Abstract
The association between pretreatment albumin-to-alkaline phosphatase ratio (AAPR) and clinicopathological parameters and prognosis in lung cancer is unclear. The study aimed to identify the clinical role of pretreatment AAPR among lung cancer patients. Several databases were searched for relevant studies. The primary outcome and secondary outcome were long-term survival including the overall survival (OS) and progression-free survival (PFS) and clinicopathological characteristics, respectively. The hazard ratios (HRs) and relative risks (RRs) with 95% confidence intervals (CIs) were combined. A total of 11 publications involving 10,589 participants were included in this meta-analysis. The pooled results manifested that a lower pretreatment AAPR predicted poorer OS (HR = 0.65, 95% CI 0.59–0.71, P < 0.001) and PFS (HR = 0.68, 95% CI 0.59–0.78, P < 0.001). Furthermore, subgroup analysis for the OS and PFS based on the pathological type and treatment showed similar results and pretreatment AAPR was significantly associated with worse prognosis. Besides, pretreatment AAPR was significantly associated with male (RR = 1.08, 95% CI 1.03–1.13, P < 0.001), poor differentiation (RR = 1.33, 95% CI 1.03–1.73, P = 0.029), advanced T stage (RR = 1.25, 95% CI 1.03–1.52, P = 0.026), N stage (RR = 1.34, 95% CI 1.15–1.55, P < 0.001) and TNM stage (RR = 1.14, 95% CI 1.06–1.223, P < 0.001). Therefore, pretreatment AAPR is significantly related to prognosis and tumor stage in lung cancer and patients with a lower pretreatment AAPR are more likely to experience poor survival and advanced tumor stage.
Similar content being viewed by others
Introduction
Lung cancer remains the most commonly diagnosed cancer type and leading cause of tumor-related death worldwide1,2. Although great advances in treatment strategies including the surgical techniques and anti-tumor drugs have been achieved in the past decade, the overall survival of lung cancer patients remains unsatisfied3. Besides, it is still difficult to predict the prognosis of lung cancer patients accurately and formulate the most appropriate therapy strategy now. Therefore, identifying more useful and reliable indicators contributing to the survival prediction of lung cancer patients is one of the urgent problems to be solved.
Albumin, as the most abundant protein in the blood, can reflect the body’s systemic inflammatory response and basic nutritional status. The serum albumin content in cancer patients usually decreases gradually with the progression of the disease, and studies have confirmed that the decrease of serum albumin indicates poor clinical prognosis4,5. Serum alkaline phosphatase is a hydrolytic enzyme concentrated in the liver, bile ducts, and kidneys, and serum levels can be significantly elevated when cancer affects the bone or liver. Studies of patients with hepatocellular carcinoma have demonstrated that the ratio of serum albumin to serum alkaline phosphatase as a prognostic factor can provide additional guidance compared with a single marker6,7. Therefore, the novel index, albumin-to-alkaline phosphatase ratio (AAPR), is believed to serve as a valuable prognostic indicator in cancer patients.
Xie et al. included 16 eligible studies with 5716 patients and manifested that low pretreatment AAPR was related to poor prognosis in patients with cancer8. However, only five studies focused on lung cancer cases in their meta-analysis. Thus, whether pretreatment AAPR is a reliable prognostic indicator in lung cancer is unclear.
This meta-analysis aimed to further identify the clinical role of pretreatment AAPR based on current evidence, contributing to the accurate prediction of survival and also formulation of treatment strategy of lung cancer patients.
Materials and methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Review and Meta-Analyses 20209.
Literature retrieval
The PubMed, EMBASE, Web of Science and CNKI electronic databases were searched from inception to October 14, 2022. During the literature search, the following terms were used: albumin-to-alkaline phosphatase ratio, AAPR, lung, pulmonary, tumor, cancer, carcinoma, neoplasm, survival, prognosis and prognostic. In detail, the specific search strategy was as follows: (albumin-to-alkaline phosphatase ratio OR AAPR) AND (lung OR pulmonary) AND (tumor OR cancer OR carcinoma OR neoplasm) AND (survival OR prognosis OR prognostic). Meanwhile, the free texts and MeSH terms were used during the search. References cited in included studies were also reviewed for availability.
