. Similarly, several previous studies1,64,65 demonstrated that the presence of plant secondary compounds such as flavonoid, phlorotannin, and condensed tannin resulted in a reduction in the abundance of R. albus and R. flavefaciens compared to the control. Moreover, this study interestingly showed an enhancement in the population of another major cellulolytic bacterium, F. succinogenes, at 24 h after incubation with MiEn-LEDRAGON supplementation at 3 or 4% DM. Given that phenolic and flavonoids typically exert antimicrobial effects by inhibiting cytoplasmic membrane function, hindering bacterial cell wall synthesis, or impeding nucleic acid synthesis66,67, differences in sensitivity to these plant secondary compounds may arise due to variations in the cell wall structures of Gram-negative bacteria (F. succinogenes) and Gram-positive bacteria (Ruminococcus species)1,9. Therefore, after a reduction in the populations of the two cellulolytic Ruminococcus species, there might be a compensatory rise in F. succinogenes populations. Kim et al.63 confirmed that an increase in F. succinogenes diversity when exposed to flavonoid plant extract, whereas populations of R. albus and R. flavefaciens decreased compared to the control. Similarly, Chen et al.1 revealed that the population of F. succinogenes increased with tannin treatments, while the populations of R. albus and R. flavefaciens decreased. This study's findings indicate that supplementing MiEn-LEDRAGON increased the population of Butyrivibrio fibrisolvens at 12 h. This enhancement might be attributed to MiEn-LEDRAGON promoting the breakdown of cellulose and protein by boosting the populations of bacteria involved in decomposing these plant bioactive components58,67. Matra et al.22 demonstrated a similar outcome with the addition of microencapsulated bioactive compounds from Mitragyna leaf extracts at 6% DM, which stimulated the B. fibrisolvens population. Additionally, Zhan et al.58 showed that alfalfa flavonoids also contribute to the growth of B. fibrisolvens in dairy cows. Meanwhile, this study indicates a decrease in the methanogen group (Methanobacteriales) when MiEn-LEDRAGON was added to the diet, which could be attributed to the presence of bioactive compounds rich in MiEn-LEDRAGON. Plant secondary compounds promptly influences rumen methanogens by interacting with the proteinaceous adhesin, suppressing methanogen growth, decreasing interspecies carbon and hydrogen transfer, and preventing the formation of the methanogen-protozoa complex1,68,69. Patra et al.70 found that extracts containing phenolics led to a reduction in ruminal methane emission and the number of protozoa. Similarly, Patra and Saxena9 demonstrated that flavonoids exert direct effects against methanogens, leading to a decrease in protozoa associated with ruminal methanogenesis. Matra et al.22 reported that the supplementation of microencapsulated bioactive compounds from Mitragyna leaf extracts has potential to decrease the Methanobacteriales population.
In this study, a higher total VFA at 24 h after incubation was observed in the MiEn-LEDRAGON supplementation groups (at 2 to 4% DM). This increase could be attributed to enhanced degradability. Furthermore, the plant secondary compounds in MiEn-LEDRAGON likely played a significant role in supporting microbial fermentation, contributing to the increase and benefiting the production of ruminal fermentation end-products (VFA)5,10,12. Matra and Wanapat11 revealed that the phytonutrients from dragon fruit peel pellets promoted total VFA production. Particularly, MiEn-LEDRAGON supplementation groups (at 2 to 4% DM) exhibited a higher proportion of C3, while a lower proportion of C2, C2:C3 ratio, and CH4 production. This may be attributed to the potential of plant secondary compounds to influence the production of C3 in conditions of hydrogen excess10. In such situations, hydrogen may be utilized to generate propionate instead of serving as the primary substrate for methane production71. Furthermore, the increased C3 production due to MiEn-LEDRAGON supplementation might be associated with the elevated population of F. succinogenes, known as a succinate-producing microbe in the rumen1. This heightened C3 production can be attributed to the succinate–propionate pathway, a predominant route for the C3 production in the rumen72. Additionally, this probably due to a consequence of the reduction in the population of R. albus and R. flavefaciens, which are recognized as substantial hydrogen (H2) producers and have been shown to produce CH4 when co-cultured with methanogens1,64. On the contrary, the increased abundance of F. succinogenes, a non-hydrogen producer, contributes to elevated C3 production, a decreased C2:C3 ratio, and a reduction in CH4 production1,73. Matra and Wanapat11 demonstrated that phytonutrients from dragon fruit peel pellets increased the proportion of C3, while decreasing the proportion of C2 and CH4 production. Similarly, Matra et al.22 reported that the supplementation of microencapsulated bioactive compounds from Mitragyna leaf extracts has the potential to enhance C3 production and reduce CH4 production. This study, a higher C4 proportion at 12 h could be due to an increasing of B. fibrisolvens, a major butyrate-producing bacterium in the rumen at 12 h after incubation74. Similarly, Matra et al.22 revealed that the addition of microencapsulated bioactive compounds from Mitragyna leaf extracts in the diet was increased C4 proportion.
Inconclusion, this study suggests that supplementing MiEn-LEDRAGON at 3% of total DM substrate enhances cumulative gas production, in vitro degradability, and NH3-N concentration as well as improved microbial diversity and fermentation end-products, particularly C3 production, while decreasing Methanobacteriales (methanogen) and methane production. Therefore, MiEn-LEDRAGON has the potential to be used as a feed additive rich in phytonutrients for ruminants. Nevertheless, additional in vivo trials are necessary to evaluate its effectiveness in animal production.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Chen, L. et al. Effects of hydrolysable tannin with or without condensed tannin on alfalfa silage fermentation characteristics and in vitro ruminal methane production, fermentation patterns, and microbiota. Animals 11, 1967. https://doi.org/10.3390/ani11071967 (2021).
Króliczewska, B., Pecka-Kiełb, E. & Bujok, J. Strategies used to reduce methane emissions from ruminants: Controversies and issues. Agriculture 13, 602. https://doi.org/10.3390/agriculture13030602 (2023).
Beauchemin, K. A. et al. Invited review: Current enteric methane mitigation options. J. Dairy Sci. 105, 9297–9326 (2022).
Palangi, V. & Lackner, M. Management of enteric methane emissions in ruminants using feed additives: A review. Animals 12, 3452. https://doi.org/10.3390/ani12243452 (2021).
Phesatcha, B., Phesatcha, K. & Wanapat, M. Mitragyna speciosa Korth leaf pellet supplementation on feed intake, nutrient digestibility, rumen fermentation, microbial protein synthesis and protozoal population in Thai native beef cattle. Animals 12, 3238. https://doi.org/10.3390/ani12233238 (2022).
Wanapat, M. et al. Supplementation of fruit peel pellet containing phytonutrients to manipulate rumen pH, fermentation efficiency, nutrient digestibility and microbial protein synthesis. J. Sci. Food Agric. 101, 4543–4550 (2021).
Hassan, A., Akmal, Z. & Khan, N. The phytochemical screening and antioxidants potential of Schoenoplectus triqueter L. Palla. J. Chem. 2020, 3865139. https://doi.org/10.1155/2020/3865139 (2020).
Suriyapha, C. et al. Manipulating rumen fermentation, microbial protein synthesis, and mitigating methane production using bamboo grass pellet in swamp buffaloes. Trop. Anim. Health Prod. 52, 1609–1615 (2020).
Patra, A. K. & Saxena, J. A new perspective on the use of plant secondary metabolites to inhibit methanogenesis in the rumen. Phytochemistry 71, 1198–1222 (2010).
Ampapon, T., Viennasay, B., Matra, M., Totakul, P. & Wanapat, M. Phytonutrients in red Amaranth (Amaranthus cruentus, L.) and feed ratios enhanced rumen fermentation dynamics, suppress protozoal population, and methane production. Front. Anim. Sci. 3, 741543. https://doi.org/10.3389/fanim.2022.741543 (2022).