Inclusion criteria and exclusion criteria
The inclusion criteria were as follows: (1) patients were diagnosed with primary lung cancer; (2) the serum albumin and alkaline phosphatase levels were detected before any anti-tumor treatment; (3) patients were divided into two groups according to the pretreatment AAPR and the primary outcomes including the overall survival (OS) and progression-free survival (PFS), were compared between the two groups; (4) the hazard ratios (HRs) and 95% confidence intervals (CIs) were directly provided in the articles; 5) high-quality studies with the Newcastle–Ottawa Scale (NOS) score > 510.
Exclusion criteria: (1) duplicated or overlapped data; (2) letters, editorials, case reports, animal trials or reviews.
Data collection
The following data were extracted from each included studies: the name of first author, year, country, sample size, pathologic type, treatment, tumor stage, cutoff value of AAPR, endpoint, age, gender, smoking history, differentiation degree, T stage, N stage, TNM stage, HR and 95% CI.
Study quality assessment
The quality of all included studies was assessed according to the NOS score tool and only high-quality studies with the NOS of 6 or higher were included as above mentioned10.
In our meta-analysis, the literature search, selection, data extraction and quality assessment were all performed by two independent authors.
Statistical analysis
In this meta-analysis, all statistical analyses were performed using STATA 15.0 software. Heterogeneity among studies was evaluated using I2 statistics and the Q-test. When obvious heterogeneity was detected, representing I2 > 50% and/or P < 0.1, the random effects model was used; otherwise, the fixed effects model was used. The HRs, relative risks (RRs) and 95% CIs were combined. Sensitivity analysis was performed to detect sources of heterogeneity and assess the stability of the pooled results. Besides, the Begg’s funnel plot and Egger’s test were performed to detect publication bias, and significant publication bias was defined as P < 0.0511,12. If we detected significant publication bias, then the nonparametric trim-and-fill method was used to re-estimate a corrective effect size after publication bias was adjusted13.
Ethical statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies that involved human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Results
Literature search and selection
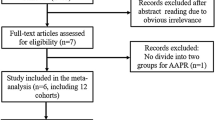
Sixty-seven records were searched from the four databases initially and 23 duplicated records were removed. Then the titles and abstracts of remaining 44 records were reviewed and full texts of 16 studies were further reviewed. Finally, 11 relevant studies were included our meta-analysis14,15,16,17,18,19,20,21,22,23,24. The detailed process was displayed in the Fig. 1.
Basic characteristics of included studies
A total of 10,589 patients were enrolled in the analysis with the sample size ranged from 116 to 7078. Except the study by Birgitte et al.21, all included studies were from China. Eight and four studies focused on the NSCLC and SCLC patients, respectively. The cutoff values of AAPR ranged from 0.238 to 0.64. Other detailed information was presented in Table 1.
The association between pretreatment AAPR and OS
All 11 included studies explored the predictive role of pretreatment AAPR for OS of lung cancer14,15,16,17,18,19,20,21,22,23,24. The pooled results demonstrated that a lower pretreatment AAPR was significantly associated with poorer OS (HR = 0.65, 95% CI 0.59–0.71, P < 0.001; I2 = 52.0%, P = 0.018) (Fig. 2). Then, subgroup analysis based on the pathological type (NSCLC vs SCLC) and treatment (non-surgery vs. surgery vs. mixed) also showed that a lower pretreatment AAPR was related to worse OS (NSCLC: HR = 0.65, 95% CI 0.58–0.73, P < 0.001; SCLC: HR = 0.62, 95% CI 0.54–0.71, P < 0.001; non-surgery: HR = 0.60, 95% CI 0.53–0.68, P < 0.001; surgery: HR = 0.46, 95% CI 0.33–0.65, P < 0.001; mixed: HR = 0.65, 95% CI 0.59–0.71, P < 0.001) (Table 2).
The association between pretreatment AAPR and PFS
Six studies explored the association between pretreatment AAPR and PFS of lung cancer patients15,16,17,18,22,23. The pooled results indicated that pretreatment AAPR was obviously related to poor PFS (HR = 0.68, 95% CI 0.59–0.78, P < 0.001; I2 = 32.5%, P = 0.192) (Fig. 3). Subgroup analysis stratified by the pathological type and treatment manifested similar results (NSCLC: HR = 0.70, 95% CI 0.60–0.80, P < 0.001; SCLC: HR = 0.59, 95% CI 0.40–0.86, P = 0.007; non-surgery: HR = 0.72, 95% CI 0.62–0.84, P < 0.001; surgery: HR = 0.55, 95% CI 0.40–0.74, P < 0.001) (Table 2).