Matra, M. & Wanapat, M. Phytonutrient pellet supplementation enhanced rumen fermentation efficiency and milk production of lactating Holstein-Friesian crossbred cows. Anim. Nutr. 9, 119–126 (2022).
Phesatcha, K., Phesatcha, B. & Wanapat, M. Mangosteen peel liquid-protected soybean meal can shift rumen microbiome and rumen fermentation end-products in lactating crossbred Holstein Friesian cows. Front. Vet. Sci. 8, 772043. https://doi.org/10.3389/fvets.2021.772043 (2022).
Phesatcha, K., Phesatcha, B., Wanapat, M. & Cherdthong, A. Mitragyna speciosa Korth leaves supplementation on feed utilization, rumen fermentation efficiency, microbial population, and methane production in vitro. Fermentation 8, 8. https://doi.org/10.3390/fermentation8010008 (2021).
Totakul, P. et al. Supplemental effect of Chaya (Cnidoscolus aconitifolius) leaf pellet on rumen fermentation, nutrients digestibility and microbial protein synthesis in growing crossbred bulls. Ital. J. Anim. Sci. 20, 279–287 (2021).
Rahayuningsih, E., Setiawan, F. A., Kasyfur Rahman, A. B., Siahaan, T. & Bayu Murti Petrus, H. T. Microencapsulation of betacyanin from red dragon fruit (Hylocereus polyrhizus) peels using pectin by simple coacervation to enhance stability. J. Food Sci. Technol. 58, 3379–3387 (2021).
Matra, M., Wanapat, M., Cherdthong, A., Foiklang, S. & Mapato, C. Dietary dragon fruit (Hylocereus undatus) peel powder improved in vitro rumen fermentation and gas production kinetics. Trop. Anim. Health Prod. 51, 1531–1538 (2019).
Matra, M., Totakul, P., Viennasay, B., Phesatcha, B. & Wanapat, M. Dragonfruit (Hylocereus undatus) peel pellet as a rumen enhancer in Holstein cross bred bulls. Anim. Biosci. 34, 594e602. https://doi.org/10.5713/ajas.20.0151 (2020).
Bhatt, R. et al. Dietary inclusion of mature lemon grass and curry leaves affects nutrient utilization, methane reduction and meat quality in finisher lambs. Anim. Feed Sci. Technol. 278, 114979. https://doi.org/10.1016/j.anifeedsci.2021.114979 (2021).
Wanapat, M., Cherdthong, A., Pakdee, P. & Wanapat, S. Manipulation of rumen ecology by dietary lemongrass (Cymbopogon citratus Stapf.) powder supplementation. J. Anim. Sci. 86, 3497–3503 (2008).
Phupaboon, S. et al. Extraction, characterization, and chitosan microencapsulation of bioactive compounds from Cannabis sativa L., Cannabis indica L., and Mitragyna speiosa K. Antioxidants 11, 2103. https://doi.org/10.3390/antiox11112103 (2022).
Mohammadalinejhad, S. & Kurek, M. A. Microencapsulation of anthocyanins—Critical review of techniques and wall materials. Appl. Sci. 11, 3936. https://doi.org/10.3390/app11093936 (2021).
Matra, M. et al. Microencapsulation from Mitragyna leaves extracts as a bioactive compound source to enhance in vitro fermentation characteristics and microbial dynamics. Anim. Biosci. https://doi.org/10.5713/ab.23.0200 (2023).
Association of Official Analytical Chemists (AOAC). The Official Methods of Analysis 19th edn. (AOAC International, 2012).
Van Soest, P. J., Robertson, J. B. & Lewis, B. A. Methods for dietary fiber neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597 (1991).
Singleton, V. L. & Rossi, J. A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic ccid reagents. Am. J. Enol. Viticult. 16, 144–158 (1965).
Braca, A. et al. Antioxidant and free radical scavenging activity of flavonol glycosides from different aconitum species. J. Ethnopharmacol. 86, 63–67 (2003).
Brand-Williams, W., Cuvelier, M. E. & Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 28, 25–30 (1995).
Dudonnédudonn, S. et al. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 57, 1768–1774 (2009).
Benzie, I. F. F. & Strain, J. J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 239, 70–76 (1996).
Makkar, H. P. S., Blümmel, M. & Becker, K. Formation of complexes between polyvinyl pyrrolidones or polyethylene glycols and tannins, and their implication in gas production and true digestibility in vitro techniques. Br. J. Nutr. 73, 897–913 (1995).
Suriyapha, C., Cherdthong, A., Suntara, C. & Polyorach, S. Utilization of yeast waste fermented citric waste as a protein source to replace soybean meal and various roughage to concentrate ratios on in vitro rumen fermentation, gas kinetic, and feed digestion. Fermentation 7, 120. https://doi.org/10.3390/fermentation7030120 (2021).
Ørskov, E. R. & McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 92, 499–503 (1979).
Tilley, J. M. A. & Terry, R. A. A two-stage technique for the digestion of forage crops. J. Br. Grassl. Soc. 18, 104–111 (1963).
Kaewpila, C. et al. Characterization of green manure Sunn Hemp crop silage prepared with additives: Aerobic instability, nitrogen value, and in vitro rumen methane production. Fermentation 8, 104. https://doi.org/10.3390/fermentation8030104 (2022).
Fawcett, J. & Scott, J. A rapid and precise method for the determination of urea. J. Clin. Pathol. 13, 156–159 (1960).
So, S., Cherdthong, A. & Wanapat, M. Growth performances, nutrient digestibility, ruminal fermentation and energy partition of Thai native steers fed exclusive rice straw and fermented sugarcane bagasse with Lactobacillus, cellulase and molasses. J. Anim. Physiol. Anim. Nutr. 106, 45–54 (2022).
Koike, S. & Kobayashi, Y. Development and use of competitive PCR assays for the rumen cellulolytic bacteria: Fibrobacter succinogenes, Ruminococcus albus and Ruminococcus flavefaciens. FEMS Microbiol. Lett. 204, 361–366 (2001).
Fernando, S. C. et al. Rumen microbial population dynamics during adaptation to a high-grain diet. Appl. Environ. Microbiol. 76, 7482–7490 (2010).
Ouwerkerk, D., Klieve, A. V. & Forster, R. J. Enumeration of Megasphaera elsdenii in rumen contents by real-time Taq nuclease assay. J. Appl. Microbiol. 92, 753–758 (2002).
Yu, Y., Lee, C., Kim, J. & Hwang, S. Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol. Bioeng. 89, 670–679 (2005).
SAS. User’s Guide: Statistics Version 9.4. (SAS Inc., 2013).
Steel, R. G. & Torrie, J. H. Principles and Procedures of Statistics (McGraw-Hill Book Co Inc., 1980).
Soriano, A. P. et al. Effect of Lactobacillus mucosae on in vitro rumen fermentation characteristics of dried brewers grain, methane production and bacterial diversity. Asian-Australas. J. Anim. Sci. 27, 1562–1570 (2014).
Chanthakhoun, V. & Wanapat, M. The in vitro gas production and ruminal fermentation of various feeds using rumen liquor from swamp buffalo and cattle. Asian J. Anim. Vet. Adv. 7, 54–60 (2012).
Chumpawadee, S., Sommart, K., Vongpralub, T. & Pattarajinda, V. Nutritional evaluation of non-forage high fibrous tropical feeds for ruminant using in vitro gas production technique. Walailak J. Sci. Technol. 2, 209–218 (2005).
Khazaal, K., Dentinho, M., Ribeiro, J. & Ørskov, E. A comparison of gas production during incubation with rumen contents in vitro and nylon bag degradability as predictors of the apparent digestibility in vivo and the voluntary intake of hays. Anim. Sci. 57, 105–112 (1993).