The association between pretreatment AAPR and clinicopathological parameters
Based on available data, we identified the relationship between pretreatment AAPR and age, gender, smoking history, differentiation degree, T stage, N stage and TNM stage. Pooled results indicated that lower pretreatment AAPR was significantly associated with male (RR = 1.08, 95% CI 1.03–1.13, P < 0.001; I2 = 34.3%, P = 0.124), poor differentiation (RR = 1.33, 95% CI 1.03–1.73, P = 0.029; I2 = 0.0%, P = 0.380), advanced T stage (RR = 1.25, 95% CI 1.03–1.52, P = 0.026; I2 = 59.6%, P = 0.060), N stage (RR = 1.34, 95% CI 1.15–1.55, P < 0.001; I2 = 0.0%, P = 0.610) and TNM stage (RR = 1.14, 95% CI 1.06–1.223, P < 0.001; I2 = 87.9%, P < 0.001) (Table 3).
Sensitivity analysis and publication bias
The sensitivity analysis for the OS showed that our results were stable and reliable (Fig. 4). Due to the asymmetrical Begg’s funnel plot (Fig. 5) and P < 0.001 of Egger’s test, obvious publication bias was detected. Therefore, the nonparametric trim-and-fill method was used to identify potentially unpublished studies. However, no potentially unpublished studies were detected (Fig. 6). Thus, more high-quality studies are still needed to verify our findings.
Discussion
The current meta-analysis demonstrated that pretreatment AAPR was significantly associated with prognosis and tumor stage and lung cancer patients with a lower pretreatment AAPR experienced poorer survival and advanced tumor stage. According to our findings, the pretreatment AAPR is a novel and reliable prognostic factor in lung cancer. However, more prospective high-quality studies are still needed to verify our findings due to limitations existed in this meta-analysis.
AAPR, as a novel parameter which has been reported in the past few years, is defined as the ratio of the serum albumin and serum alkaline phosphatase. It has been well manifested that the albumin level is related to the development, progression and prognosis among cancer patients25,26,27. Albumin concentration could reflect the nutritional status, liver function and human defense capabilities23. Decreased albumin concentration indicates the sign of malnutrition and decrease in immunity and the production of albumin is suppressed by the activation of inflammatory cytokines28. Besides, it has been also reported that the alteration in protein binding shows an impact on the drug half-life, which means hypoalbuminemia could cause a poor response to anti-tumor therapeutics29. Therefore, albumin is closely related to the disease progression and prognosis of cancers30,31,32. On the other hand, alkaline phosphatase is a novel factor predicting the survival of cancer patients. It is a hydrolase enzyme that widely exists in the kidney, liver and bone. Thus, alkaline phosphatase is usually applied as a biomarker reflecting the liver and bone health. In recent years, alkaline phosphatase has been demonstrated to play an important role in the anti-inflammation and immune response33. Meanwhile, numerous studies indicate that alkaline phosphatase concentration is also significantly related to the development and disease progression in cancers34,35. Besides, alkaline phosphatase has also been reported to associated with the survival in several types of cancers including the lung cancer36.
Although the association of serum and alkaline phosphatase concentration with prognosis in tumor patients have been reported29,30,31,32,36. Some studies suggest that AAPR is more effective in predicting the survival and prognosis of lung cancer patients compared to individual biomarkers15,16,17,18,22,23. It demonstrates greater sensitivity in capturing the patient’s physical condition and disease progression, thus holding stronger predictive value15,16,17,18,22,23. Moreover, monitoring changes in AAPR during the course of treatment allows for a more comprehensive understanding of the patient’s response to therapy. The effectiveness of treatment may manifest in various aspects, including cellular activity, inflammation levels, and protein metabolism, and AAPR, as a composite indicator, is better positioned to reflect these complex biological changes. Therefore, AAPR is considered to show greater clinical value than the individual levels of albumin and alkaline phosphatase.