Yuanita, I. et al. The effect of red dragon fruit (Hylocereus polyrhizus) peel meal on internal organs, ileal coliform, and growth performances in broiler chickens. Indones. J. Anim. Sci. 32, 174–182 (2022).
Wichienchot, S., Jatupornpipat, M. & Rastall, R. Oligosaccharides of pitaya (dragon fruit) flesh and their prebiotic properties. Food Chem. 120, 850–857 (2010).
Sabater-Molina, M., Larqué, E., Torrella, F. & Zamora, S. Dietary fructooligosaccharides and potential benefits on health. J. Physiol. Biochem. 65, 315–328. https://doi.org/10.1007/BF03180584 (2009).
Parente, J. F., Sousa, V. I., Marques, J. F., Forte, M. A. & Tavares, C. J. Biodegradable polymers for microencapsulation Systems. Adv. Polym. Technol. 2022, 4640379. https://doi.org/10.1155/2022/4640379 (2022).
Getachew, G., Blümmel, M., Makkar, H. & Becker, K. In vitro gas measuring techniques for assessment of nutritional quality of feeds: A review. Anim. Feed Sci. Technol. 72, 261–281 (1998).
Nopharatana, A., Pullammanappallil, P. C. & Clarke, W. P. Kinetics and dynamic modelling of batch anaerobic digestion of municipal solid waste in a stirred reactor. Waste Manag. 27, 595–603 (2006).
Amin, N., Tagliapietra, F., Arango, S., Guzzo, N. & Bailoni, L. Free and microencapsulated essential oils incubated in vitro: Ruminal stability and fermentation parameters. Animals 11, 180. https://doi.org/10.3390/ani11010180 (2020).
Lira-Casas, R. et al. Designing and evaluation of urea microcapsules in vitro to improve nitrogen slow release availability in rumen. J. Sci. Food Agric. 99, 2541–2547 (2019).
Ibrahim, S. L. & Hassen, A. Characterization, density and in vitro controlled release properties of Mimosa (Acacia mearnsii) tannin encapsulated in palm and sunflower oils. Animals 11, 2919. https://doi.org/10.3390/ani11102919 (2021).
Pal, K., Patra, A. K. & Sahoo, A. Evaluation of feeds from tropical origin for in vitro methane production potential and rumen fermentation in vitro. Span. J. Agric. Res. 13, e0608. https://doi.org/10.5424/sjar/2015133-7467 (2015).
Sommai, S. et al. In vitro fermentation characteristics and methane mitigation responded to flavonoid extract levels from Alternanthera sissoo and dietary ratios. Fermentation 7, 109. https://doi.org/10.3390/fermentation7030109 (2021).
Zhan, J. et al. Effects of alfalfa flavonoids on the production performance, immune system, and ruminal fermentation of dairy cows. Asian-Australas. J. Anim. Sci. 30, 1416–1424 (2017).
Makkar, H. P. S. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 49, 241–256 (2003).
Wanapat, M., Gunun, P., Anantasook, N. & Kang, S. Changes of rumen pH, fermentation and microbial population as influenced by different ratios of roughage (rice straw) to concentrate in dairy steers. J. Agric. Sci. 152, 675–685 (2014).
Viennasay, B., Totakul, P., Matra, M., Phesatcha, B. & Wanapat, M. Influence of bamboo grass (Tiliacora triandra, Diels) pellet supplementation on in vitro fermentation and methane mitigation. J. Sci. Food Agric. 102, 4927–4932 (2022).
Ahmed, E., Fukuma, N., Hanada, M. & Nishida, T. The efficacy of plant-based bioactives supplementation to different proportion of concentrate diets on methane production and rumen fermentation characteristics in vitro. Animals 11, 1029. https://doi.org/10.3390/ani11041029 (2021).
Kim, E. T. et al. Effects of Flavonoid-rich plant extracts on in vitro ruminal methanogenesis, microbial populations and fermentation characteristics. Asian-Australas. J. Anim. Sci. 28, 530–537 (2015).
Khiaosa-ard, R. et al. Fortification of dried distillers grains plus solubles with grape seed meal in the diet modulates methane mitigation and rumen microbiota in Rusitec. J. Dairy Sci. 98, 2611–2626 (2015).
Wang, Y., Alexander, T. W. & McAllister, T. A. In vitro effects of phlorotannins from Ascophyllum nodosum (brown seaweed) on rumen bacterial populations and fermentation. J. Sci. Food Agric. 89, 2252–2260 (2009).
Cushnie, T. P. T. & Lamb, A. J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 26, 343–356 (2005).
McAllister, T. A. et al. Characterization of condensed tannins purified from legume forages: Chromophore production, protein precipitation, and inhibitory effects on cellulose digestion. J. Chem. Ecol. 31, 2049–2068 (2005).
Li, M. et al. Mulberry flavonoids modulate rumen bacteria to alter fermentation kinetics in water buffalo. PeerJ 10, e14309. https://doi.org/10.7717/peerj.14309 (2022).
Manasri, N., Wanapat, M. & Navanukraw, C. Improving rumen fermentation and feed digestibility in cattle by mangosteen peel and garlic pellet supplementation. Livest. Sci. 148, 291–295 (2012).
Patra, A., Kamra, D. & Agarwal, N. Effect of plant extracts on in vitro methanogenesis, enzyme activities and fermentation of feed in rumen liquor of buffalo. Anim. Feed Sci. Technol. 128, 276–291 (2006).
Newbold, C. J. et al. Propionate precursors and other metabolic intermediates as possible alternative electron acceptors to methanogenesis in ruminal fermentation in vitro. Br. J. Nutr. 94, 27–35 (2005).
Lan, W. & Yang, C. Ruminal methane production: Associated microorganisms and the potential of applying hydrogen-utilizing bacteria for mitigation. Sci. Total Environ. 654, 1270–1283 (2019).
Naumann, H. D., Tedeschi, L. O., Zeller, W. E. & Huntley, N. F. The role of condensed tannins in ruminant animal production: Advances, limitations and future directions. Rev. Brasil. Zootec. 46, 929–949 (2017).
Sawanon, S. & Kobayashi, Y. Studies on fibrolytic bacterium Butyrivibrio fibrisolvens isolated from sheep rumen. Songklanakarin J. Sci. Technol. 29, 351–361 (2007).
Acknowledgements
The authors express our most sincere gratitude to the Fundamental Fund (FF) (No. 66A103000057), the Ministry of Higher Education, Science, Research, and Innovation (MHESI), and Khon Kaen University, Thailand for making this manuscript possible. In addition, the authors would like to thank the Tropical Feed Resources Research and Development Center (TROFREC), Department of Animal Science, Faculty of Agriculture, Khon Kaen University, Thailand for their facilitation and execution.
Author information
Authors and Affiliations
Contributions
C. Suriyapha, Project administration, Conceptualization, Investigation, Methodology, Visualization, C. Suriyapha, S. Phupaboon, G. Dagaew, S. Sommai, M. Matra, and R. Prachumchai: Data curation, Formal analysis, and Software, M. Wanapat and T. Haitook: Resources, Supervision, Conceptualization, Validation, Visualization, Project administration and Funding acquisition, C. Suriyapha: Roles/Writing – original draft, C. Suriyapha, S. Phupaboon, G. Dagaew, S. Sommai, M. Matra, R. Prachumchai, T. Haitook and M. Wanapat: Writing – review & editing. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suriyapha, C., Phupaboon, S., Dagaew, G. et al. In vitro fermentation end-products and rumen microbiome as influenced by microencapsulated phytonutrient pellets (LEDRAGON) supplementation. Sci Rep 14, 14425 (2024). https://doi.org/10.1038/s41598-024-59697-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59697-x
- Springer Nature Limited