Actually, the prognosis role of pretreatment AAPR has been verified in some cancers37,38. An et al. conducted a meta-analysis by including 18 studies involving 25 cohorts with 7019 cancer patients and revealed that decreased AAPR was significantly related to poor OS (HR = 2.14, 95% CI 1.83–2.51), disease-free survival (DFS) (HR = 1.81, 95% CI 1.60–2.04), PFS (HR = 1.71, 95% CI 1.31–2.22) and cancer-specific survival (CSS) (HR = 2.22, 95% CI 1.67–2.95)37. Notably, in their meta-analysis only five included studies focused on lung cancer37. Besides, Zhang et al. included 12 cohorts and demonstrated that lower AAPR predicted significantly poorer OS (HR = 2.02, 95% CI 1.78–2.30) and RFS (HR = 1.88, 95% CI 1.37–2.57) in hepatocellular carcinoma patients37. This was the first meta-analysis to identify the prognosis role of AAPR in lung cancer and our results strongly indicated the association of lower pretreatment AAPR with worse survival of lung cancer patients.
According to current evidence, we speculate some application of AAPR in clinics. AAPR is used as a prognostic indicator in assessing lung cancer patients. A lower AAPR is typically associated with an unfavorable prognosis, indicating a poorer physical condition in patients and a higher susceptibility to malignant tumor-related complications. Physicians can monitor changes in AAPR before and after treatment to assess the effectiveness of therapy and the overall condition of the patient. AAPR can serve as an auxiliary metric, aiding doctors in devising more personalized treatment plans. For patients with a lower AAPR, more proactive supportive therapies, such as nutritional support or adjustments to the treatment plan, may be necessary to enhance treatment tolerance. Changes in AAPR can also be utilized to monitor disease recurrence in lung cancer patients. Following treatment, a rapid decline in AAPR may suggest tumor recurrence or progression, prompting physicians to undertake further examinations or adjust the treatment plan. However, AAPR should be considered as part of a comprehensive evaluation alongside other clinical and laboratory indicators, rather than being used in isolation. Other factors influencing AAPR, such as infections, inflammation, and other chronic diseases, should also be taken into account. Additionally, caution is advised when interpreting AAPR results, as they may be influenced by various factors. Clinical practitioners should integrate AAPR with the overall patient profile and other relevant indicators for a more accurate assessment of the patient’s disease status and the formulation of individualized treatment plans.
Several limitations existed in our meta-analysis. First, all included studies are retrospective with relatively small sample sizes. Second, most included studies are from China, limiting the generalizability of our results. Third, it was unable to conduct more subgroup analysis based on other important parameters such as the tumor stage and age. Four, it is not feasible to determine the optimal threshold of AAPR due to the lack of original data.
Conclusion
Pretreatment AAPR is significantly related to prognosis and tumor stage in lung cancer and patients with a lower pretreatment AAPR are more likely to experience poor survival and advanced tumor stage. Therefore, the pretreatment AAPR could serve as a novel and valuable prognostic factor in lung cancer patients.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Torre, L. A., Siegel, R. L. & Jemal, A. Lung cancer statistics. Adv. Exp. Med. Biol. 893, 1–19. https://doi.org/10.1007/978-3-319-24223-1_1 (2016).
Release Notice - Canadian Cancer Statistics: A 2020 special report on lung cancer. (2020). Health Promot Chronic Dis Prev Can 40, 325. Doi: https://doi.org/10.24095/hpcdp.40.10.05.
Jenkins, R., Walker, J. & Roy, U. B. 2022 Cancer statistics: Focus on lung cancer. Future Oncol. https://doi.org/10.2217/fon-2022-1214 (2023).
Danan, D. et al. Prognostic value of albumin in patients with head and neck cancer. Laryngoscope 126, 1567–1571. https://doi.org/10.1002/lary.25877 (2016).
Guner, A. et al. Prognostic value of postoperative neutrophil and albumin: Reassessment one month after gastric cancer surgery. Front Oncol. 11, 633924. https://doi.org/10.3389/fonc.2021.633924 (2021).
Namikawa, T. et al. Prognostic significance of serum alkaline phosphatase and lactate dehydrogenase levels in patients with unresectable advanced gastric cancer. Gastric Cancer 22, 684–691. https://doi.org/10.1007/s10120-018-0897-8 (2019).
Mori, K. et al. Prognostic value of alkaline phosphatase in hormone-sensitive prostate cancer: A systematic review and meta-analysis. Int. J. Clin. Oncol. 25, 247–257. https://doi.org/10.1007/s10147-019-01578-9 (2020).
Xie, H., Wei, L., Tang, S. & Gan, J. Prognostic value of pretreatment albumin-to-alkaline phosphatase ratio in cancer: A meta-analysis. Biomed. Res. Int. 2020, 6661097. https://doi.org/10.1155/2020/6661097 (2020).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clin. Res. Ed.) 372, n71. https://doi.org/10.1136/bmj.n71 (2021).
Wang, Y., Li, J., Chang, S., Dong, Y. & Che, G. Risk and influencing factors for subsequent primary lung cancer after treatment of breast cancer: A systematic review and two meta-analyses based on four million cases. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 16, 1893–1908. https://doi.org/10.1016/j.jtho.2021.07.001 (2021).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin. Res. Ed.) 315, 629–634. https://doi.org/10.1136/bmj.315.7109.629 (1997).
Wang, Y., Huang, D., Xu, W. Y., Wang, Y. W. & Che, G. W. Prognostic value of pretreatment lymphocyte-to-monocyte ratio in non-small cell lung cancer: A meta-analysis. Oncol. Res. Treat. 42, 523–531. https://doi.org/10.1159/000501726 (2019).
Li, D., Yu, H. & Li, W. Albumin-to-alkaline phosphatase ratio at diagnosis predicts survival in patients with metastatic non-small-cell lung cancer. Oncotargets Therapy 12, 5241–5249. https://doi.org/10.2147/ott.s203321 (2019).
Li, S. J. et al. Albumin-to-alkaline phosphatase ratio as a novel prognostic indicator for patients undergoing minimally invasive lung cancer surgery: A prospective propensity score matching study. Int. J. Surg. 69, 32 (2019).
Li, X. et al. Prognostic value of dynamic albumin-to-alkaline phosphatase ratio in limited stage small-cell lung cancer. Future Oncol. 15, 995–1006. https://doi.org/10.2217/fon-2018-0818 (2019).
Zhang, L. et al. The prognostic value of the preoperative albumin to alkaline phosphatase ratio in patients with non-small cell lung cancer after surgery. Thorac. Cancer 10, 1581–1589. https://doi.org/10.1111/1759-7714.13107 (2019).
Li, B. et al. Prognostic value of a nomogram based on the dynamic albumin-to-alkaline phosphatase ratio for patients with extensive-stage small-cell lung cancer. Oncotargets Therapy 13, 9043–9057. https://doi.org/10.2147/ott.s262084 (2020).
Zhou, S., Jiang, W., Wang, H., Wei, N. & Yu, Q. Predictive value of pretreatment albumin-to-alkaline phosphatase ratio for overall survival for patients with advanced non-small cell lung cancer. Cancer Med. 9, 6268–6280. https://doi.org/10.1002/cam4.3244 (2020).
Zhou, S., Wang, H., Jiang, W., Yu, Q. & Zeng, A. Prognostic value of pretreatment albumin-to-alkaline phosphatase ratio in extensive-disease small-cell lung cancer: A retrospective cohort study. Cancer Manag. Res. 12, 2015–2024. https://doi.org/10.2147/cmar.s247967 (2020).
Birgitte, S., Ninna, A. & Anne, W. Pretreatment albumin-to-alkaline phosphatase ratio is a prognostic marker in lung cancer patients: A registry-based study of 7077 lung cancer patients. Cancers 13, 6133 (2021).
Liu, X. et al. Albumin-to-alkaline phosphatase ratio: A novel prognostic index for patients with driver mutation-negative advanced non-small cell lung cancer. Clin. Respir. J. 15, 540–549. https://doi.org/10.1111/crj.13339 (2021).
Gan, Y. et al. Prognostic value of albumin-to-alkaline phosphatase ratio for EGFR-mutated advanced non-small-cell lung cancer patients treated with first-line EGFR-TKIs: A large population-based study and literature review. Int. J. Gen. Med. 15, 3405–3416. https://doi.org/10.2147/ijgm.s348912 (2022).
Hu, S., Li, X., Xiang, M. & Zhang, X. Value of albumin to alkaline phosphatase ratio in predicting clinical outcome of patients with non-small cell lung cancer. J. Med. Forum 43, 1–4+9 (2022).
Lorigo, J. et al. C reactive protein/albumin ratio as predictor of prognosis in castration resistant metastatic prostate cancer. Arch. Ital. Urol. Androl. https://doi.org/10.4081/aiua.2023.11242 (2023).
Roberts, W. S., Delladio, W., Price, S., Murawski, A. & Nguyen, H. The efficacy of albumin–globulin ratio to predict prognosis in cancer patients. Int. J. Clin. Oncol. https://doi.org/10.1007/s10147-023-02380-4 (2023).
Wu, W. et al. The prognostic value of the preoperative albumin/globulin and monocyte ratio in resected early-stage non-small cell lung cancer. Asian J. Surg. https://doi.org/10.1016/j.asjsur.2023.06.068 (2023).
McMillan, D. C. et al. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr. Cancer 39, 210–213. https://doi.org/10.1207/S15327914nc392_8 (2001).
Rabbani, G. & Ahn, S. N. Structure, enzymatic activities, glycation and therapeutic potential of human serum albumin: A natural cargo. Int. J. Biol. Macromol. 123, 979–990. https://doi.org/10.1016/j.ijbiomac.2018.11.053 (2019).
Wang, Y. T. et al. Low pretreatment albumin-to-globulin ratios predict poor survival outcomes in patients with head and neck cancer: A systematic review and meta-analysis. J. Cancer 14, 281–289. https://doi.org/10.7150/jca.80955 (2023).
Utsumi, M. et al. Preoperative C-reactive protein-to-albumin ratio as a prognostic factor in biliary tract cancer: A systematic review and meta-analysis. Medicine 102, e33656. https://doi.org/10.1097/md.0000000000033656 (2023).
Dai, M. & Wu, W. Prognostic role of C-reactive protein to albumin ratio in cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Front. Oncol. 13, 1148786. https://doi.org/10.3389/fonc.2023.1148786 (2023).
Rader, B. A. Alkaline phosphatase, an unconventional immune protein. Front. Immunol. 8, 897. https://doi.org/10.3389/fimmu.2017.00897 (2017).
Han, Y., Yoo, H. J., Jee, S. H. & Lee, J. H. High serum levels of L-carnitine and citric acid negatively correlated with alkaline phosphatase are detectable in Koreans before gastric cancer onset. Metabolomics 18, 62. https://doi.org/10.1007/s11306-022-01922-7 (2022).
Qu, F. et al. Construction and validation of a serum albumin-to-alkaline phosphatase ratio-based nomogram for predicting pathological complete response in breast cancer. Front. Oncol. 11, 681905. https://doi.org/10.3389/fonc.2021.681905 (2021).
Yang, T. et al. Pretreatment levels of serum alkaline phosphatase are associated with the prognosis of patients with non-small cell lung cancer receiving immune checkpoint inhibitors. Oncol. Lett. 25, 154. https://doi.org/10.3892/ol.2023.13740 (2023).
Zhang, X., Xin, Y., Chen, Y. & Zhou, X. Prognostic effect of albumin-to-alkaline phosphatase ratio on patients with hepatocellular carcinoma: A systematic review and meta-analysis. Sci. Rep. 13, 1808. https://doi.org/10.1038/s41598-023-28889-2 (2023).
An, L., Yin, W. T. & Sun, D. W. Albumin-to-alkaline phosphatase ratio as a promising indicator of prognosis in human cancers: Is it possible?. BMC Cancer 21, 247. https://doi.org/10.1186/s12885-021-07921-6 (2021).
Acknowledgements
None.
Funding
This work is supported by Sichuan Provincial Health Committee Research Project Grand (20PJ289) and Natural Science Foundation of Sichuan Province (2023NSFSC1893).
Author information
Authors and Affiliations
Contributions
X.X. designed the study. Y.Y. and Y.W. established the process of literature selection and screened the abstracts and articles. Y.W. and X.L. analyzed data. Y.Y. and X.X. wrote the main manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, Y., Wang, Y., Li, X. et al. Clinical role of pretreatment albumin-to-alkaline phosphatase ratio in lung cancer: a meta-analysis. Sci Rep 14, 1166 (2024). https://doi.org/10.1038/s41598-024-51844-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-51844-8
- Springer Nature Limited